Abstract
Using a step-wise, reductionist approach we characterized the time course and degree to which mechanical, vasodilatory and cardiac mechanisms contribute to the increase in leg blood flow (LBF) at the onset of dynamic knee-extensor exercise. Heart rate (HR) and LBF (ultrasound Doppler) were evaluated during (1) voluntary and (2) passive exercise in the seated position, (3) passive exercise in the supine position with the leg above the heart, and (4) passive exercise with measurements made in the non-moving leg. In trials 2 and 3, the degree of change and time course of peak ΔHR (8.7 ± 2 bpm, seated; 10 ± 1 bpm, supine) and peak ΔLBF (518 ± 135 ml min−1, seated; 448 ± 179 ml min−1, supine) were similar, supporting the concept that the skeletal muscle pump was minimized. Even with the reduction of skeletal muscle pump and metabolic influences (trials 2, 3 and 4) a significant cardio-acceleration and hyperaemia was seen. In the first 5 s of seated passive exercise, the retrograde component of the blood velocity profile was significantly greater than rest or the 5–20 s interval, which may suggest an arterial inflow that initially exceeded leg vasodilatation. Steady-state LBF (minutes 2 and 3) remained elevated during voluntary exercise, but returned to near baseline during passive movement. Taken together, these data suggest that cardio-acceleration (i.e. tachycardia) and mechanical forces other than the skeletal muscle pump play a role in reducing vascular resistance and ultimately increasing LBF at the onset of exercise, followed by steady-state LBF which matches muscle metabolic demand.
During the transition from rest to exercise, a host of factors act collectively to increase blood flow to skeletal muscle, and many attempts have been made both in animals and humans to address the mechanisms which govern the onset of exercise hyperaemia. From these studies, the potential contributions of the skeletal muscle pump (Folkow et al. 1970, 1971; Laughlin, 1987; Sheriff et al. 1993; Sheriff, 2003), vasodilatation (Skinner & Costin, 1970; Tschakovsky et al. 1996; Buckwalter et al. 1998; Segal, 2000; Clifford & Hellsten, 2004), and cardio-acceleration (i.e. tachycardia) from feed-forward and feed-back input (Rowell, 1992; Nobrega & Araujo, 1993; Williamson et al. 1995; Gladwell & Coote, 2002) to onset exercise hyperaemia have been proposed. However, this obvious abundance and apparent redundancy of mechanisms have made the task of experimentally dissecting the individual contributions of these factors extremely difficult.
The intrigue surrounding the cause of exercise hyperaemia has been fostered by differences in the temporal nature of the potential mechanisms. A substantial increase in blood flow is generally observed within the first 5 s following the onset of voluntary exercise, yet it has been suggested that few vasodilatory mechanisms can be fully realized within this brief period of time (Clifford & Hellsten, 2004). At present, the predominant mechanisms implicated to increase blood flow during the first few seconds of voluntary exercise have remained the skeletal muscle pump and rapid vasodilatation. A recent review comprehensively outlined experimental evidence for and against both mechanisms (Tschakovsky & Sheriff, 2004), emphasizing that these factors are not mutually exclusive. A large body of literature indicates that the time course and mechanisms responsible for vasodilatation remain disputed and appear heavily dependant upon both species and experimental model (Tschakovsky et al. 1996; Tschakovsky & Sheriff, 2004), with compelling evidence for a significant (4–6 s) time delay before vasodilatation in isolated microvessels (Wunsch et al. 2000). In addition to the impact of skeletal muscle pumping on the venous circulation, recent studies have suggested that mechanical factors which cause physical distortion of the skeletal muscle vasculature during muscle lengthening and shortening may alter endothelial (Hamann et al. 2004) and vascular smooth muscle properties (Tschakovsky et al. 2004), resulting in vasodilatation. Interestingly, discussion regarding the potential impact of alternative contributors such as cardio-acceleration to onset exercise hyperaemia is largely absent from these debates.
To our knowledge, no human studies have applied a step-wise, reductionist approach to investigate the time course and degree to which these putative factors contribute to onset exercise hyperaemia in the human leg. Thus, the aim of the present study was to characterize the contribution of mechanical, vasodilatory, and cardiac mechanisms to the increase in leg blood flow (LBF) at the onset of isolated dynamic knee-extensor exercise. To address these mechanisms, subjects performed (1) voluntary exercise in the seated position, thereby involving all mechanical and vasodilatory mechanisms, (2) passive exercise in the seated position, which reduced skeletal muscle pump and metabolic influences, (3) passive exercise in the supine position with the leg positioned above heart level, which promoted venous emptying and thus further reduced the skeletal muscle pump, and (4) passive exercise with measurements in the non-moving contralateral leg. In each experimental condition, we focused upon the time course for changes in heart rate and the blood velocity profile, with a dissection of the anterograde and retrograde components. We sought to test the general hypothesis that the magnitude and time course of onset exercise hyperaemia would differ according to the contribution of each mechanism.
Methods
Subjects and general procedures
Seven young (20–38 year) non-smoking men participated in the current study. Written, informed consent was obtained according to the University of California San Diego Human Subjects Protection Program requirements, and the study was in accordance with the Declaration of Helsinki. As part of the initial subject screening, subjects were familiarized with the knee-extensor ergometer and practiced both the passive and voluntary exercise protocols. Health histories and physical examinations were also completed. All studies were performed in a thermoneutral environment. Subjects reported to the lab in the fasted state, and had not performed exercise within the past 24 h.
Exercise protocols
The single-leg knee-extensor exercise protocol was utilized, as previously described (Richardson & Saltin, 1998; Richardson et al. 2004). The ergometer was adjusted so that contraction of the quadriceps muscles (voluntary exercise) or passive muscle shortening and lengthening (passive exercise) turned a flywheel producing a ∼90–170 deg arc of the lower leg. Subjects were seated in a semirecumbent position (∼60 deg reclined) for all trials except the supine (leg positioned above heart) exercise condition. For all trials, a blood pressure cuff was placed below the knee and inflated to supra-systolic values (>250 mmHg) 2 min prior to and during exercise to limit blood flow to the lower leg. To avoid ordering effects, the sequence of exercise bouts was randomized, with 30 min of recovery between each trial.
Voluntary exercise
Voluntary exercise was performed at a workload of 10 W, and subjects were instructed to raise the cadence to 1 Hz within the first two to three kicks. Exercise was then performed for 3 min.
Seated passive exercise
During the last minute preceding passive exercise, subjects were encouraged to relax and close their eyes, and noise-cancelling headphones were used to avoid anticipation of the start of passive exercise. To perform passive exercise, a member of the research team pushed and pulled the subject's leg through the normal range of motion by holding onto the knee-extensor ergometer boot, with the objective of achieving a rate of 1 Hz within the first two to three duty cycles.
Supine passive exercise
To promote leg venous emptying, the ergometer was tilted back ∼45 deg so that the subject was effectively supine, with the upper leg (quadriceps) above heart level. Passive exercise was then performed as described above.
Non-exercising leg
Seated passive exercise was repeated with ultrasound Doppler measurements made in the contralateral, nonmoving leg.
Measurements
The ultrasound system (Logiq 7, GE Medical Systems, Milwaukee, WI, USA) was equipped with a linear array transducer operating at an imaging frequency of 7 MHz. Vessel diameter was determined at a perpendicular angle along the central axis of the scanned area, where the best spatial resolution was achieved. The common femoral artery was insonated distal to the inguinal ligament, approximately 2–3 cm proximal to the bifurcation. The blood velocity profile was obtained using the same transducer with a Doppler frequency of 4.0–5.0 MHz, operated in the high-pulsed repetition frequency mode (2–25 kHz) and a sample volume of 1.5–3.5 cm in depth. Care was taken to avoid aliasing using scale adjustments, especially during the rest-to-exercise transition. In duplex mode, real-time ultrasound imaging and pulse-wave velocity profile were viewed simultaneously. All blood velocity measurements were obtained with the probe appropriately positioned to maintain an insonation angle of 60 deg or less. The sample volume was maximized according to vessel size and centred, verified by real-time ultrasound visualization of the vessel.
Heart rate (HR) was recorded simultaneously from standard three-lead ECGs sent to the Doppler system (Logiq 7) and the Bipoac A/D board (BioPac Systems Inc., Goleta, CA, USA). Knee-extensor force was measured with a transducer installed in the pole connecting the leg to the ergometer (Transducer Techniques, Temecula, CA, USA), and erogemeter r.p.m. was displayed to ensure constant cadence and for calculation of work rate. Arterial blood pressure was measured using automated radial tonometry (Medwave Vasotrac APM205A, BioPac Systems), with one measurement every 8–10 s. Analog signals for all of these variables underwent A/D conversion and were recorded at 300 Hz using commercially available data acquisition software (AcqKnowledge, BioPac Systems).
Data Analyses
At all sample points, arterial diameter and angle-corrected, time-and space-averaged, and intensity-weighted mean velocity (Vmean) values were calculated using commercially available software (Logiq 7). Two ultrasound digital images and velocity spectra segments of 20 s each were recorded and saved to the GE Logiq 7 hard drive for off-line image and waveform analysis. Measurements were taken at rest with the leg cuff inflated below the knee, during the first 20 s of exercise (all trials), and at the end of minutes 2 (1 : 40–2 : 00 min), and 3 (2 : 40–3 : 00 min) of exercise (voluntary and seated passive exercise trials only). Ultrasound vessel diameter measurements were evaluated during diastole, and were in the relaxation phase of muscle contraction during the voluntary exercise protocol, with one diameter measurement used for each 10-s clip.
Vmean values were first analysed on the ultrasound system (GE Logiq 7) for each pulse interval to identify beat-to-beat changes. These values were then processed with 1-s averaging to allow direct comparison between subjects as a function of time rather than pulse interval. In addition to this analysis, Doppler audio output was recorded to provide an index of anterograde and retrograde blood velocity. This information underwent A/D conversion and was recorded at 300 Hz with real-time signal integration to produce a waveform of anterograde and retrograde velocity (AcqKnowledge). While these data were not used for quantification of Vmean, they served to identify changes in anterograde and retrograde velocity in relation to time (Figs 1 and 3). Using artery diameter and mean blood velocity (Vmean), blood flow in the CFA was calculated as:
where blood flow is in millilitres per minute.
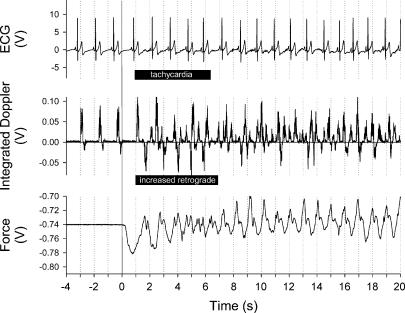
Figure 1. Sample tracing of electrocardiogram, integrated blood velocity profile and force applied to the ergometer at the onset of seated passive exercise in one subject.
Note the shortened pulse interval and predominance of the retrograde portion of the blood velocity signal during the first 5 s of leg movement.
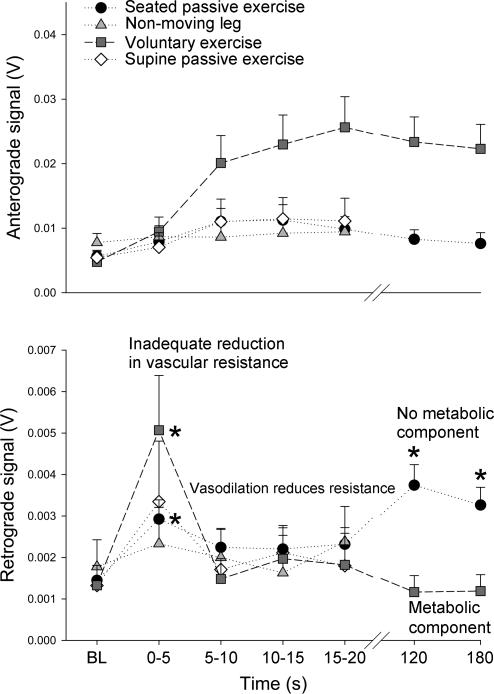
Figure 3. Temporal nature of the blood velocity profile.
Elevated retrograde velocity may imply an increased arterial inflow against an adequate reduction in leg vascular resistance, while reduced retrograde velocity may suggest a greater degree of vasodilatation. *Significantly different from baseline (BL).
Statistics
Statistics were performed with the use of commercially available software (SigmaStat, Systat Software Inc., Point Richmond, CA, USA). Repeated measures analysis of variance was performed on all measurements to compare each second of exercise onset against baseline values, and post hoc analysis was performed using Bonferroni's test for multiple comparison against control. All group data are expressed as the mean ± standard error of the mean. Significance was established at P < 0.05.
Results
Immediate response: the 0–5 s interval
Resting heart rate (HR) was similar during the voluntary exercise (58.3 ± 3 bpm), seated passive (57.3 ± 4 bpm), supine passive (53.1 ± 4 bpm), and non-moving leg (53.9 ± 3 bpm) trials. A significant cardio-acceleration was observed during the initial 5 s of all trials (bpm: voluntary, +18.4 ± 3; seated passive, +4.6 ± 1; supine passive, +7.6 ± 2; non-moving = +5.8 ± 2; Figs 1, 2 and 4).
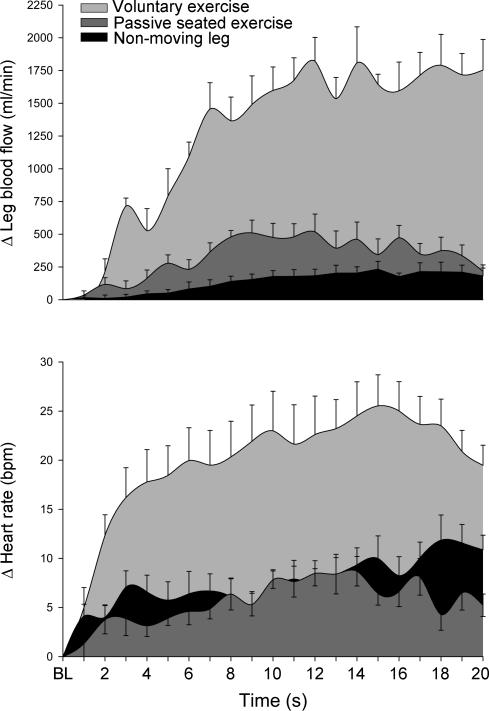
Figure 2. Dynamic changes in leg blood flow and heart rate.
Changes in leg blood flow (top panel) and heart rate (bottom panel) during voluntary exercise (light grey fill), seated passive leg movement (dark grey fill), and measurements in the non-moving leg (black fill).
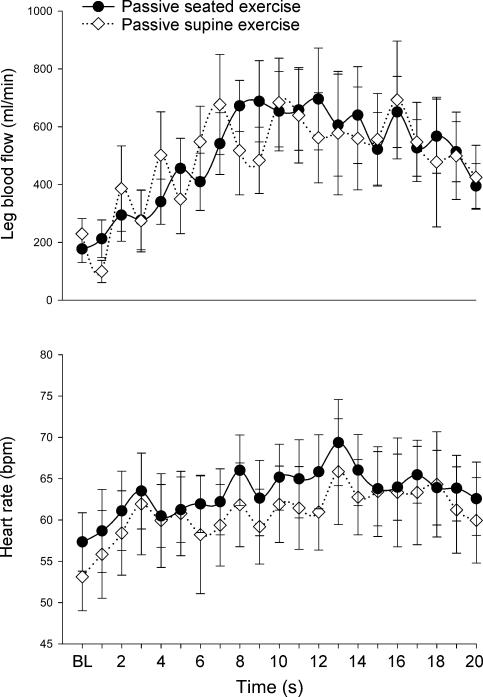
Figure 4. Hydrostatic influence at the onset of exercise.
During passive exercise, leg blood flow (top panel) and heart rate (bottom panel) were similar whether the leg was positioned above (supine trial, ⋄) or below (seated trial, •) the heart. This suggests a minimal contribution for the skeletal muscle pump to the onset hyperaemia seen with passive movement in either scenario.
Resting leg blood flow (LBF) was not different between trials (ml min−1: voluntary, 186.9 ± 22; seated passive, 177.3 ± 47; supine passive, 229.2 ± 54; non-moving leg, 166.9 ± 27). A significant increase in LBF was seen within the first 5 s of voluntary exercise (+791.6 ± 209 ml min−1), seated passive exercise (+278.4 ± 64 ml min−1) and supine passive exercise (+272.4 ± 99 ml min−1), while the change in LBF in the non-moving leg (+90.6 ± 51 ml min−1) did not achieve statistical significance within this time period.
Delayed response: the 5–20 s interval
Heart rate continued to rise in the 5–20 s interval of all trials, with clear between-trial differences in time and peak HR attained. During voluntary exercise, HR peaked (83.9 ± 4 bpm) within 15 s, while peak HR (69.3 ± 5 bpm, seated; 65.8 ± 6 bpm, supine) occurred at second 13 for both seated and supine passive exercise trials. During the non-moving leg trial, HR peak (67.5 ± 4 bpm) was reached at second 18 (Figs 2 and 4).
Like HR, LBF continued to change during the 5–20 s period. During voluntary exercise, peak LBF (2009 ± 191 ml min−1) occurred within 12 s, while passive and seated exercise produced a smaller but similar peak hyperaemia (seated passive = 695.9 ± 176 ml min−1, second 12; supine passive = 683.7 ± 153 ml min−1, second 10). A comparatively modest but sustained hyperaemia was observed in the non-moving leg, with the rise in blood flow becoming significantly greater than rest at second 11 and peak flow (402.6 ± 83 ml min−1) occurring at second 15.
Steady-state: minutes 2 and 3
Cardio-acceleration persisted during minutes 2 (74.1 ± 3 bpm) and 3 (74.4 ± 4 bpm) of voluntary exercise. Likewise, a small but significantly higher HR was sustained during minutes 2 (61.4 ± 3 bpm) and 3 (62.5 ± 4 bpm) of steady-state passive seated exercise. LBF remained elevated during steady-state voluntary exercise (1967 ± 154 ml min−1, minute 2; 1909 ± 205 ml min−1, minute 3). In contrast, the hyperaemia of the seated passive trial returned to near baseline values (P < 0.05) during steady-state passive exercise (267.4 ± 57 ml min−1, minute 2; 247.3 ± 62 ml min−1, minute 3).
Blood velocity profile
Anterograde and retrograde portions of the blood velocity profile were assessed during onset (0–5 s, 5–10 s, 10–15 s, and 15–20 s) and steady-state (min 2 and min 3) exercise (Fig. 3). As expected, anterograde velocity increased at the onset of exercise in all trials, with a dramatic rise seen during voluntary exercise. During voluntary exercise, retrograde velocity increased significantly in the initial 0–5 s period, and then returned to baseline levels (5–20 s, min 2 and 3). In contrast, during seated passive exercise, retrograde velocity increased significantly (∼+15%) during the initial 0–5 s period, returned to baseline levels during the 5–20 s interval, and then was elevated significantly during minute 2 (∼+23%) and minute 3 (∼+18%) (Fig. 3).
Arterial blood pressure
Mean arterial blood pressure did not change significantly during voluntary exercise (97.6 ± 3, 95.8 ± 3, 98.7 ± 2 and 99.8 ± 2 mmHg at rest, onset, minute 2 and minute 3, respectively), seated passive (95.4 ± 2, 94.1 ± 2, 96.1 ± 2 and 94.3 ± 3 mmHg at rest, onset, minute 2 and minute 3, respectively), supine passive (93.3 ± 3, 95.4 ± 3, 94.6 ± 3 and 94.1 ± 3 mmHg at rest, onset, minute 2 and minute 3, respectively), and non-moving leg (93.5 ± 2 and 93.9 ± 4 mmHg at rest and onset, respectively) trials.
Discussion
We have identified contrasting heart rate (HR) and leg blood flow (LBF) responses between passive and voluntary knee-extensor exercise onset due to differences in the relative contribution of mechanical, vasodilatory and cardiac mechanisms (Fig. 5). In contrast to voluntary exercise, the skeletal muscle pump was minimized during passive movement, demonstrated by similar changes in LBF and HR between upright and supine conditions (Fig. 4). Even with the reduction of skeletal muscle pump and metabolic influences a significant hyperaemia was seen, which may have been due to mechanically induced changes in muscle and vessel length and cardio-acceleration (i.e. tachycardia). LBF also increased in the non-moving leg during contralateral leg passive movement, though the putative mechanisms governing this hyperaemia remain unclear. Steady-state LBF remained elevated during voluntary exercise, but returned to near baseline during passive movement. Taken together, these data suggest that cardio-acceleration and mechanical forces other than the skeletal muscle pump contribute to the increased LBF at the onset of exercise, followed by steady-state LBF which matches muscle metabolic demand.
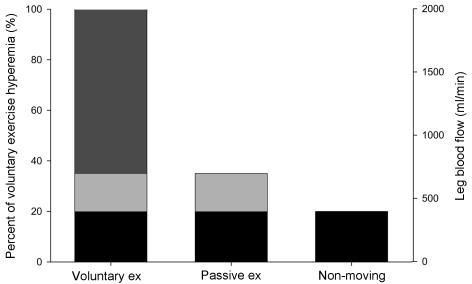
Figure 5. Graphical representation of the ‘reductionist’ experimental approach.
Separate exercise modalities were utilized to evaluate the relative contribution of factors to onset exercise hyperaemia. Bars indicate; the multiple factors (i.e. metabolic and endothelium-mediated vasodilatation, skeletal muscle pump, central command) associated with hyperaemia at the onset of voluntary exercise (dark grey fill), hyperaemia persisting after removal of metabolic and skeletal muscle pump influences, which may be due to vessel deformation and cardio-acceleration (light grey shade), and hyperaemia remaining after removal of the established contributors to onset hyperaemia, which may be attributable to cardiac, endothelial and/or neural influences (black fill).
Hydrostatic influence at the onset of exercise
We compared haemodynamic responses during seated and supine passive knee-extensor exercise in an effort to eliminate the venous hydrostatic column (Tschakovsky et al. 1996; Shoemaker et al. 1998). Positioning the leg above heart level did not change LBF or HR responses at the onset of passive exercise, thereby supporting the minimal impact of the skeletal muscle pump in these trials. These findings differ from Rådergran et al. (1998), who reported an increase in blood velocity and intramuscular pressure (IMP) during passive knee-extensor exercise, which the authors attributed to the skeletal muscle pump. However, we suggest that while IMP is a dominant factor during voluntary knee-extensor exercise, during passive exercise equal but opposite changes in IMP should occur in the opposing quadriceps and hamstrings muscle groups, and that this alternating oscillation of IMP throughout the duty cycle may partially offset any skeletal muscle pump effect on the vessels perfusing these muscles.
Mechanisms of hyperaemia during voluntary and passive exercise
Voluntary exercise hyperaemia is thought to involve the skeletal muscle pump, metabolic vasodilatation, flow-mediated vasodilatation and cardio-acceleration. (Laughlin, 1987; Delp, 1999; Joyner & Proctor, 1999; Wunsch et al. 2000; Hughson, 2003). The passive leg movement model employed in the current study was designed to limit the contribution of metabolic and mechanical factors to onset hyperaemia, and thus the observed increase in LBF during passive exercise could only occur due to augmented arterial inflow. This can only be achieved by an increase in the pressure gradient across the leg vasculature or a decrease in leg vascular resistance (Laughlin et al. 1996). As systemic arterial blood pressure did not increase, the most likely mechanism for onset hyperaemia in the passively moving leg was reduced leg vascular resistance, which may have been achieved in the following ways.
Mechanical changes in vessel dimension and volume produced during muscle shortening and lengthening may have lowered vascular resistance in the distensible leg vasculature, increasing volume flow into the passively moving leg. It has been reported that varying muscle length may significantly alter the geometry of arterioles in animals (Nakao & Segal, 1995), while others have discussed the concept of ‘mechanically induced vasodilatation’ through deformation of vascular smooth muscle cells (Tschakovsky et al. 2004) and shown that stretch (Dopico et al. 1994) and increased laminar shear stress (Olesen et al. 1988) can hyperpolarize vascular smooth muscle cells. While the present data do not directly address each specific mechanism, our cardiovascular measurements support mechanical vessel deformation as a potential contributor to hyperaemia at the onset of exercise.
Passive leg movement generated a significant cardio-acceleration, presumably evoked by the muscle mechanoreceptor reflex (Nobrega & Araujo, 1993; Nurhayati & Boutcher, 1998), raising the additional possibility of a cardiac contribution to onset hyperaemia. When viewed in conjunction with the presumed changes in vessel dimension and the assumed reduction in vascular resistance, we propose that cardio-acceleration could conceptually impart enough additional kinetic energy to the blood to contribute to the observed increase in LBF. This concept is further supported by the increased LBF in the non-moving leg, though the time course for changes in HR and LBF differ from the passively moving leg trial (Fig. 2).
LBF in the non-moving leg could have theoretically been altered by neural and endothelial influences. Passive leg movement may have triggered a reflex sympathetic withdrawal which could reduce systemic vascular resistance in the resting limb, as noted by others during voluntary leg (Callister et al. 1994) and arm (Jacobsen et al. 1994) exercise. While the semirecumbent body position lessened venous hydrostatic influences, without central venous pressure measurements we cannot exclude the possibility that some cardiopulmonary baroreflex-mediated sympathoinhibition may have occurred. Finally, although flow-mediated dilatation is not thought to be essential for hyperaemia at the onset of exercise (Shoemaker et al. 1997; Brock et al. 1998), in the non-moving leg this process may account for the hyperaemia observed during seconds 5–20 (Fig. 2). Further investigation is needed to determine the exact mechanisms governing hyperaemia observed in the resting limb.
Blood velocity profile during voluntary and passive exercise
Additional information regarding haemodynamic changes may be derived from analysis of the anterograde and retrograde portions of the blood velocity profile (Figs 1 and 3). While the increase in LBF during the first 5 s of passive exercise is best explained by a decrease in leg vascular resistance, the sharp rise in retrograde velocity may be suggestive of increased arterial inflow against an inadequate reduction in leg vascular resistance (Risoe & Wille, 1978; Green et al. 2005). During the remainder of the exercise onset period, the velocity profile demonstrated a gradual decline in the retrograde signal, while the anterograde signal remained elevated, which we interpret as indicative of delayed vasodilatation. Sustained hyperaemia and low retrograde velocity were seen during voluntary steady-state exercise, which contrasts with the passive exercise bout, where retrograde velocity returned to a significantly elevated level and LBF decayed to near baseline levels, thus demonstrating the tight relationship between perfusion and muscle metabolism (Fig. 3).
Summary
We sought to dissect the individual contributions of mechanical, vasodilatory and cardiac mechanisms to the hyperaemia seen at the onset of passive and voluntary exercise. LBF measurements in the passively moving and non-moving contralateral leg partitioned the contributions of the muscle pump and metabolic vasodilatation, with the remaining mechanical changes in vessel geometry and cardio-acceleration implicated as possible contributors to onset hyperaemia. The increased LBF seen at the onset of voluntary exercise was sustained during steady-state exercise but decayed during the passive movement trial, demonstrating the tight relationship between perfusion and muscle metabolism.
Acknowledgments
This work was funded in part by NIH HL-17731 and the Stein Institute for Research on Ageing.
References
- Brock RW, Tschakovsky ME, Shoemaker JK, Halliwill JR, Joyner MJ, Hughson RL. Effects of acetylcholine and nitric oxide on forearm blood flow at rest and after a single muscle contraction. J Appl Physiol. 1998;85:2249–2254. doi: 10.1152/jappl.1998.85.6.2249. [DOI] [PubMed] [Google Scholar]
- Buckwalter JB, Ruble SB, Mueller PJ, Clifford PS. Skeletal muscle vasodilation at the onset of exercise. J Appl Physiol. 1998;85:1649–1654. doi: 10.1152/jappl.1998.85.5.1649. [DOI] [PubMed] [Google Scholar]
- Callister R, Ng AV, Seals DR. Arm muscle sympathetic nerve activity during preparation for and initiation of leg-cycling exercise in humans. J Appl Physiol. 1994;77:1403–1410. doi: 10.1152/jappl.1994.77.3.1403. [DOI] [PubMed] [Google Scholar]
- Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol. 2004;97:393–403. doi: 10.1152/japplphysiol.00179.2004. [DOI] [PubMed] [Google Scholar]
- Delp MD. Control of skeletal muscle perfusion at the onset of dynamic exercise. Med Sci Sports Exerc. 1999;31:1011–1018. doi: 10.1097/00005768-199907000-00014. [DOI] [PubMed] [Google Scholar]
- Dopico AM, Kirber MT, Singer JJ, Walsh JV., Jr Membrane stretch directly activates large conductance Ca2+-activated K+ channels in mesenteric artery smooth muscle cells. Am J Hypertens. 1994;7:82–89. doi: 10.1093/ajh/7.1.82. [DOI] [PubMed] [Google Scholar]
- Folkow B, Gaskell P, Waaler BA. Blood flow through limb muscles during heavy rhythmic exercise. Acta Physiol Scand. 1970;80:61–72. doi: 10.1111/j.1748-1716.1970.tb04770.x. [DOI] [PubMed] [Google Scholar]
- Folkow B, Haglund U, Jodal M, Lundgren O. Blood flow in the calf muscle of man during heavy rhythmic exercise. Acta Physiol Scand. 1971;81:157–163. doi: 10.1111/j.1748-1716.1971.tb04887.x. [DOI] [PubMed] [Google Scholar]
- Gladwell VF, Coote JH. Heart rate at the onset of muscle contraction and during passive muscle stretch in humans: a role for mechanoreceptors. J Physiol. 2002;540:1095–1102. doi: 10.1113/jphysiol.2001.013486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DJ, Bilsborough W, Naylor LH, Reed C, Wright J, O'Driscoll G, Walsh JH. Comparison of forearm blood flow responses to incremental handgrip and cycle ergometer exercise: Relative contribution of nitric oxide. J Physiol. 2005;562:617–628. doi: 10.1113/jphysiol.2004.075929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann JJ, Buckwalter JB, Clifford PS, Shoemaker JK. Is the blood flow response to a single contraction determined by work performed? J Appl Physiol. 2004;96:2146–2152. doi: 10.1152/japplphysiol.00779.2003. [DOI] [PubMed] [Google Scholar]
- Hughson RL. Regulation of blood flow at the onset of exercise by feed forward and feedback mechanisms. Can J Appl Physiol. 2003;28:774–787. doi: 10.1139/h03-058. [DOI] [PubMed] [Google Scholar]
- Jacobsen TN, Hansen J, Nielsen HV, Wildschiodtz G, Kassis E, Larsen B, Amtorp O. Skeletal muscle vascular responses in human limbs to isometric handgrip. Eur J Appl Physiol Occup Physiol. 1994;69:147–153. doi: 10.1007/BF00609407. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Proctor DN. Muscle blood flow during exercise: The limits of reductionism. Med Sci Sports Exerc. 1999;31:1036–1040. doi: 10.1097/00005768-199907000-00017. [DOI] [PubMed] [Google Scholar]
- Laughlin MH. Skeletal muscle blood flow capacity: Role of muscle pump in exercise hyperemia. Am J Physiol. 1987;253:H993–H1004. doi: 10.1152/ajpheart.1987.253.5.H993. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Korthuis RJ, Duncker DJ, Bache RJ. Control of blood flow to cardiac and skeletal muscle during exercise. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 705–769. [Google Scholar]
- Nakao M, Segal SS. Muscle length alters geometry of arterioles and venules in hamster retractor. Am J Physiol. 1995;268:H336–H344. doi: 10.1152/ajpheart.1995.268.1.H336. [DOI] [PubMed] [Google Scholar]
- Nobrega AC, Araujo CG. Heart rate transient at the onset of active and passive dynamic exercise. Med Sci Sports Exerc. 1993;25:37–41. [PubMed] [Google Scholar]
- Nurhayati Y, Boutcher SH. Cardiovascular response to passive cycle exercise. Med Sci Sports Exerc. 1998;30:234–238. doi: 10.1097/00005768-199802000-00010. [DOI] [PubMed] [Google Scholar]
- Olesen SP, Clapham DE, Davies PF. Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature. 1988;331:168–170. doi: 10.1038/331168a0. [DOI] [PubMed] [Google Scholar]
- Radegram G, Saltin B. Muscle blood flow at onset of dynamic exercise in humans. Am J Physiol. 1998;274:H314–H322. doi: 10.1152/ajpheart.1998.274.1.H314. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Leek BT, Gavin TP, Haseler LJ, Mudaliar SR, Henry R, Mathieu-Costello O, Wagner PD. Reduced mechanical efficiency in chronic obstructive pulmonary disease but normal peak VO2 with small muscle mass exercise. Am J Respir Crit Care Med. 2004;169:89–96. doi: 10.1164/rccm.200305-627OC. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Saltin B. Human muscle blood flow and metabolism studied in the isolated quadriceps muscles. Med Sci Sports Exerc. 1998;30:28–33. doi: 10.1097/00005768-199801000-00005. [DOI] [PubMed] [Google Scholar]
- Risoe C, Wille SO. Blood velocity in human arteries measured by a bidirectional ultrasonic doppler flowmeter. Acta Physiol Scand. 1978;103:370–378. doi: 10.1111/j.1748-1716.1978.tb06230.x. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Reflex control of the circulation during exercise. Int J Sports Med. 1992;13(Suppl. 1):S25–S27. doi: 10.1055/s-2007-1024583. [DOI] [PubMed] [Google Scholar]
- Segal SS. Integration of blood flow control to skeletal muscle: Key role of feed arteries. Acta Physiol Scand. 2000;168:511–518. doi: 10.1046/j.1365-201x.2000.00703.x. [DOI] [PubMed] [Google Scholar]
- Sheriff DD. Muscle pump function during locomotion: Mechanical coupling of stride frequency and muscle blood flow. Am J Physiol Heart Circ Physiol. 2003;284:H2185–H2191. doi: 10.1152/ajpheart.01133.2002. [DOI] [PubMed] [Google Scholar]
- Sheriff DD, Rowell LB, Scher AM. Is rapid rise in vascular conductance at onset of dynamic exercise due to muscle pump. Am J Physiol. 1993;265:H1227–H1234. doi: 10.1152/ajpheart.1993.265.4.H1227. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Halliwill JR, Hughson RL, Joyner MJ. Contributions of acetylcholine and nitric oxide to forearm blood flow at exercise onset and recovery. Am J Physiol. 1997;273:H2388–H2395. doi: 10.1152/ajpheart.1997.273.5.H2388. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Tschakovsky ME, Hughson RL. Vasodilation contributes to the rapid hyperemia with rhythmic contractions in humans. Can J Physiol Pharmacol. 1998;76:418–427. doi: 10.1139/cjpp-76-4-418. [DOI] [PubMed] [Google Scholar]
- Skinner NS, Jr, Costin JC. Interactions of vasoactive substances in exercise hyperemia: O2, K+, and osmolality. Am J Physiol. 1970;219:1386–1392. doi: 10.1152/ajplegacy.1970.219.5.1386. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Rogers AM, Pyke KE, Saunders NR, Glenn N, Lee SJ, Weissgerber T, Dwyer EM. Immediate exercise hyperemia in humans is contraction intensity dependent: Evidence for rapid vasodilation. J Appl Physiol. 2004;96:639–644. doi: 10.1152/japplphysiol.00769.2003. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Sheriff DD. Immediate exercise hyperemia: Contributions of the muscle pump vs. rapid vasodilation. J Appl Physiol. 2004;97:739–747. doi: 10.1152/japplphysiol.00185.2004. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Shoemaker JK, Hughson RL. Vasodilation and muscle pump contribution to immediate exercise hyperemia. Am J Physiol. 1996;271:H1697–H1701. doi: 10.1152/ajpheart.1996.271.4.H1697. [DOI] [PubMed] [Google Scholar]
- Williamson JW, Nobrega AC, Winchester PK, Zim S, Mitchell JH. Instantaneous heart rate increase with dynamic exercise: Central command and muscle-heart reflex contributions. J Appl Physiol. 1995;78:1273–1279. doi: 10.1152/jappl.1995.78.4.1273. [DOI] [PubMed] [Google Scholar]
- Wunsch SA, Muller-Delp J, Delp MD. Time course of vasodilatory responses in skeletal muscle arterioles: Role in hyperemia at onset of exercise. Am J Physiol Heart Circ Physiol. 2000;279:H1715–H1723. doi: 10.1152/ajpheart.2000.279.4.H1715. [DOI] [PubMed] [Google Scholar]