Abstract
In the immature hippocampus, the so-called ‘giant depolarizing potentials’ (GDPs) are network-driven synaptic events generated by the synergistic action of glutamate and GABA. Here we tested the hypothesis that ATP, a widely distributed neurotransmitter, directly contributes to the network activity during the first postnatal week. We found that in CA3 pyramidal cells, in the presence of the adenosine antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), ATP produced a transient facilitation of GDPs followed by a depressant effect. A similar biphasic effect was produced by blockade of the ectoATPase activity with 6-N,N-diethyl-d-β,γ-dibromomethylene ATP (ARL-67156). The effects of exogenous and endogenous ATP on GDPs were prevented by the P2X receptor antagonist pyridoxal phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS). On pyramidal cells, ATP upregulated spontaneous action-potential-dependent GABAA-mediated synaptic events (GABA-SPSPs), suggesting a network-driven effect. Recordings from interneurones allowed comparison of ATP effects on GABAergic and glutamatergic synaptic activity. While ATP depressed GABA-SPSPs via metabotropic P2Y1 receptors, it up- and downregulated glutamatergic SPSPs via PPADS-sensitive receptors. Thus, ATP exerts an excitatory action on CA3 pyramidal cells via facilitation of GDPs and SPSPs. This excitatory drive is propagated to pyramidal cells by interneurons that represent the ‘common pathway’ for generation of GDPs and SPSPs. Our results show that ATP operating via distinct P2X and P2Y receptors directly contributes to modulate network activity at the early stages of postnatal development.
During the first postnatal week, hippocampal activity is characterized by spontaneous network-driven membrane oscillations, the so-called giant depolarizing potentials (GDPs; Ben-Ari et al. 1989). GDPs, which occur at the frequency of 0.01–0.3 Hz, are characterized by recurrent membrane depolarization with superimposed fast action potentials. The mechanism of GDP induction is not fully understood. It is commonly accepted that GDPs depend on the synergistic action of GABA and glutamate acting on GABAA, AMPA and NMDA receptors (Ben-Ari et al. 1989; Bolea et al. 1999). Early in postnatal life, GABA depolarizes and excites postsynaptic cells (Cherubini et al. 1991; Ben-Ari et al. 1997; Ben-Ari, 2002) through an outward flux of chloride. The depolarizing action of GABA during GDPs results in Ca2+ influx through N-methyl-d-aspartate receptors and voltage-dependent Ca2+ channels (Leinekugel et al. 1995, 1997; Garaschuk et al. 1998). Hence, as in many others developing systems (Yuste & Katz, 1991; Wong et al. 1995; Garaschuk et al. 2000; Spitzer et al. 2000), GDPs ensure large Ca2+ oscillations that may act as coincident detector signals for enhancing synaptic efficacy (Kasyanov et al. 2004).
ATP is a widely distributed neurotransmitter and neuromodulator in the peripheral (Giniatullin & Sokolova, 1998; Sokolova et al. 2003) and central nervous system (Burnstock, 2004; Illes & Ribeiro, 2004). Here, we explored the possibility that endogenous ATP released in the hippocampus during GDPs may affect network activity, thus contributing to sculpt the neural circuit at early stages of postnatal development.
In adult neurones, ATP is coreleased with glutamate and GABA (Ralevic & Burnstock, 1998; Burnstock, 2004), and mediates fast excitatory synaptic transmission (Edwards et al. 1992; Jo & Schlichter, 1999; Mori et al. 2001; Pankratov et al. 2002). ATP exerts its physiological action through ionotropic (P2X) or metabotropic (P2Y) receptor subtypes (Ralevic & Burnstock, 1998), which are highly expressed in the hippocampus (Rubio & Soto, 2001; Armstrong et al. 2002; Sperlagh et al. 2002; Illes & Ribeiro, 2004). While ionotropic receptors are mainly excitatory, metabotropic receptors participate in ATP-mediated inhibition of synaptically released glutamate (Mendoza-Fernandez et al. 2000). Moreover, ATP can be hydrolysed by ectonucleotidases to adenosine (Dunwiddie et al. 1997; Cunha et al. 1998; Cunha, 2001), which in turn may activate P1 receptors to inhibit transmitter release (Zimmermann & Braun, 1996; Cunha, 2001).
We have found that in the presence of the adenosine receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), ATP transiently increases and then decreases the frequency of GDPs, an effect that is mediated by ionotropic P2X receptors. Moreover, in principal cells, ATP, through the activation of metabotropic P2Y1 receptors, facilitates GABA release via disinhibition of GABAergic interneurones.
Methods
Slice preparation
Experiments were performed on hippocampal slices obtained from postnatal (P) P2–P6 Wistar rats (P0 was taken as the day of birth) as previously described (Maggi et al. 2001). The procedure was in accordance with the European Community Council Directive of 24 November 1986 (86/609EEC), and was approved by the local authority veterinary service. Briefly, animals were decapitated after being anaesthetized with an i.p. injection of urethane (2 g kg−1). The brain was quickly removed from the skull and placed in ice-cold artificial cerebrospinal fluid (ACSF) containing (mm): NaCl 130, KCl 3.5, NaH2PO4 1.2, NaHCO3 25, MgCl2 1.3, CaCl2 2, glucose 25, saturated with 95% O2 and 5% CO2 (pH 7.3–7.4). Transverse hippocampal slices (500 μm thick) were cut with a vibratome and incubated at room temperature (22–25°C) in oxygenated ACSF. After 1 h, an individual slice was transferred to a submerged recording chamber where it was continuously superfused at 33–34°C with oxygenated ACSF at a rate of 2–3 ml min−1.
Patch-clamp whole-cell recordings
Electrophysiological experiments were performed from CA3 pyramidal cells and from GABAergic interneurones localized on stratum radiatum using the whole-cell configuration of the patch-clamp technique in current-clamp mode. Neurones were visualized using infrared Nomarski optics on an upright microscope (Leica DMLFS, Wetzlar, Germany). Pyramidal cells and interneurones were identified on the basis of their localization and their particular firing patterns (see Figs 3A and 6A). Moreover, in the majority of the cases, neurones were injected with 3–4% biocytin (Nɛ-biotinyl-l-lysine; Sigma) for later morphological identification (Figs 3A and 6A). GDPs and spontaneous (action potential-dependent and -independent) ongoing synaptic potentials (SPSPs) were recorded from a holding potential of −70 ± 5 mV with a standard patch-clamp amplifier (Axoclamp 2B; Axon Instruments, Union City, CA, USA). In some experiments, miniature postsynaptic potentials (mSPSPs) were recorded in the presence of tetrodotoxin (TTX, 1 μm). Patch electrodes were pulled from borosilicate glass capillaries (Hilgenberg, Malsfeld, Germany). They had a resistance of 5–7 MΩ when filled with an intracellular solution containing (mm): KCl 140, MgCl2 1, NaCl 1, EGTA 1, Hepes 2 and K2ATP 2; the pH was adjusted to 7.3 with KOH.
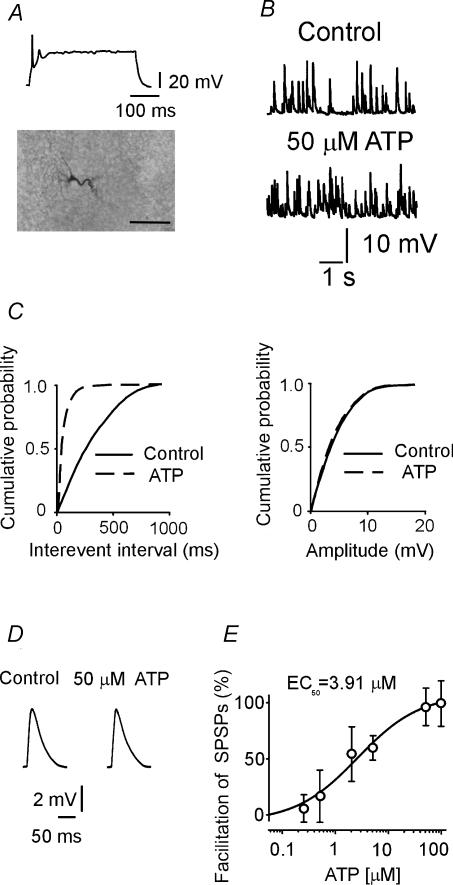
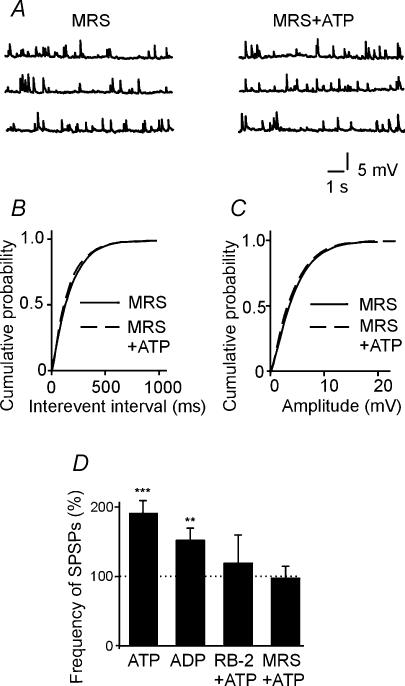
Figure 3. ATP enhances the frequency of spontaneous synaptic events (SPSPs) recorded in CA3 pyramidal cells.
A, representative trace showing the typical firing pattern of a CA3 pyramidal neurone at P3 in response to a long depolarizing current pulse. Below, the neurone labelled with biocytin (calibration bar, 50 μm). B, SPSPs in control and during application of ATP (50 μm). C, cumulative distributions of inter-event intervals (left) and amplitude (right) of SPSPs before (continuous line) and during (dashed line) application of ATP for the cell shown in B. Note that ATP increased the frequency but not the amplitude of SPSPs. D, average traces (each one from 100 events) in control and in the presence of ATP (cell shown in B). E, the frequency of SPSPs, normalized to control, is plotted against different ATP concentrations (data from 35 neurones).
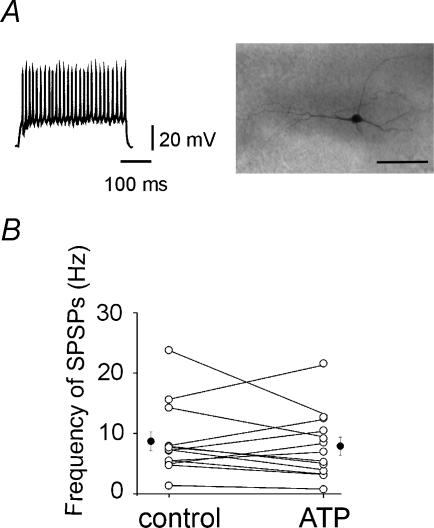
Figure 6. ATP increases or decreases the frequency of SPSPs recorded from GABAergic interneurones.
A, representative trace showing the characteristic firing pattern of a GABAergic interneurone in response to a long depolarizing current pulse. Right, the neurone labelled with biocytin (calibration bar, 50 μm). B, each line represents the frequency of SPSPs recorded from individual interneurones in the control and in the presence of ATP (open circles). Average values obtained in the control and during ATP are shown (filled circles).
The whole-cell capacitance was fully compensated and the series resistance (10–20 MΩ) was compensated at 75–80%. The stability of the patch was checked by repetitively monitoring the input and series resistance during the experiment. Cells exhibiting 15–20% changes were excluded from the analysis. Membrane input resistance was measured during recordings from the amplitude of the electrotonic potentials (300 ms duration) evoked by passing hyperpolarizing current steps of 100–200 pA across the cell membrane.
Drugs
All substances were prepared as 1000 times concentrated stock solutions. ATP (Sigma, Milan, Italy), 6-N,N-diethyl-d-β,γ-dibromomethylene ATP (ARL-67156; Tocris Cookson), adenosine (Sigma), adenosine 5′-O-(thiotriphosphate) (ATP-γ-S; Sigma), adenosine 5′-diphosphate sodium salt (ADP; Sigma), apyrase (grade III; Sigma), reactive blue-2 (Sigma), 2′-deoxy-N6-methyl adenosine 3′,5′-diphosphate diammonium salt (MRS-2179; Tocris Cookson), pyridoxal phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS; Tocris Cookson) and TTX (Latoxan) were prepared in distilled water. DPCPX (Tocris Cookson), picrotoxin (Sigma), bicuculline (Sigma) and 6,7-dinitroquinoxaline-2,3-dione (DNQX; Sigma) were dissolved in dimethylsulphoxide (DMSO). Control experiments using a solution containing 0.25% DMSO did not affect GDPs or SPSPs. Stock solutions were stored at −20°C. The substances to be tested were dissolved at their final concentrations in extracellular solution just before the recording session. All substances were applied through a three-way tap system. Bath volume exchange was completed within 1.5 min.
Data acquisition and analysis
Data were stored on a magnetic tape and transferred to a computer after digitization with an A/D converter (Digidata 1200; Axon Instruments). Data were sampled at 20 kHz and filtered with a cut-off frequency of 1 kHz. Acquisition and analysis were performed with Clampex 7 (Axon Instruments).
The analysis of SPSPs and mSPSPs was performed with Mini Analysis Program (Synaptosoft, Inc., USA). General principles and details on the analysis of synaptic currents can be found elsewhere (Poisbeau et al. 1996; Jo et al. 1998). The analysis of miniature synaptic currents was performed on sequences with at least 100 events each. Signals were repeatedly averaged for at least 15 min before infusion of agonists or antagonists to ensure stable baseline conditions. Spontaneous events were detected using amplitude thresholds set as a multiple (4–5 times) of the s.d. of the noise (0.05–0.1 mV).
The cumulative amplitude and inter-event plots obtained for cells in controls and after drug application were compared using the Kolmogorov–Smirnov test.
For each cell, the mean GDP frequency was calculated in control conditions and during drug application (starting 3 min after the onset of drug perfusion). For each cell, GDP area was measured in control conditions and after application of drugs using Clampfit 7.0. Statistical comparisons were made between mean values (control versus drug treatment). The numerical data are given as means ± s.e.m. and compared using Student's t test. The differences were considered significant when P < 0.05.
Measurement of ATP concentration
Hippocampal slices were prepared as described, and after 1 h they were transferred to the recording chamber, where they were washed at 33–34°C with oxygenated ACSF for 5 min. For measurements of basal ATP release, five 1 ml samples were collected from the bathing medium (bubbled with 95% O2 and 5% CO2) in a sterile Eppendorf. Then, after two additional controls TTX (1 μm) or ARL-67156 (50 μm) were added to the bathing solution. After 10 min in TTX- or ARL-67156-containing solution, 1 ml samples were collected and placed immediately into ice. Luciferase/luciferin reagent (Sigma) was reconstituted with the necessary amount of sterile bi-distilled water to obtain a 10 mg ml−1 solution at 25°C. Samples (20 μl) were assayed for their ability to promote ATP-dependent bioluminescence using a Turner Designs TD-20/20 (version 2020–1F 0302) luminometer according to the manufacturer's instructions. An ATP standard calibration curve was first constructed with fresh aliquots of ATP concentrations, and then assays of test samples were performed. The samples and ATP standard curve were run at least in duplicate to assess the reliability of the responses.
Results
Unless otherwise stated, all experiments reported in this study were performed in the presence of the adenosine receptor antagonist DPCPX (10 μm) to rule out the involvement of adenosine receptors (Khakh et al. 2003; Bowser & Khakh, 2004). Whole-cell recordings were obtained from CA3 pyramidal cells and stratum radiatum interneurones in slices from P2–P6 rats. These neurones exhibited spontaneous membrane oscillations (GDPs) typical of the immature brain. GDPs lasted 0.5–1 s and their amplitude ranged between 50 and 65 mV. In the presence of DPCPX (10 μm), GDP frequency was 0.15 ± 0.04 Hz (n = 32). In principal cells, GDPs were intermingled with small-amplitude SPSPs that occurred at frequencies of 5.5 ± 0.7 Hz (without DPCPX) and 5.4 ± 0.9 Hz (with DPCPX).
ATP firstly increases and then decreases the frequency of GDPs via P2X receptors
As shown in the representative example of Fig. 1A, bath-application of ATP (50 μm) produced a transient increase in GDP frequency followed by a depressant effect. Figure 1A shows also that the reduction in GDP frequency was associated with an increase in frequency of spontaneous synaptic events (see below). Altogether, in 14 cells, ATP (50 μm) increased the frequency of GDPs by 0.18 ± 0.001 to 0.28 ± 0.002 Hz (P < 0.001). This effect, which lasted a few minutes, was followed by a persistent depression (0.09 ± 0.002 Hz; P < 0.001; Fig. 1C). A full recovery was obtained 5–10 min after reintroducing the control solution. Both the up- and downregulation of GDP frequency occurred in the absence of any change in membrane potential, membrane input resistance or shape of GDPs. In five cells, GDP area was measured before and during ATP application. ATP did not modify GDP area, which was 14.8 ± 2.7 mV s−1 in control, and 14.2 ± 1.6 mV s−1 in the presence of ATP (P > 0.05).
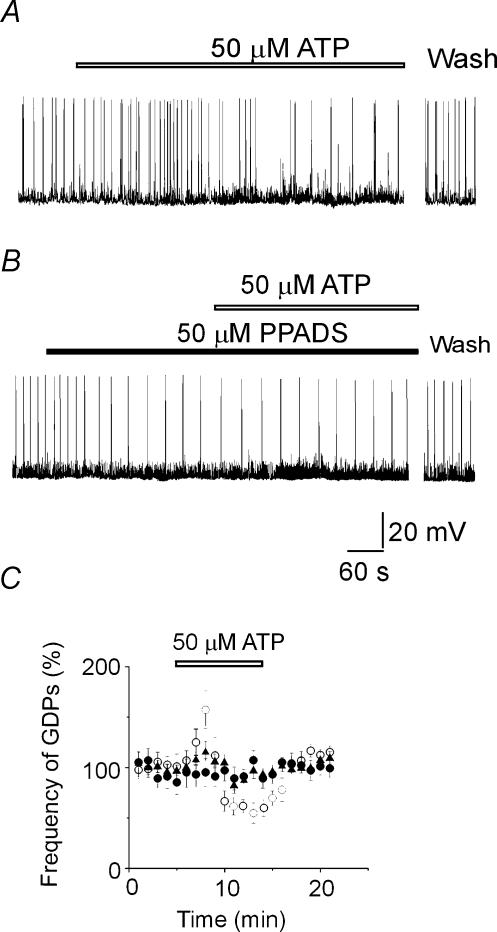
Figure 1. ATP transiently increases and then decreases frequency of giant depolarizing potentials (GDPs) via PPADS-sensitive receptors.
A, representative trace of GDPs recorded in the presence of 8-cyclopentyl-1,3-dipropylxanthine (DPCPX; 10 μm) at −75 mV from a CA3 pyramidal neurone before and during application of ATP (50 μm, open bar). Notice that ATP first increased and then decreased GDP frequency. The effect was fully reversed 5 min after ATP washout. B, in another cell, application of the P2X receptor antagonist pyridoxal phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS; filled bar) prevented the action of ATP (open bar) on GDP frequency. Note that PPADS per se reduced GDP frequency. C, effects of ATP (open bar) on the frequency of GDPs, expressed as a percentage of control versus time, in the absence (open circles; n = 14), and in the presence of 10 μm PPADS (filled triangles; n = 4) and 50 μm PPADS (filled circles; n = 6).
The potentiating and depressing effects of ATP on GDPs were prevented by PPADS (Fig 1B and C), an antagonist of ionotropic P2X1,2,3,5 receptors (North, 2002). In the presence of PPADS (50 μm), the frequency of GDPs was 0.09 ± 0.002 and 0.10 ± 0.002 Hz, before and during ATP application, respectively (P > 0.05; n = 6). Notably, PPADS per se diminished the frequency of GDPs (from 0.15 ± 0.002 to 0.09 ± 0.002 Hz; P < 0.01; n = 6; Fig. 1B) indicating that endogenous ATP is involved in GDP regulation. PPADS neither affected nor prevented the effects of ATP on SPSPs, which at this developmental stage have a predominantly GABAergic nature (see below; Hosokawa et al. 1994; Tyzio et al. 1999). Since PPADS at 50 μm can have some nonspecific effects (Ralevic & Burnstock, 1998), additional experiments were performed using a lower concentration of this antagonist. PPADS (10 μm) still reduced GDP frequency (from 0.14 ± 0.007 to 0.07 ± 0.001 Hz; P < 0.01; n = 4) and prevented both the facilitatory and inhibitory action of ATP on GDPs (Fig. 1C). Thus, GDP frequency was 0.07 ± 0.001 Hz in the presence of PPADS, and 0.06 ± 0.001 Hz in the presence of PPADS and ATP 50 μm (P > 0.05; n = 4). PPADS did not modify GDP area (in four cells this was 19.0 ± 2.1 and 18.5 ± 1.7 mV s−1 in control and in the presence of PPADS, respectively; P > 0.05). In the presence of PPADS (10 μm), ATP (50 μm) still produced a facilitation of SPSP frequency (from 4.9 ± 0.3 to 10.4 ± 0.9 Hz; P < 0.05; n = 3) in the absence of any change in amplitude, thus ruling out the possibility of a direct effect of this drug on GABAA receptors (North, 2002). On average, the amplitude of SPSPs was 4.6 ± 0.6 mV in control versus 5.0 ± 0.5 mV in the presence of PPADS (n = 7; P > 0.05).
Interestingly, the degree of GDP depression by PPADS was not different (P > 0.05; Mann–Whitney test) from that observed during the inhibitory phase of ATP action. Consistent with this observation, PPADS (10 μm) did not change the frequency of GDPs (0.09 ± 0.001 versus 0.08 ± 0.002 Hz; n = 4; P > 0.05) when it was applied after development of the inhibitory effect of ATP, suggesting that the latter was related to desensitization of PPADS-sensitive P2X receptors.
The potentiating effect of ATP on GDPs was not reproduced by 50 μm ADP, an agonist of several subtypes of metabotropic P2Y receptors (Ralevic & Burnstock, 1998; Illes & Ribeiro, 2004), suggesting that these receptors were not involved in GDP modulation. In the absence and presence of ADP, GDP frequency was 0.15 ± 0.001 and 0.14 ± 0.001 Hz, respectively (n = 8; P > 0.05). Consistent with this observation, the frequency of GDPs was not affected by MRS-2179 (10 μm) and reactive blue-2 (50 μm), antagonists of metabotropic P2Y receptors (data not shown).
Endogenous ATP is involved in GDP modulation
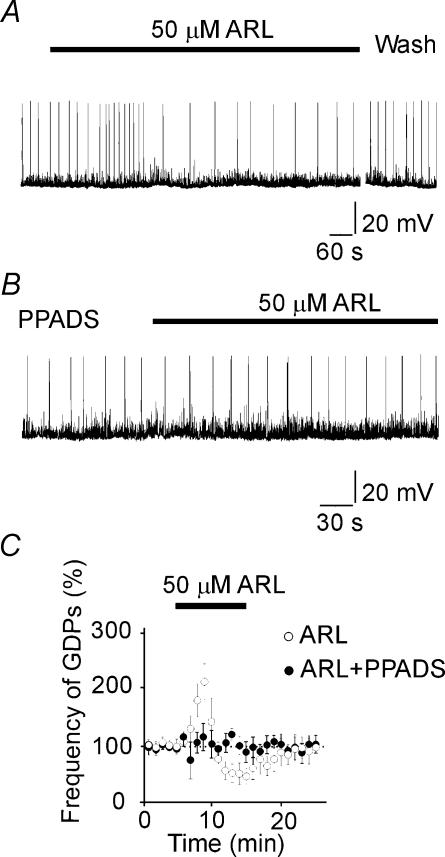
The reduction of GDP frequency by PPADS suggests the involvement of endogenous ATP in GDP generation. To further address this point, in the following experiments, ARL-67156, a selective ectoATPase inhibitor (Crack et al. 1995), was applied in order to increase the level of endogenous ATP. ARL-67156 (50 μm) first enhanced (from 0.03 ± 0.001 to 0.06 ± 0.006 Hz; P < 0.05) and then decreased (to 0.01 ± 0.003 Hz; P < 0.05; n = 7) the frequency of GDPs (Fig. 2A and C) in the same way as exogenously applied ATP. Like ATP, the effects of ARL-67156 on GDPs were associated with an increase in frequency of SPSPs (from 5.3 ± 0.2 to 6.1 ± 0.2 Hz; n = 4; P < 0.05; Fig. 2A). PPADS (50 μm) prevented the effect of ARL-67156 on GDPs, but not that on SPSPs, indicating that the latter were mediated by a different ATP receptor subtype. In the presence of PPADS, the frequency of GDPs was 0.02 ± 0.001 and 0.06 ± 0.004 Hz, before and during ARL-67156, respectively (P > 0.05; n = 4; Fig. 2B).
Figure 2. The ectoATPase inhibitor ARL-67156 produced a biphasic excitatory and inhibitory effect on GDPs.
A, bath application of 6-N,N-diethyl-d-β,γ-dibromomethylene ATP (ARL-67156; filled bar) first increased and then decreased the frequency of GDPs. B, the effect of ARL-67156 (filled bar) was prevented by PPADS (50 μm). C, effects of ARL-67156 (50 μm; filled bar) on the frequency of GDPs, expressed as a percentage of control versus time, in the absence (open circles; n = 7) and in the presence (filled circles; n = 4) of PPADS.
To further test the contribution of endogenous ATP to GDP generation, we applied (in presence of DPCPX) apyrase, an enzyme that hydrolyses endogenous ATP (Bowser & Khakh, 2004). Apyrase (20 U ml−1) decreased GDP frequency from 0.15 ± 0.001 to 0.10 ± 0.002 Hz (P < 0.05; n = 5; supplementary Fig. 1). This is consistent with a tonic action of endogenous ATP on GDPs. Apyrase did not significantly modify the frequency of SPSPs (5.29 ± 1.55 Hz in control, and 4.50 ± 2.16 Hz in the presence of apyrase; P > 0.05; n = 5). Taken together, these results indicate that endogenous ATP participates in the control of GDPs.
ATP increases the frequency of SPSPs recorded from pyramidal cells
As already mentioned, the effects of ATP on GDPs were associated with an increase in frequency of SPSPs. This effect was concentration dependent and reversible, since it completely recovered after washing out ATP. Thus, ATP (0.5 μm) enhanced the frequency of SPSPs from 5.6 ± 0.6 to 6.4 ± 1.3 Hz (n = 5; P < 0.05), while ATP (50 μm) enhanced the frequency of SPSPs from 5.6 ± 0.6 to 10.9 ± 0.9 Hz (n = 15; P < 0.01; Fig. 3B). As shown in the cumulative probability plots of Fig. 3C, in spite of a clear effect on the frequency, ATP did not modify the amplitude of spontaneous events, suggesting a presynaptic site of action. On average, the peak amplitude of SPSPs was 5.01 ± 0.73 and 5.04 ± 0.56 mV before and after ATP (50 μm), respectively. ATP did not modify the kinetics of spontaneous events (Fig. 3D). The EC50 value for the facilitatory effect of ATP on the frequency of SPSPs was 3.91 μm (n = 38; Fig. 3E). The facilitating effect of ATP on SPSPs was mimicked by ATP-γ-S, a relatively stable analogue of ATP (Ralevic & Burnstock, 1998). Thus, bath application of ATP-γ-S (50 μm) increased SPSP frequency from 5.5 ± 0.5 to 10.4 ± 0.8 Hz (n = 3; P < 0.01; data not shown). Another P2Y receptor agonist, ADP (50 μm), increased the frequency of SPSPs from 4.2 ± 0.3 to 6.2 ± 0.6 Hz (n = 6; P < 0.01). Thus, as shown in Fig. 4, the potentiating action of ATP on SPSPs recorded from pyramidal cells was clearly mediated by P2Y1 receptors, since it was mimicked by ADP and prevented by reactive blue-2, a general antagonist of P2Y receptors (Ralevic & Burnstock, 1998), or MRS-2179, a selective antagonist of P2Y1 receptor subtype (Illes & Ribeiro, 2004). MRS-2179 alone slightly reduced the frequency of SPSPs without reaching the significance level (from 5.6 ± 0.2 to 5.0 ± 0.1 Hz; P > 0.05; n = 5).
Figure 4. The potentiating effects of ATP on SPSPs recorded in principal cells is mediated by P2Y1 receptors.
A, representative traces obtained at −70 mV in the presence of MRS-2179 (10 μm; left) and MRS-2179 plus ATP (50 μm; right). Note that MRS-2179, a selective antagonist of P2Y1 receptors, prevented the potentiating action of ATP on SPSPs. Cumulative probability plots of inter-event intervals (B) and amplitude (C) of SPSPs in the presence of MRS-2179 (continuous line) or MRS-2179 plus ATP (dashed line) for the cell shown in A. D, each bar represents the mean frequency of SPSPs obtained in ATP (50 μm), ADP (50 μm), ATP plus reactive blue-2 (RB-2; 50 μm) or ATP plus MRS-2179 (10 μm). Data are from 3–8 cells normalized to control values. Note that both antagonists prevented the action of ATP on SPSPs. **P < 0.01; ***P < 0.001.
The action of ATP on SPSPs occurs at the network level
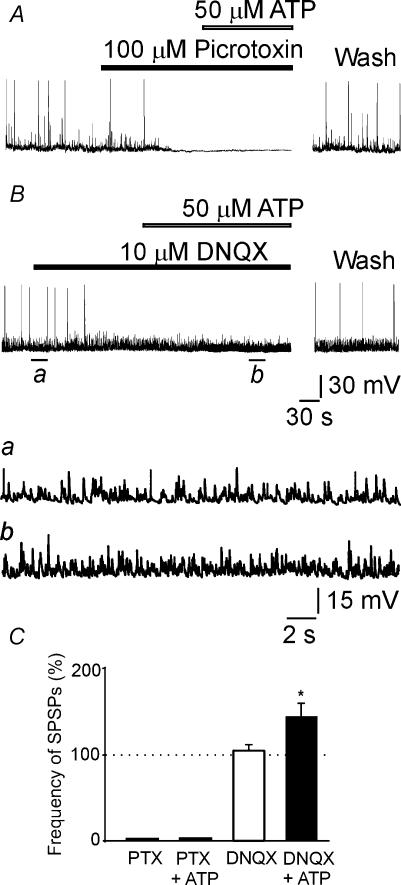
SPSPs recorded from pyramidal cells result from local network activity which depends on the activation of both GABAA and AMPA receptors (Ben-Ari et al. 1989; Cherubini et al. 1991). The GABAA receptor channel blocker picrotoxin (100 μm) completely eliminated SPSPs (along with GDPs) indicating that during the first postnatal week pyramidal cells are driven by GABA released from GABAergic terminals (Fig. 5A; see also Hosokawa et al. 1994).
Figure 5. ATP facilitates SPSPs mainly mediated by GABA release into principal cells.
A, trace showing GDPs and spontaneous ongoing synaptic activity at −70 mV before and during application of the GABAA channel blocker picrotoxin (filled bar) and picrotoxin plus ATP (open bar). Note that picrotoxin fully abolished GDPs and background synaptic activity, which was not restored by addition of ATP. B, in another cell, application of the AMPA/kainate receptor antagonist 6,7-dinitroquinoxaline-2,3-dione (DNQX; 10 μm; filled bar) blocked GDPs but not spontaneous events, indicating that they were mainly GABAA mediated. Further application of ATP (open bar) increased the frequency of GABAA-mediated SPSPs. These are shown below (a and b) in an expanded time scale. C, each bar represents the mean frequency value of SPSPs obtained in the presence of picrotoxin (PTX), PTX plus ATP (50 μm), DNQX, and DNQX plus ATP, normalized to pre-drug control values. Data were obtained from 3–4 cells. *P < 0.05.
DNQX, a blocker of AMPA/kainate receptors, abolished GDPs but failed to block the potentiating effect of ATP on SPSPs (Fig. 5B and C, see insets Ba and Bb for better time resolution). Thus, in the presence of DNQX (10 μm), ATP (50 μm) was still able to increase the frequency of SPSPs from 8.6 ± 1.9 to 13.0 ± 3.9 Hz (n = 3; P < 0.05). This result suggests a direct action of ATP on GABAergic interneurones.
It is known that a prolonged exposure to GABAA antagonists produces low-frequency interictal-like events which involve recurrent glutamatergic collaterals (Khazipov et al. 2004). To see whether ATP can modulate interictal activity generated by glutamatergic recurrent collaterals in the absence of GABAA-mediated inhibition, bicuculline (20 μm) was applied (in the presence of DPCPX) for 10–15 min. In these conditions, interictal events developed at 0.03 ± 0.007 Hz (n = 6). Further application of ATP (50 μm) did not modify the frequency of interictal events (0.04 ± 0.007 Hz; P > 0.05), indicating that recurrent glutamatergic collaterals were not involved in ATP action (see supplementary Fig. 2).
In order to test whether the effects of ATP on SPSPs were action-potential dependent, in three cells ATP was applied in the presence of TTX (1 μm). In these conditions, ATP (50 μm) failed to modify the frequency of SPSPs (2.8 ± 0.4 and 2.6 ± 0.5 Hz, before and after ATP, respectively; P > 0.05). Likewise, ATP did not change the amplitude of miniature events (4.8 ± 0.7 and 4.9 ± 0.6 mV, before and after ATP, respectively). These results indicate that ATP acts at the network level upstream of pyramidal cells, most probably on interneurones projecting to principal cells.
Effects of ATP on GABAergic interneurones
In order to identify at the network level where the action of ATP originated from, direct recordings from interneurones were performed in the presence of DPCPX (10 μm; n = 14). Interneurones were all localized on stratum radiatum, in close vicinity to stratum lucidum. They had a resting membrane potential ranging from −44 to −70 mV (on average −54.7 ± 2.4 mV), input resistance ranging from 129 to 400 MΩ (on average 293 ± 23 MΩ). They discharged at high frequency (63 ± 7 Hz) in response to long depolarizing current pulses (100 pA, 400 ms; Fig. 6A).
In contrast with pyramidal cells, ATP had two different effects in interneurones: it decreased or increased the frequency of SPSPs. In 8 out of 13 cells, ATP (50 μm) reduced the frequency of SPSPs from 9.8 ± 2.4 to 5.3 ± 1.3 Hz (Fig. 6B), while in the remaining five cells, it increased the frequency of SPSPs from 8.2 ± 1.9 to 12 ± 2 Hz (Fig. 6B). These effects occurred in the absence of any change in membrane potential, input resistance, amplitude or time course of spontaneous events (not shown), suggesting a presynaptic site of action. Moreover, this effect was action-potential dependent since it was not observed in the presence of TTX. TTX (1 μm) significantly reduced the frequency of SPSPs (from 6.7 ± 0.8 to 0.8 ± 0.1 Hz; P > 0.05; n = 4; see also Kawamura et al. 2004). Moreover, application of ATP (50 μm) in the presence of TTX did not affect the frequency of miniature events that remained almost unchanged (0.7 ± 0.2 Hz; P > 0.05; n = 4).
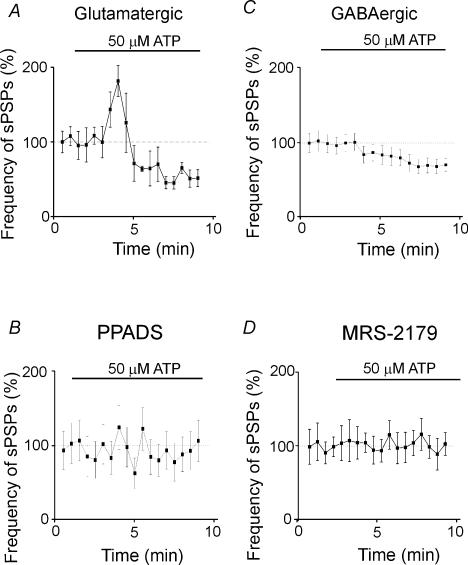
Effect of ATP on the glutamatergic and GABAergic inputs to interneurones
ATP (50 μm) was applied in the presence of DPCPX (10 μm) plus DNQX (20 μm), or DPCPX (10 μm) plus picrotoxin (100 μm), to block adenosine and AMPA/kainate or GABAA receptors, respectively. In keeping with the late development of glutamatergic connections (Hosokawa et al. 1994; Tyzio et al. 1999; Hennou et al. 2002) in the presence of picrotoxin, spontaneous glutamatergic events were merely detectable. However, in comparison with pyramidal cells, they occurred more often at the frequency of 1.1 ± 0.18 Hz. Application of ATP (50 μm) to GABAergic interneurones increased the frequency of AMPA/kainate-mediated SPSPs from 1.1 ± 0.18 to 1.6 ± 0.26 Hz (P < 0.05; n = 3). ATP-induced facilitation of SPSPs was transient and was followed by a persistent depression below control level (0.6 ± 0.2 Hz; P < 0.05; n = 3; Fig. 7A). ATP did not modify the amplitude of SPSPs (this was 1.4 ± 0.3 and 1.5 ± 0.2 mV in the presence and absence of ATP, respectively; P > 0.05; n = 3). As in the case of GDPs, both the potentiating and depressant effects of ATP on spontaneous glutamatergic events were prevented by PPADS (50 μm; Fig. 7B), suggesting the involvement of P2X receptor subtypes.
Figure 7. ATP differentially modulates glutamatergic and GABAergic inputs into interneurones.
A, the frequency of glutamatergic SPSPs (recorded in the presence of 10 μm DPCPX and 100 μm PTX) is plotted against time before and during application of ATP. Each point represents the mean frequency of SPSPs in 30 s (n = 3). Note the ATP induced a transient increase followed by a decrease in frequency of SPSPs. B, PPADS (50 μm) prevented both the effects of ATP (n = 3). C, the frequency of GABAergic SPSPs (recorded in the presence of 10 μm DPCPX and 20 μm DNQX) is plotted against time before and during application of ATP (n = 9). Note that ATP slowed down the frequency of SPSPs. D, MRS-2179, an antagonist of P2Y1 receptors, prevented the effect of ATP on GABA-mediated SPSPs (n = 3).
In additional experiments from nine interneurones, ATP was applied in the presence of DNQX (10 μm) to reveal spontaneous GABAergic events. ATP (50 μm) reduced the frequency of GABAA-mediated synaptic responses from 6.5 ± 0.9 to 4.3 ± 0.7 Hz (P < 0.05; Fig. 7C). As for glutamatergic events, ATP did not affect the amplitude of spontaneous synaptic responses (this was 6.2 ± 0.7 and 5.9 ± 0.6 mV in the presence or absence of ATP, respectively; P > 0.05). The effect of ATP on GABAergic events was prevented by 10 μm MRS-2179 (n = 3; Fig. 7D), suggesting that it was mediated by P2Y1 receptor subtype. Consistent with the involvement of P2Y1 receptors, ADP (50 μm) reduced the frequency of spontaneous GABAergic events from 6.8 ± 0.5 to 5.6 ± 0.4 Hz (P < 0.05; n = 4).
In summary, it appears that P2X receptor subtypes are involved in both up- and downregulation of GDPs and spontaneous glutamatergic events, while the P2Y1 receptor subtype seems to exert a positive control on GABA released into principal cells.
Release of endogenous ATP during GDPs
In the hippocampus of adult animals, endogenous ATP can be released following stimulation of the Schaffer collateral (Wieraszko et al. 1989). To see whether in the immature hippocampus ATP can be released during GDPs, in the following experiments the lucifirase/luciferin method (see Methods; Cunha et al. 1998) was used to measure the endogenous levels of ATP present in control conditions. The concentration of ATP present in the extracellular space was 80 ± 6 pm. In the presence of TTX, the concentration of ATP dropped to 68 ± 7% of the control value (n = 5; P < 0.05). In contrast, ATP levels increased to 144 ± 13%(n = 5; P < 0.05) after application of the ectoATPases inhibitor ARL-67156 (50 μm for 10 min). Since TTX blocks both GDPs and SPSPs, these results indicate that ATP is released, in an activity-dependent way during GDPs and SPSPs.
Additional evidence that ATP is released during GDPs was given by the experiments in which the frequency of SPSPs was measured immediately after GDPs, in the presence or absence of the ectoATPase inhibitor ARL-67156. In the presence of ARL-67156 (50 μm), the frequency of SPSPs occurring in a 1 s period following a GDP was significantly higher than the average frequency (9.0 ± 0.8 versus 6.3 ± 0.5 Hz; n = 5; P < 0.05; data not shown). It was also higher than the frequency of SPSPs occurring in a 1 s period taken every 3 min (6.6 ± 0.4 Hz; n = 5; P < 0.05). This suggests that endogenous ATP, released in an activity-dependent way, regulates SPSPs. In the absence of ARL-67156, no facilitation of SPSPs was observed after GDPs.
Discussion
The main finding of the present experiments is that in the neonatal rat hippocampus, endogenous ATP modulates the expression of GDPs, which appear to play a key role in the development of the adult circuit (Kasyanov et al. 2004). In particular, ATP regulates the occurrence of GDPs via ionotropic P2X receptors. Moreover, ATP, through metabotropic P2Y1 receptors, exerts a powerful excitatory action on GABAA-mediated SPSPs recorded from principal cells. At the network level, the combined activation of these two types of ATP receptors leads to an increase of spontaneous synaptic activity and GDPs in CA3 pyramidal cells.
The potentiating effect of ATP on GDPs and SPSPs is mediated by ionotropic P2X and metabotropic P2Y1 receptors
ATP is broken down by ectoATPases into adenosine, which in turn could act on adenosine receptor masking the direct action of ATP. Therefore, the present experiments were done in the presence of the adenosine antagonist DPCPX, which at the concentration used blocks all types of adenosine receptors (Khakh et al. 2003; Bowser & Khakh, 2004). In these conditions, we found a significant facilitatory action of ATP on GDPs followed by a depressant effect. This biphasic type of modulation was replicated (with the same time profile) in experiments with ARL-67156, an ectoATPase inhibitor, which presumably increases the concentration of endogenous ATP in the extracellular space (Crack et al. 1995). Consistent with the involvement of endogenous ATP in GDP generation, apyrase, an enzyme which specifically destroys ATP (Khakh et al. 2003; Kawamura et al. 2004), significantly reduced GDP frequency.
Remarkably, effects of both ATP and ARL-67156 were prevented by PPADS, an antagonist of ionotropic P2X receptors (Ralevic & Burnstock, 1998; North, 2002). ADP, an agonist of metabotropic P2Y receptors (Ralevic & Burnstock, 1998), did not affect GDPs. Thus, P2X receptor subtypes appear to be responsible not only for the initial facilitation, but also for the delayed depression of GDP frequency, which was observed during prolonged action of ATP. It is tempting to speculate that the enhancement of GDP frequency with ATP or ARL-67156 results from activation of P2X receptors with consequent desensitization, as suggested by the occlusion experiments with PPADS.
The exact subtype of P2X receptor controlling GDP generation remains to be established. However, a widespread localization of ionotropic P2X2 receptor subtype in hippocampus (Illes & Ribeiro, 2004) suggests that P2X2 receptors, with their high sensitivity to PPADS (North, 2002), most probably mediate the action of ATP on GDPs.
While the potentiating effects of ATP on GDPs was mediated by P2X receptors, that on GABAergic SPSPs recorded from pyramidal cells was dependent on the activation of metabotropic P2Y1 receptors, since it was prevented by MRS-2179, a specific antagonist of this receptor subtype (Scemes et al. 2003; Illes & Ribeiro, 2004). However, this effect occurred within the network since it was abolished by TTX.
GABAergic and glutamatergic inputs to interneurones control distinct ATP receptors
Surprisingly, in contrast to pyramidal cells, SPSPs occurring in interneurones were differentially modulated by ATP: the frequency of spontaneous events was either increased or decreased by this nucleotide. The reason for this dual effect may be due to the predominant effect of ATP either on the GABAergic or glutamatergic pathway. Thus, when we examined these inputs separately, it became clear that glutamatergic events were transiently facilitated by ATP. As in the case of GDPs, the action of ATP on glutamatergic synaptic currents was biphasic and was prevented by PPADS but not by the P2Y1 receptor antagonist MRS-2179, indicating that it was mediated via ionotropic P2X receptor subtypes. On the contrary, ATP exerted a clear inhibitory effect on the GABA-mediated SPSP that was insensitive to PPADS but was blocked by MRS-2179, suggesting the involvement of metabotropic P2Y1 receptors. Consistent with the involvement of P2Y1 receptors, the inhibitory action of ATP was mimicked by ADP, an agonist of metabotropic P2Y receptors (Ralevic & Burnstock, 1998; Illes & Ribeiro, 2004). In agreement with the present findings, it has been recently reported that ATP, via P2Y1 receptors, facilitates GABAergic IPSCs recorded from pyramidal cells (Bowser & Khakh, 2004; Kawamura et al. 2004). However, while in the case of Kawamura et al. (2004) facilitation of GABAA-mediated SPSPs was attributed to a direct depolarization of interneurones, in our case it was consistent with disinhibition of principal cells because we did not observe any direct postsynaptic effect of ATP on GABAergic interneurones (see below). It should be stressed that, in contrast to the present experiments, previous results were obtained from older animals using relative high doses of P2 agonists.
Two types of network activity converge into pyramidal cells
Synchronized oscillatory activity such as GDPs constitutes a peculiar feature of developing brain. GDPs have also been recorded in vivo in rat pups, where they occur during immobility periods, sleep and feeding (Leinekugel et al. 2002). In this respect, GDPs can be seen as a primordial form of synchrony between neurones, which precedes the development of the theta- and gamma-rhythm (Leblanc & Bland, 1979; Avignone & Cherubini, 1999). Spontaneous synaptic activity is also crucial for neurodevelopment, since block of this type of activity with tetanus toxin produces a severe impairment of hippocampal network (Groc et al. 2002).
Taken together, our data are consistent with the role of interneurones as integrators of two types of network activity: GDPs and SPSPs originating from glutamatergic and GABAergic inputs. These two types of activity, GDPs and GABAergic SPSPs, are relatively independent since they are controlled by distinct ATP receptor subtypes located at presynaptic level. However, functionally, they both provide an excitatory drive to pyramidal cells, since at this developmental stage GABAergic interneurones impinging into principal cells exert a depolarizing and excitatory action (Ben-Ari et al. 1989; Cherubini et al. 1991). In previous work (Khazipov et al. 1997), it was shown that activation of GABAA receptors contributes to action-potential generation in CA3 interneurones. However, it is conceivable that early in postnatal life different interneurones express different degrees of maturation, and that those contacting other interneurones are predominantly inhibitory. We hypothesize, therefore, that ATP-induced facilitation of GDPs results from disinhibition of GABAergic inputs to excitatory interneurones, in accord with previous work (Maggi et al. 2001). It is clear, however, that the coincident ATP-induced excitation of glutamatergic inputs to GABAergic interneurones via P2X receptors contributes to GDP generation. The present results allow, however, exclusion of the contribution of glutamatergic recurrent collaterals in such an effect, since ATP did not modify the interictal discharges induced by the prolonged exposure of the slice to bicuculline (Khazipov et al. 2004). Therefore, the balance between the activation of P2X and P2Y1 receptor types by endogenous ATP would differently regulate the release of glutamate and GABA within the CA3 network, thus contributing to increase synaptic activity and GDP generation. The level of network activity would, in turn, control the concentration of endogenous ATP at synapses.
Does endogenous ATP control synchronized network activity?
The experiments with ARL-67156 and apyrase clearly demonstrate that endogenous ATP is involved in GDP regulation. Further support in favour of ATP as an endogenous modulator of GDPs is given by the observation that PPADS by itself slowed down GDP frequency. The sensitive luciferase/luciferine method also revealed release of ATP in conditions in which network activity was not blocked.
Basic questions are: from where is endogenous ATP released, and how does it control GDPs? Although ATP can be released from neurones and/or astrocytes (Bowser & Khakh, 2004), the reduction of endogenous ATP by TTX favours a vesicular type of release from neuronal elements. It is known that ATP is stored in synaptic vesicles (Zimmermann & Braun, 1996) from where it can be released with GABA or glutamate (Jo & Schlichter, 1999; Burnstock, 2004). Our biochemical assay has most likely underestimated the real concentration of ATP attained at synaptic level since, in synaptic vesicles, ATP is stored in millimolar concentrations (Zimmermann & Braun, 1996; Burnstock, 2004) and, being released, the ATP concentration drops due to diffusion and hydrolysis. Although we don't know the exact concentration of ATP at the synaptic level, from the present experiments it is clear that this nucleotide plays a crucial role in regulating network activity early in postnatal development.
Acknowledgments
We are grateful to Andrea Nistri for helpful discussion, and to Massimo Righi for excellent technical assistance. This work was supported by a grant from Ministero Istruzione Universita′ e Ricerca (MIUR & COFI 2003 to EC), FIRB (Italy) and RFBR (Russia) to V.F.S. and R.G.
Supplemental material
The online version of this paper can be accessed at: 10.1113/jphysiol.2005.085621 http://jp.physoc.org/cgi/content/full/jphysiol.2005.085621/DC1 and contains supplemental material consisting of a figure entitled:Apyrase reduces the frequency of GDPs;and ATP does not affect interictal discharges
This material can also be found as part of the full-text HTML version available from http://www.blackwell-synergy.com
References
- Armstrong JN, Brust TB, Lewis RG, MacVicar BA. Activation of presynaptic P2X7-like receptors depresses mossy fiber–CA3 synaptic transmission through p38 mitogen-activated protein kinase. J Neurosci. 2002;15:5938–5945. doi: 10.1523/JNEUROSCI.22-14-05938.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avignone E, Cherubini E. Muscarinic receptor modulation of GABA-mediated giant depolarizing potentials in the neonatal rat hippocampus. J Physiol. 1999;518:97–107. doi: 10.1111/j.1469-7793.1999.0097r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa JL. GABAA, NMDA and AMPA receptors: a developmentally regulated ‘ménage à trois’. Trends Neurosci. 1997;20:523–529. doi: 10.1016/s0166-2236(97)01147-8. [DOI] [PubMed] [Google Scholar]
- Bolea S, Avignone E, Berretta N, Sanchez-Andres JV, Cherubini E. Glutamate controls the induction of GABA-mediated giant depolarizing potentials through AMPA receptors in neonatal rat hippocampal slices. J Neurophysiol. 1999;81:2095–2102. doi: 10.1152/jn.1999.81.5.2095. [DOI] [PubMed] [Google Scholar]
- Bowser DN, Khakh BS. ATP excites interneurons and astrocytes to increase synaptic inhibition in neuronal networks. J Neurosci. 2004;24:8606–8620. doi: 10.1523/JNEUROSCI.2660-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Cotransmission. Curr Opin Pharmacol. 2004;4:47–52. doi: 10.1016/j.coph.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Cherubini E, Gaiarsa JL, Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Crack B, Pollard C, Beukers M, Roberts S, Hunt S, Ingall A. Pharmacological and biochemical analysis of FPL 67156, a novel, selective inhibitor of ecto-ATPase. et al. 1995;114:475–481. doi: 10.1111/j.1476-5381.1995.tb13251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA. Regulation of the ecto-nucleotidase pathway in rat hippocampal nerve terminals. Neurochem Res. 2001;26:979–991. doi: 10.1023/a:1012392719601. [DOI] [PubMed] [Google Scholar]
- Cunha R, Sebastião A, Ribeiro J. Inhibition by ATP of hippocampal synaptic transmission requires localized extracellular catabolism by ecto-nucleotidases into adenosine and channeling to adenosine A1 receptors. J Neurosci. 1998;18:1987–1995. doi: 10.1523/JNEUROSCI.18-06-01987.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie TV, Diao L, Proctor WR. Adenine nucleotides undergo rapid, quantitative conversion to adenosine in the extracellular space in rat hippocampus. J Neurosci. 1997;17:7673–7682. doi: 10.1523/JNEUROSCI.17-20-07673.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards FA, Gibb AJ, Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992;359:144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- Garaschuk O, Hanse E, Konnerth A. Developmental profile and synaptic origin of early network oscillations in the CA1 region of rat neonatal hippocampus. J Physiol. 1998;15:219–236. doi: 10.1111/j.1469-7793.1998.219bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaschuk O, Linn J, Eilers J, Konnerth A. Large-scale oscillatory calcium waves in the immature cortex. Nat Neurosci. 2000;3:452–459. doi: 10.1038/74823. [DOI] [PubMed] [Google Scholar]
- Giniatullin R, Sokolova E. ATP and adenosine inhibit transmitter release at the frog neuromuscular junction through distinct presynaptic receptors. Br J Pharmacol. 1998;124:839–844. doi: 10.1038/sj.bjp.0701881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc L, Petanjek Z, Gustafsson B, Ben-Ari Y, Hanse E, Khazipov R. In vivo blockade of neural activity alters dendritic development of neonatal CA1 pyramidal cells. Eur J Neurosi. 2002;16:1931–1938. doi: 10.1046/j.1460-9568.2002.02264.x. [DOI] [PubMed] [Google Scholar]
- Hennou S, Khalilov I, Diabira D, Ben-Ari Y, Gozlan H. Early sequential formation of functional GABAA and glutamatergic synapses on CA1 interneurons of the rat foetal hippocampus. Eur J Neurosci. 2002;16:197–208. doi: 10.1046/j.1460-9568.2002.02073.x. [DOI] [PubMed] [Google Scholar]
- Hosokawa Y, Sciancalepore M, Stratta F, Martina M, Cherubini E. Developmental changes in spontaneous GABAA-mediated synaptic events in rat hippocampal CA3 neurons. Eur J Neurosci. 1994;6:805–813. doi: 10.1111/j.1460-9568.1994.tb00991.x. [DOI] [PubMed] [Google Scholar]
- Illes P, Ribeiro JA. Molecular physiology of P2 receptors in the central nervous system. Eur J Pharmacol. 2004;483:5–17. doi: 10.1016/j.ejphar.2003.10.030. [DOI] [PubMed] [Google Scholar]
- Jo YH, Schlichter R. Synaptic corelease of ATP and GABA in cultured spinal neurons. Nat Neurosci. 1999;2:241–245. doi: 10.1038/6344. [DOI] [PubMed] [Google Scholar]
- Jo YH, Stoeckel ME, Schlichter R. Electrophysiological properties of cultured neonatal rat dorsal horn neurons containing GABA and met-enkephalin-like immunoreactivity. J Neurophysiol. 1998;79:1583–1586. doi: 10.1152/jn.1998.79.3.1583. [DOI] [PubMed] [Google Scholar]
- Kasyanov AM, Safiulina VF, Voronin LL, Cherubini E. GABA-mediated giant depolarizing potentials as coincidence detectors for enhancing synaptic efficacy in the developing hippocampus. Proc Natl Acad Sci U S A. 2004;101:3967–3972. doi: 10.1073/pnas.0305974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura M, Gachet C, Inoue K, Kato F. Direct excitation of inhibitory interneurons by extracellular ATP mediated by P2Y1 receptors in the hippocampal slice. J Neurosci. 2004;24:10835–10845. doi: 10.1523/JNEUROSCI.3028-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Gittermann D, Cockayne DA, Jones A. ATP modulation of excitatory synapses onto interneurons. J Neurosci. 2003;23:7426–7437. doi: 10.1523/JNEUROSCI.23-19-07426.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazipov R, Khalilov I, Tyzio R, Morozova E, Ben-Ari Y, Holmes GL. Developmental changes in GABAergic actions and seizure susceptibility in the rat hippocampus. Eur J Neurosci. 2004;19:590–600. doi: 10.1111/j.0953-816x.2003.03152.x. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Leinekugel X, Khalilov I, Gaiarsa JL, Ben-Ari Y. Synchronization of GABAergic interneuronal network in CA3 subfield of neonatal rat hippocampal slices. J Physiol. 1997;498:763–772. doi: 10.1113/jphysiol.1997.sp021900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc MO, Bland BH. Developmental aspects of hippocampal electrical activity and motor behavior in the rat. Exp Neurol. 1979;66:220–237. doi: 10.1016/0014-4886(79)90076-1. [DOI] [PubMed] [Google Scholar]
- Leinekugel X, Khazipov R, Cannon R, Hirase H, Ben-Ari Y, Buzsaki G. Correlated bursts of activity in the neonatal hippocampus in vivo. Science. 2002;296:2049–2052. doi: 10.1126/science.1071111. [DOI] [PubMed] [Google Scholar]
- Leinekugel X, Medina I, Khalilov I, Ben-Ari Y, Khazipov R. Ca2+ oscillations mediated by the synergistic excitatory actions of GABAA and NMDA receptors in the neonatal hippocampus. Neuron. 1997;18:243–255. doi: 10.1016/s0896-6273(00)80265-2. [DOI] [PubMed] [Google Scholar]
- Leinekugel X, Tseeb V, Ben-Ari Y, Bregestovski P. Synaptic GABAA activation induces Ca2+ rise in pyramidal cells and interneurons from rat neonatal hippocampal slices. J Physiol. 1995;487:319–329. doi: 10.1113/jphysiol.1995.sp020882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi L, Sher E, Cherubini E. Regulation of GABA release by nicotinic acetylcholine receptors in the neonatal rat hippocampus. J Physiol. 2001;536:89–100. doi: 10.1111/j.1469-7793.2001.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Fernandez V, Andrew RD, Barajas-Lopez C. ATP inhibits glutamate synaptic release by acting at P2Y receptors in pyramidal neurons of hippocampal slices. J Pharmacol Exp Ther. 2000;293:172–179. [PubMed] [Google Scholar]
- Mori M, Heuss C, Gahwiler BH, Gerber U. Fast synaptic transmission mediated by P2X receptors in CA3 pyramidal cells of rat hippocampal slice cultures. J Physiol. 2001;535:115–123. doi: 10.1111/j.1469-7793.2001.t01-1-00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Pankratov YV, Lalo UV, Krishtal OA. Role for P2X receptors in long-term potentiation. J Neurosci. 2002;22:8363–8369. doi: 10.1523/JNEUROSCI.22-19-08363.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poisbeau P, Rene F, Egles C, Felix JM, Feltz P, Schlichter R. Characterization of functional GABAergic synapses formed between rat hypothalamic neurons and pituitary intermediate lobe cells in coculture: Ca2+ dependence of spontaneous IPSCs. J Neurosci. 1996;16:4835–4845. doi: 10.1523/JNEUROSCI.16-16-04835.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Rubio ME, Soto F. Distinct localization of P2X receptors at excitatory postsynaptic specializations. J Neurosci. 2001;21:641–653. doi: 10.1523/JNEUROSCI.21-02-00641.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scemes E, Duval N, Meda P. Reduced expression of P2Y1 receptors in connexin43-null mice alters calcium signaling and migration of neural progenitor cells. J Neurosci. 2003;23:11444–11452. doi: 10.1523/JNEUROSCI.23-36-11444.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolova E, Grishin S, Shakirzyanova A, Talantova M, Giniatullin R. Distinct receptors and different transduction mechanisms for ATP and adenosine at the frog motor nerve endings. Eur J Neurosci. 2003;18:1254–1264. doi: 10.1046/j.1460-9568.2003.02835.x. [DOI] [PubMed] [Google Scholar]
- Sperlagh B, Kofalvi A, Deuchars J, Atkinson L, Milligan CJ, Buckley NJ, Vizi ES. Involvement of P2X7 receptors in the regulation of neurotransmitter release in the rat hippocampus. J Neurochem. 2002;81:1196–1211. doi: 10.1046/j.1471-4159.2002.00920.x. [DOI] [PubMed] [Google Scholar]
- Spitzer NC, Lautermilch NJ, Smith RD, Gomez TM. Coding of neuronal differentiation by calcium transients. Bioassays. 2000;22:811–817. doi: 10.1002/1521-1878(200009)22:9<811::AID-BIES6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Tyzio R, Represa A, Jorquera I, Ben-Ari Y, Gozlan H, Aniksztejn L. The establishment of GABAergic and glutamatergic synapses on CA1 pyramidal neurons is sequential and correlates with the development of the apical dendrite. J Neurosci. 1999;19:10372–10382. doi: 10.1523/JNEUROSCI.19-23-10372.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieraszko A, Goldsmith G, Seyfried TN. Stimulation-dependent release of adenosine triphosphate from hippocampal slices. Brain Res. 1989;485:244–250. doi: 10.1016/0006-8993(89)90567-2. [DOI] [PubMed] [Google Scholar]
- Wong RO, Chernjavsky A, Smith SJ, Shatz CJ. Early functional neural networks in the developing retina. Nature. 1995;374:716–718. doi: 10.1038/374716a0. [DOI] [PubMed] [Google Scholar]
- Yuste R, Katz LC. Control of postsynaptic Ca2+ influx in developing neocortex by excitatory and inhibitory neurotransmitters. Neuron. 1991;6:333–344. doi: 10.1016/0896-6273(91)90243-s. [DOI] [PubMed] [Google Scholar]
- Zimmermann H, Braun N. Extracellular metabolism of nucleotides in the nervous system. J Auton Pharmacol. 1996;16:397–400. doi: 10.1111/j.1474-8673.1996.tb00062.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.