Abstract
Dipeptidyl-aminopeptidase-like protein 6 (DPPX) was recently shown in the brain to modulate the kinetics of transient A-type currents by accelerating inactivation and recovery from inactivation. Since the kinetics of human cardiac transient outward current (Ito) are not mimicked by coexpression of the α-subunit Kv4.3 with its known β-subunit KChIP2, we have tested the hypothesis that DPPX may serve as an additional β-subunit in the human heart. With quantitative real-time RT-PCR strong mRNA expression of DPPX was detected in human ventricles and was verified at the protein level in human but not in rat heart by a DPPX-specific antibody. Co-expression of DPPX with Kv4.3 in Chinese hamster ovary cells produced Ito-like currents, but compared with expression of KChIP2a and Kv4.3, the time constant of inactivation was faster, the potential of half-maximum steady-state inactivation was more negative and recovery from inactivation was delayed. Co-expression of DPPX in addition to Kv4.3 and KChIP2a produced similar current kinetics as in human ventricular myocytes. We therefore propose that DPPX is an essential component of the native cardiac Ito channel complex in human heart.
The transient outward K+ current (Ito) plays an essential role in initiating the early repolarization phase of the cardiac action potential (AP). In the human heart the respective channel-forming α-subunit is Kv4.3 (Dixon et al. 1996) and several accessory β-subunits like KChIPs, MiRPs and KChAPs can significantly modify the electrophysiological properties of Ito when coexpressed in heterologous expression systems (reviewed by Pourrier et al. 2003). Nevertheless, expression of Kv4.3 alone or in combination with the β-subunits KChIP2 or KCNE2 (Deschenes & Tomaselli, 2002; Jiang et al. 2004) does not reproduce the characteristic kinetics of the native human cardiac Ito, for instance fast time courses of inactivation and recovery from inactivation, suggesting that additional accessory β-subunits may be required to mimick the characteristics of native Ito. One potential candidate is the dipeptidyl-aminopeptidase-like protein 6 (DPPX; EC 3.4.14.5), which has recently been discovered in neuronal brain tissue of rat where it substantially accelerates inactivation and recovery from inactivation of A-type currents encoded by Kv4.2 and Kv4.3, which are the brain correlates of Ito in rat (Nadal et al. 2003).
DPPX belongs to the prolyl-oligopeptidase family of serine proteases but lacks enzymatic activity (Wada et al. 1992; Kin et al. 2001). In rat, expression of DPPX mRNA was detected in several organs, including brain, kidney, ovary, prostate and testis, but not heart (Wada et al. 1992). While the long mRNA isoform (DPPX-L) is expressed almost exclusively in brain, the short isoform (DPPX-S) is detected also in the above-mentioned peripheral tissues (Wada et al. 1992). In human, DPPX has been identified in the brain (Yokotani et al. 1993), but it is not known whether it is also expressed in the heart.
In order to test the hypothesis that DPPX could serve as an Ito-modulating β-subunit in human heart we have examined the expression of the molecule at the mRNA and protein level in human right and left ventricular tissue and studied the functional effects of DPPX on Ito in a stable Chinese hamster ovary cell (CHO) expression system for Kv4.3 and KChIP2a.
Methods
Molecular biology
cDNAs encoding the long and the short isoforms of DPPX (DPPX-L; DPPX-S) cloned into pRC/RSV vector (Invitrogen) were a kind gift from Professor Keiji Wada (Tokyo, Japan). The pEGFP-N1 vector was from Clontech (Palo Alto, CA, USA). The DPPX-S isoform was used for transfection because in rat studies it was reported to be more widely expressed in different organs while the long isoform was found almost exclusively in brain (Wada et al. 1992; Kin et al. 2001).
Cardiac tissue samples were collected from explanted hearts of NYHA IV patients who had given written consent or from non-failing donor hearts which could not be used for transplantation due to technical reasons. Tissues from other organs were collected from autopsies. (The study conformed with the Declaration of Helsinki.) Total RNA was isolated using the LiCl method (Sambrook & Russel, 2001). Real-time RT-PCR was performed using the QuantiTect SYBR® Green RT-PCR kit (Qiagen, Hilden, Germany) and Rotor Gene thermal cycler (Corbett Research, Mortlake, Australia). In order to detect total DPPX we used the primers DPPX_F (5′-AGGAGAAGGACCAGATGGAG-3′) and DPPX_R (5′-GAAAACGCAGAGGCATAGAG-3′) and for DPPX-S the primers DPPX-S_F (5′-AAATCCGTGCAGCAGCAGGAAC-3′) and DPPX-S_R (5′-AGGTGACGATCAAGGAGCAGATG-3′). Real-time RT-PCR conditions were: 50°C for 30 min, 95°C for 15 min 30 s at 94°C, 30 s at 61°C, 30 s at 72°C and 15 s at 80°C. cRNA standards were produced by T7 in vitro transcription with the mMessage machine kit (Ambion, Austin, TX, USA) from RT-PCR products produced as above but using a modified forward primer containing an additional T7 promoter sequence (GGCCGCGG). Standards of known concentration of RNA included in each dilution series (105–1012 molecules μl−1) allowed calculation of cRNAs. Results were analysed by Rotorgene software version 4.6 (Corbett Research). Qualitative RT-PCR was performed following standard procedures.
DPPX antibody
cDNA encoding 69 amino acids located at the C-terminus of DPPX (EEKDQMEAVRTMLKEQYIDRTRVAVFGKDYGGYLSTYILPAKGENQGQTFTCGSALSPITDFKLYASAF) was amplified by RT-PCR from human atrium total RNA. The PCR product was cloned in frame with the thioredoxin gene and a His-Tag in the pET32a(+) vector (Novagen, Madison, WI, USA). Recombinant protein was expressed in E. coli BL21(DE3), according to the pET System handbook (Novagen). The DPPX fusion protein was purified on a Ni-NTA agarose affinity resin (Qiagen) according to the manufacturer's instructions. The purified protein was used to raise an antibody in rabbits and to produce an affinity column by coupling the recombinant DPPX protein to a HiTrap™ NHS-activated HP column (Amersham, Freiburg, Germany) for further purification.
Western blotting
Tissue samples and DPPX-transfected cells were homogenized in phosphate-buffered saline (PBS) containing 8 m urea. Protein extracts were run on a 7.5% SDS-PAGE gel (18 μg of cells and 75 μg of tissues) and blotted onto a nitrocellulose membrane. Membranes were blocked in Tris-buffered-saline (TBS), 1% milk and probed overnight with the anti-DPPX antibody (diluted 1 : 200). Membranes were washed, probed with a secondary antibody (anti-rabbit conjugated with horseradish peroxidase (HRP), diluted 1 : 5000, Amersham), and detected by chemiluminescence. For the antigen competition assay, 15 μg of antibody was incubated with 150 μg of antigen for 3 h before probing the blotted membrane.
Cell culture, transfection and patch clamp experiments
Chinese hamster ovary (CHO) cell lines stably transfected with hKv4.3 or hKv4.3/hKChIP2a were gifts from Dr K. Steinmeyer (Sanofi-Aventis Pharma, Frankfurt, Germany). Cells were maintained at 37°C, 5% CO2, in basal Iscove's medium (Biochrom, Berlin, Germany) supplemented with 10% fetal calf serum (FCS), 1% glutamine, 100 μg ml−1 Zeocin and 400 μg ml−1 G418. Cells were seeded on 24-well plates at a density of 75 000 cells per well. Transfections were done with the Roti®-Fect transfection reagent (Carl Roth, Karlsruhe, Germany) according to the manufacturer's instructions, using 2.5 μl of transfection reagent, 0.5 μg of DPPX-S vector and 0.2 μg of pEGFP-N1 per well. After 48 h of incubation to allow protein synthesis, cells were harvested by trypsinization. Transient outward currents in CHO cells were measured with standard patch clamp techniques. Raw data were digitized at 10 kHz sampling rate, pulse protocols were generated by ISO2 software (MFK, Niedernhausen, Germany). Mean series resistance was 8.4 ± 0.1 MΩ (n = 66) and compensated by at least 70%. Mean cell capacitance was 33.7 ± 2.7 pF (n = 61) in non-transfected and transfected cells. Currents were normalized to cell capacitance and expressed in pA pF−1. Superfusion solution contained (mm): NaCl 150, KCl 5.4, MgCl2 2, CaCl2 1.8, Hepes 10, glucose 11, pH 7.4. Pipette solution contained (mm): KCl 40, potassium aspartate 80, NaCl 8, CaCl2 2, MgATP 5, EGTA 5, GTP 0.1, Hepes 10, pH 7.4 adjusted with KOH. The liquid junction potential for this solution was 11 mV as calculated with the software JPCalc (P. H. Barry, Sydney, Australia). Data are not corrected for the junction potential.
Statistical analysis
All results are given as means ± s.e.m. Statistical analysis was performed with Student's t test or one-way ANOVA with Bonferroni's post hoc test (GraphPad Prism software, V4.1; San Diego, CA, USA). Differences were considered to be significant if P < 0.05.
Results
mRNA expression of DPPX in human heart
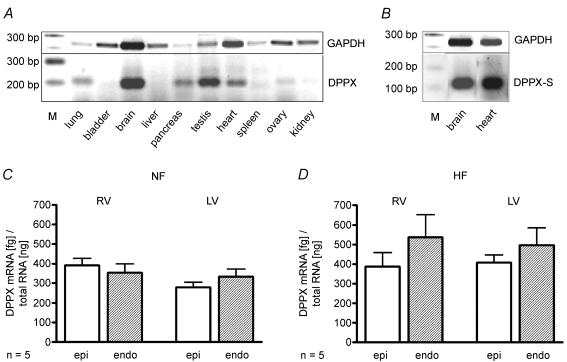
Qualitative RT-PCR exhibited clear positive mRNA expression of DPPX in human brain (3/3), pancreas (3/3), testes (2/2) and heart (5/5). No expression was detected in bladder (0/2), liver (0/3) and spleen (0/3), and uncertain results were found for lung (2/3), ovary (1/2) and kidney (1/3), the numbers in brackets indicating number of positive samples from number of autopsies (Fig. 1A). The short isoform of DPPX has been shown to be expressed not only in brain but also in peripheral tissues (Wada et al. 1992). Since this isoform was used for the expression experiments we also tested cardiac expression of DPPX-S, which could clearly be detected by RT-PCR (Fig. 1B).
Figure 1. mRNA expression of DPPX in human tissues.
A, distribution of DPPX mRNA among different human tissues as analysed by RT-PCR. Lanes: M: DNA ladder (Roche XIV); 1–10: organs as indicated. Expression of GAPDH served as control for mRNA integrity. B, expression of DPPX-S isoform in human brain and heart. mRNA expression of DPPX in 5 non-failing (C) and 5 failing human hearts (D), Quantification by real-time RT-PCR in samples from epi- and endocardial layers of right (RV) and left ventricles (LV).
Since cardiac Ito exhibits a transmural gradient (subepicard > subendocard; Wettwer et al. 1994) distribution of DPPX expression between subepi- and subendocardial tissue layers of left and right ventricles was determined. Large amounts of DPPX were detected in all samples investigated and differences between the regions did not reach the level of statistical significance. Pooled data yielded a mean mRNA content of 453 ± 59 fg (ng of total RNA)−1 (Fig. 1D). DPPX was also detected in tissue samples from non-failing hearts, however, the pooled data yielded a significantly lower expression level of 340 ± 20 fg ng−1 (Fig. 1C).
Western blot analysis of DPPX
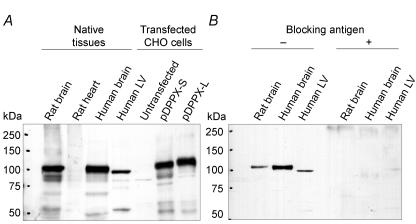
Our antibody was validated with total protein extracts from human and rat brain and DPPX-transfected CHO cells as positive controls. In both human and rat brain, the antibody recognized a band with an apparent molecular mass of about 100 kDa as expected (Kin et al. 2001; Nadal et al. 2003; Zagha et al. 2005) (Fig. 2A). The antibody recognized two bands at about 107 and 115 kDa in CHO cells, corresponding to the short and long isoforms, respectively. In human heart preparations, the antibody recognized one band at 100 kDa which was absent in rat heart and differed from bands in brain preparations by about 2 kDa (all apparent molecular weights were calculated with the Phoretics 1D gel analysis software). Specificity of the DPPX antibody was tested by Western blot after preincubation with the blocking antigen (Fig. 2B), which completely abolished all bands from brain and heart preparations.
Figure 2. Western blot analysis of DPPX.
A, total protein extracts from the indicated tissues (75 μg) or CHO cells (18 μg) transfected with DPPX as positive controls were separated by 7.5% SDS-PAGE, blotted onto nitrocellulose membranes and probed with our DPPX antibody. B, test for antibody specificity by preincubation with the antigen; 150 μg of antigen were used to block 15 μg of antibody. Protein standards: Precision Plus Protein™. (Biorad). LV: left ventricle.
Electrophysiological recordings of Ito in CHO cells
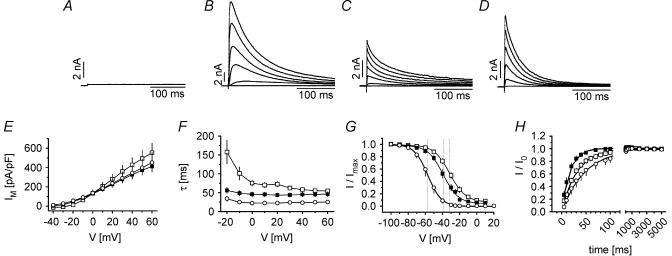
Typical test pulses from −80 mV to test pulses between −40 and +60 mV did not activate any Ito in wild-type CHO control cells or minute currents in cells transfected with Kv4.3 (2.3 ± 0.7 pA pF−1 steady-state current at +50 mV, n = 6, Fig. 3A). In comparison, coexpression of Kv4.3 and KChIP2a yielded large transient outward currents (495 ± 86 pA pF−1 at +50 mV, n = 26, Fig. 3B). Transient outward current in cells coexpressing Kv4.3 with DPPX (Fig. 3C) exhibited smaller current density (399 ± 90 pA pF−1 at +50 mV, n = 6) and significantly different kinetics: the time constant of inactivation at +50 mV was smaller (25 ± 6 ms, versus 55 ± 4 ms, P < 0.05), V0.5 of steady-state inactivation was shifted to more negative potentials (−57.0 ± 3.1 mV versus−31.3 ± 2.4 mV, P < 0.01), and recovery from inactivation was slower (52 ± 16 ms versus 42 ± 8 ms). In cells expressing Kv4.3 and DPPX-S in addition to KChIP2a (Fig. 3D) current density was smaller compared to cells with KChIP2a expression alone (373 ± 50 pA pF−1, n = 15). The time constant of inactivation became slightly smaller (45 ± 6 ms, n = 10 at +50 mV), V0.5 of inactivation was more negative (−39.8 ± 2.8 mV) and the time constant for recovery from inactivation was smaller than in cells with KChIP2a expression (18 ± 3 ms, P < 0.05). Mean values for the measured parameters are depicted in Fig. 3E–H and summarized in Table 1.
Figure 3. Effects of KChIP2a and DPPX on Kv4.3 currents expressed in CHO cells.
A–D, current tracings (initial 300 ms) elicited by test steps (1000 ms) from −80 mV to −40, −20, 0, +20, +40 and +60 mV in CHO cells stably transfected with Kv4.3 (A), Kv4.3 plus KChIP2a (B, □), Kv4.3 plus DPPX (C, ○), and Kv4.3 plus KChIP2a plus DPPX (D, ▪). E, current–voltage relations of Ito in the transfected CHO cells. F, voltage dependence of fast time constant of Ito inactivation determined from fitting single exponential curves to individual current traces. G, normalized steady-state inactivation (I/I0) curves for Ito. Fitting of a Boltzmann function to mean data yielded the following potentials of half maximum inactivation (V0.5, dotted lines): −31.5 mV for Kv4.3 plus KChIP2a, −57.0 mV for Kv4.3 plus DPPX, and −39.2 mV for Kv4.3 plus KChIP2a plus DPPX, with slope factors (k) of −9.2, −7.8 and −8.9 mV, respectively. H, time course of recovery from inactivation of Ito, test pulse +50 mV, recovery potential −80 mV. Curve fitting yielded τ-values of 33 ms, 46 ms and 17 ms, respectively.
Table 1.
Ito current characteristics in CHO expression systems with coexpression of KChIP2a and DPPX, 22°C
| Kv4.3 + KChIP2aa | n | Kv4.3 + DPPXb | n | Kv4.3 + KChIP2a + DPPXc | n | Humanventricularmyocytesd | n | |
|---|---|---|---|---|---|---|---|---|
| Cm (pF) | 33 ± 3 | 40 | 12 ± 2 | 8 | 39 ± 6 | 18 | 292 ± 22 | 28 |
| Im current density at +50 mV (pA pF−1) | 495 ± 86 | 26 | 399 ± 90 | 6 | 373 ± 50 | 15 | 7.9 ± 0.7 | 28 |
| τfast of inactivation at +50 mV (ms) | 55 ± 4 | 11 | 25 ± 6* | 8 | 45 ± 6 | 10 | 54 ± 3 | 5 |
| (58 ± 3) | (28) | |||||||
| τslow of inactivation at +50 mV (ms) | 392 ± 160 | 10 | 177 ± 16 | 8 | 427 ± 213 | 10 | n.d. | — |
| V0.5 of steady-stateinactivation (mV) | −31.3 ± 2.4 | 24 | −57.0 ± 3.1* | 8 | −39.8 ± 2.8 | 9 | −45.5 ± 1.2 | 5 |
| (−30.4 ± 1.2) | (26) | |||||||
| Slope k of steady-state inactivation (mV) | −5.0 ± 0.5 | 24 | −5.5 ± 0.4 | 8 | −6.6 ± 0.6 | 9 | −4.6 ± 0.8 | 5 |
| (−4.5 ± 0.2) | (26) | |||||||
| τfast of recovery from inactivation at+50 mV, from −80 mV (ms) | 42 ± 8 | 11 | 52 ± 16 | 8 | 18 ± 3* | 11 | 24 ± 3 | 6 |
| at +20 mV,from −100 mV |
Values significantly different from other groups P < 0.05.
Stable expression of Kv4.3 and KChiP2a.
Stable expression of Kv4.3 and transient expression of DPPX.
Stable expression of Kv4.3 and KChiP2a and transient expression of DPPX.
Data from Wettwer et al. (1994) for comparison, not included in statistical analysis; subepicardial myocytes, nifedipine (1 μm) or Cd2+ (0.1 mm, data in brackets) was used for block of ICa. n.d. values not determined.
Discussion
Here we provide evidence that the dipetidyl-aminopeptidase-like protein 6 (DPPX) is expressed in human cardiac tissue on mRNA and protein level. Functional experiments in Kv4.3 expressing CHO cells revealed that additional expression of KChIP2a or DPPX alone produced transient outward current, but the characteristic Ito kinetics of native human ventricular cells were most closely mimicked in cells coexpressing both subunits in addition to Kv4.3.
Tissue distribution of DPPX
DPPX was first discovered in rat and bovine brain (Wada et al. 1992). In rat, the long isoform DPPX-L was found only in brain, whereas the short isoform DPPX-S was also detected in some peripheral tissues like kidney, ovary and testis, but transcripts of either isoform are absent in rat heart, liver or spleen (Wada et al. 1992). Human DPPX possesses more than 90% homology to bovine and rat DPPX (Yokotani et al. 1993). The predominance of DPPX expression in rat brain is confirmed by Western blot, but detection failed in other tissues particularly in rat heart (Kin et al. 2001; this study).
We found significant amounts of DPPX mRNA expressed in the human heart as well as in human brain, testes and pancreas and to a lesser extent in lung, kidney and ovary. No transcripts were detected in bladder, spleen and liver. In fact, one preliminary communication confirms our positive finding in human heart (Kloos et al. 2005). Interestingly, DPPX was significantly up-regulated in failing hearts, which could contribute to the observed alteration of Ito in heart failure (Kaab et al. 1998).
Western Blots reveal clear bands at about 100 kDa in human ventricle in good agreement with data previously reported in rat brain (Kin et al. 2001; Nadal et al. 2003; Zagha et al. 2005). However, the apparent molecular mass of DPPX in human heart differs from that of DPPX in rat brain by 2 kDa, which is probably due to a different glycosylation pattern. A similar explanation may hold true for the even higher apparent molecular weight found for DPPX expressed in CHO cells.
Functional experiments
The detection of DPPX in human heart and the functional data from coexpression experiments suggest an important role for DPPX also in native Ito although direct evidence as could be provided by gene silencing techniques is still pending.
In Xenopus oocytes expression of DPPX in addition to Kv4.3 accelerates inactivation, shifts V0.5 to more negative membrane potentials and hastens recovery from inactivation compared with Kv4.3 expression alone or with coexpression of Kv4.3 and KChIP2 (Nadal et al. 2003). In our study with CHO cells, negligible Ito was obtained with expression of Kv4.3 alone. For robust Ito amplitudes, coexpression of KChIP2a or DPPX was required (compare Fig. 3A, B and C). This difference in functional outcome of channel subunit expression between the two expression systems is most likely due to differences in endogenous expression of chaperones that promote translocation of Kv4.3 to the surface membrane. Both KChIP2a and DPPX have been associated with enhanced trafficking of the ion conducting subunit to the cell surface (Shibata et al. 2003; Nadal et al. 2003).
Co-expression of Kv4.3 with the β-subunits affected the kinetics of Ito; however, the effects were different in CHO cells and Xenopus oocytes. Unexpectedly, DPPX retarded recovery from inactivation in comparison to KChIP2a whereas recovery from inactivation was accelerated in Xenopus oocytes. The time course of inactivation was accelerated and V0.5 was substantially more negative. These effects of DPPX are consistent with those in Xenopus oocytes (Nadal et al. 2003). We hypothesized that the coexpression of two important β-subunits, KChIP2a and DPPX, should more closely reproduce Ito current characteristics of human ventricular myocytes, i.e. time constant of inactivation τi= 54 ms (+ 50 mV), V0.5 of steady-state inactivation −45 mV, and time constant of recovery from inactivation τrec 24 ms (+ 20 mV; recovery potential −100 mV; Wettwer et al. 1994). The respective values obtained in CHO cells expressing both KChIP2a and DPPX in addition to Kv4.3 were τi 45 ms (+ 50 mV), V0.5 of inactivation −40 mV, and τrec 18 ms (+ 50 mV, recovery potential −80 mV). From this surprisingly good agreement with the data reported for native cardiomyocytes we suggest that both KChIP2a and DPPX determine Ito kinetics in human ventricular myocytes.
Acknowledgments
We wish to thank Professor K. Wada, National Institute of Neurosciences, Tokyo, Japan and Dr K. Steinmeyer, Sanofi-Aventis Pharma, Frankfurt for the DPPX clones and transgenic CHO cells, respectively. A Marie Curie Host Fellowship from the EU Commission (HPMD-CT-2001-00119) supports D. Cotella. We thank M. Darna, M.Sc., for assistance with Western Blots; Professor G. Baretton, Department of Pathology, Medical Faculty, Dresden University of Technology, for providing human tissue specimen. We gratefully acknowledge the cooperation of the surgeons of the Dresden Heart Centre (Drs M. Knaut, K. Matschke, R. Cichon, U. Kappert, M. Tugtekin) and Professor A. Varro from the Department of Pharmacology and Pharmacotherapy University of Szeged, Hungary for providing access to the surgical tissue that made this study possible. We thank Dr T. Christ for indispensable technical assistance and helpful discussions.
References
- Deschenes I, Tomaselli GF. Modulation of Kv4.3 current by accessory subunits. FEBS Lett. 2002;528:183–188. doi: 10.1016/s0014-5793(02)03296-9. [DOI] [PubMed] [Google Scholar]
- Dixon JE, Shi W, Wang HS, McDonald C, Yu H, Wymore RS, Cohen IS, McKinnon D. Role of the Kv4.3 K+ channel in ventricular muscle. A molecular correlate for the transient outward current. Circ Res. 1996;78:659–668. doi: 10.1161/01.res.79.4.659. [DOI] [PubMed] [Google Scholar]
- Jiang M, Zhang M, Tang DG, Clemo HF, Liu J, Holwitt D, Kasirajan V, Pond AL, Wettwer E, Tseng GN. KCNE2 protein is expressed in ventricles of different species, and changes in its expression contribute to electrical remodeling in diseased hearts. Circulation. 2004;109:1783–1788. doi: 10.1161/01.CIR.0000124225.43852.50. [DOI] [PubMed] [Google Scholar]
- Kaab S, Dixon J, Duc J. Molecular basis of transient outward potassium current down-regulation in human heart failure: a decrease in Kv4.3 mRNA correlates with a reduction in current density. Circulation. 1998;98:1383–1393. doi: 10.1161/01.cir.98.14.1383. [DOI] [PubMed] [Google Scholar]
- Kin Y, Misumi Y, Ikehara Y. Biosynthesis and characterization of the brain-specific membrane protein DPPX, a dipeptidyl peptidase IV-related protein. J Biochem (Tokyo) 2001;129:289–295. doi: 10.1093/oxfordjournals.jbchem.a002856. [DOI] [PubMed] [Google Scholar]
- Kloos P, Barth A, Zwermann L, Kartmann H, Näbauer M. Transkriptionelle Kontrolle der funktionellen Expression des transienten Auswärtsstromes in insuffizientem humanen Myokard durch Regulation von Alpha-(Kv4.3) und Beta-Untereinheiten (KChIP2 and DPPX) Z Kardiol. 2005;94(Suppl. 1):V274. [Google Scholar]
- Nadal MS, Ozaita A, Amarillo Y, Vega-Saenz de Miera E, Ma Y, Mo W, Goldberg EM, Misumi Y, Ikehara Y, Neubert TA, Rudy B. The CD26-Related dipeptidyl aminopeptidase-like protein DPPX is a critical component of neuronal A-type K+ channels. Neuron. 2003;37:449–461. doi: 10.1016/s0896-6273(02)01185-6. [DOI] [PubMed] [Google Scholar]
- Pourrier M, Schram G, Nattel S. Properties, expression and potential roles of cardiac K+ channel accessory subunits: MinK, MiRPs, KChIP and KchAP. J Membrane Biol. 2003;194:141–152. doi: 10.1007/s00232-003-2034-8. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russel DW. Cold Spring Harbor Laboratory Press. NY, USA: Cold Spring Harbor; 2001. Molecular cloning: A laboratory manual. [Google Scholar]
- Shibata R, Misonu H, Campomanes CR, Anderson AE, Schrader LA, Doliveira LC, Carroll KI, Sweatt JD, Rhodes KJ, Trimmer JS. A fundamental role for KChIPs in determining the molecular properties and trafficking of Kv4.2 potassium channels. J Biol Chem. 2003;278:36445–36454. doi: 10.1074/jbc.M306142200. [DOI] [PubMed] [Google Scholar]
- Wada K, Yokotani N, Hunter C, Doi K, Wenthold RJ, Shimasaki S. Differential expression of two distinct forms of mRNA encoding members of a dipeptidyl aminopeptidase familiy. Proc Natl Acad Sci U S A. 1992;89:197–201. doi: 10.1073/pnas.89.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettwer E, Amos GJ, Posival H, Ravens U. Transient outward current in human ventricular myocytes of subepicardial and subendocardial origin. Circ Res. 1994;75:473–482. doi: 10.1161/01.res.75.3.473. [DOI] [PubMed] [Google Scholar]
- Yokotani N, Doi K, Wentholf RJ, Wada K. Non-conservation of a catalytic residue in a dipeptidyl aminopeptidase IV-related protein encoded by a gene on human chromosome 7. Human Mol Genet. 1993;2:1037–1039. doi: 10.1093/hmg/2.7.1037. [DOI] [PubMed] [Google Scholar]
- Zagha E, Ozaita A, Chang SY, Nadal MS, Lin U, Saganich MJ, McCormack T, Akinsanya KO, Qi SY, Rudy B. DPP10 modulates Kv4-mediated A-type potassium channels. J Biol Chem. 2005;280:18853–18861. doi: 10.1074/jbc.M410613200. [DOI] [PubMed] [Google Scholar]