Abstract
Compelling epidemiological and experimental evidence indicates that a suboptimal environment during fetal and neonatal development in both humans and animals may programme offspring susceptibility to later development of several chronic diseases including obesity and diabetes in which altered carbohydrate metabolism plays a central role. One of the most interesting and significant features of developmental programming is the evidence from several studies that the adverse consequences of altered intrauterine environments can be passed transgenerationally from mother (F0) to daughter (F1) to second generation offspring (F2). We determined whether when F0 female rats are exposed to protein restriction during pregnancy and/or lactation their F1 female pups deliver F2 offspring with in vivo evidence of altered glucose and insulin metabolism. We fed F0 virgin Wistar rats a normal control 20% casein diet (C) or a protein restricted isocaloric diet (R) containing 10% casein during pregnancy. F1 female R pups weighed less than C at birth. After delivery, mothers received C or R diet during lactation to provide four F1 offspring groups CC (first letter pregnancy diet and second lactation diet), RR, CR and RC. All F1 female offspring were fed ad libitum with C diet after weaning and during their first pregnancy and lactation. As they grew female offspring (F1) of RR and CR mothers exhibited low body weight and food intake with increased sensitivity to insulin during a glucose tolerance test at 110 days of postnatal life. Male F2 CR offspring showed evidence of insulin resistance. In contrast RC F2 females showed evidence of insulin resistance. Sex differences were also observed in F2 offspring in resting glucose and insulin and insulin : glucose ratios. These sex differences also showed differences specific to stage of development time window. We conclude that maternal protein restriction adversely affects glucose and insulin metabolism of male and female F2 offspring in a manner specific to sex and developmental time window during their mother's (the F1) fetal and neonatal development.
Compelling epidemiological and laboratory evidence indicates that a suboptimal environment during fetal and neonatal development in both humans and experimental animals may programme susceptibility in the offspring to later development of several chronic diseases including altered carbohydrate metabolism (Dahri et al. 1991; Ravelli et al. 1999; Petry & Hales, 2000; Roseboom, 2001; Kind et al. 2003). Barker and colleagues proposed the fundamental hypothesis that adverse intrauterine conditions at various stages of gestation alter the trajectory of growth and produce intrauterine growth restriction (Barker, 1995a). This altered growth trajectory compromises developing organs, which may then malfunction in later life. Thus the concept of ‘developmental programming’ proposes that challenges during an organism's development evoke a persistent physiological response during its life.
Epidemiological investigations such as those conducted on the children of the Dutch hunger winter have highlighted the association between poor maternal nutrition, lowered birth weight and subsequent adult disease (Ravelli et al. 1999; Roseboom et al. 2001). However, difficulties with socio-economic confounds and the inability to include precise contemporaneous controls in epidemiological studies demonstrate the need for carefully controlled animal investigations to address the association between maternal nutrient intake and subsequent health of the offspring. There are five main protocols that have been used for the evaluation of developmental programming of metabolism: (1) exposure of the mother to an isocaloric low protein diet (Stewart et al. 1975; Ozanne et al. 1996; Reusens & Remacle, 2001a); (2) global nutrient restriction (Garofano et al. 1997, 1998a); (3) experimentally induced maternal diabetes (Holemans et al. 1997; Holemans et al. 2003); (4) restriction of uterine blood flow (Simmons et al. 2001); and (5) over-exposure of the fetus to glucocorticoids (Nyirenda et al. 2001). Several studies have demonstrated altered glucose and insulin metabolism in the offspring of protein restricted rats and other rodents (Dahri et al. 1991; Petry et al. 2000; Kind et al. 2003). This is the model we have chosen to use.
As an extension of the concept of developmental programming, Hales & Barker (1992) proposed the ‘thrifty phenotype hypothesis’ stating that when in utero nutrition is suboptimal, the fetus makes a predictive adaptive response and develops a physiology that maximizes uptake and conservation of nutrients. These developmental changes confer a survival advantage if nutrition continues to be restricted during the offspring's life-time. However, if food supply is abundant postnatally, offspring with the thrifty phenotype may accumulate stored resources in the form of fat thereby predisposing the offspring to obesity and other metabolic problems.
One of the most interesting and important features of developmental programming is the evidence from several studies that the consequences of an altered intrauterine environment can be passed transgenerationally from mother (F0) to daughter (F1) to the F2 progeny. Stewart et al. (1975) demonstrated effects of maternal nutrient restriction across 12 generations in the rat. Two independent groups of investigators have demonstrated that the female diabetic offspring (F1) of rats treated with streptozotocin during pregnancy themselves have offspring (F2) with altered glucose and carbohydrate metabolism (Aerts et al. 1990; Oh et al. 1991; Aerts & Van Assche, 1992).
We studied F0 female rats exposed to protein restriction during pregnancy and/or lactation to determine (1) whether there are transgenerational effects on the F2 generation, (2) whether these effects show sex specificity, and (3) whether effects are dependent on the stage of development at which protein restriction occurs – pregnancy or lactation. We fed one group of F0 virgin Wistar rats a normal control diet (C) during pregnancy and lactation (CC – first letter pregnancy diet and second letter lactation diet). Additional F0 rats were fed a restricted 50% protein isocaloric diet (R) during pregnancy and/or lactation to provide three further groups RR, CR and RC. All F1 female offspring ate the control diet after weaning and during their first pregnancy and lactation as did the F2 generation after weaning.
Female offspring (F1) of mothers restricted during lactation (RR and CR) exhibited low body weight and food intake with increased sensitivity to insulin during a glucose tolerance test at 110 days of postnatal life. F1 RC offspring demonstrated increased insulin resistance. Since the aim of this paper is to report transgenerational effects, data from the F1 male offspring are not given here. F2 female offspring maintained higher glucose and lower insulin concentrations than F2 male offspring. Male F2 CR and female F2 RC offspring showed decreased insulin sensitivity. These findings indicate that maternal protein restriction adversely affects glucose and insulin metabolism of F2 offspring in a manner specific to sex and to the stage of their mother's (the F1) fetal and neonatal development.
Methods
Care and maintenance of animals
Breeding and maintenance of the first generation of female rats (F0)
Details of protein restriction and generation of the F1 pups have been given in full previously (Zambrano et al. 2005). Those details that are central to this study will be presented here. The F0 mothers were 40 virgin female albino Wistar rats aged between 10 and 12 weeks and weighing 220 ± 20 g (mean ± s.e.m.) obtained from the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (Mexico City, Mexico). Female rats with regular cycles were maintained on Purina Laboratory Chow 5001. Rats were maintained under controlled lighting (lights on from 07.00 to 19.00 h at 22–23°C). All procedures were approved by the Animal Experimentation Ethics Committee of the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City.
Female rats were mated overnight with proven male breeders and the day on which spermatozoa were present in a vaginal smear was designated as day of conception – day 0. Only rats that were pregnant within 5 days of introduction of the male were retained in the study. Pregnant rats were transferred to individual metabolism cages and allocated at random to one of two groups to be fed either a 20% casein (control) diet or a 10% casein isocaloric (restricted) diet (Table 1). Food and water were available ad libitum for all animals.
Table 1.
Composition of the two isocaloric diets
| Control diet (%) | Restricted diet (%) | |
|---|---|---|
| Casein | 20 | 10 |
| Cystine | 0.3 | 0.15 |
| Choline | 0.165 | 0.165 |
| Vitamin mix | 1 | 1 |
| Mineral mix | 5 | 5 |
| Cellulose | 5 | 5 |
| Corn oil | 5 | 5 |
| Carbohydrates | ||
| Corn starch | 31.76 | 37.34 |
| Dextrose | 31.76 | 37.34 |
| kcal g−1 diet | 4 | 4 |
F0 pregnant and lactating rats were weighed every day through pregnancy and until the pups were removed at weaning. Food was provided in the form of large flat biscuits which were retained behind a grill through which the rats nibbled the food. The amount of food provided each day was weighed as was the amount remaining after 24 h. On day 20 post-conception, pregnant rats were transferred to normal rat cages to provide optimal conditions for delivery which occurred in the early daylight hours between 09.00 h and 12.00 h on post-conceptual day 22. Day of delivery was considered as day 0. Food intake continued to be monitored during this period.
All F0 rats delivered the F1 generation by spontaneous vaginal delivery. Timing of delivery of the F1 pups, F1 litter size and pup weight were recorded at birth. Ano-genital distance was measured with calipers to enable determination of sex. Our unpublished data indicate that female pups have an ano-genital distance of 0.167 ± 0.013 mm (n = 291 pups from 43 litters; mean ± s.e.m.) and males 0.326 ± 0.022 mm (n = 252 pups from 43 litters). Thus a value of 0.25 mm is more than 2 s.d. from the mean of either group and sex was judged according to whether the ano-genital distance was greater than (male) or less than (female) 0.25 mm. To ensure homogeneity of study subjects, litters of over 14 pups were not included in the study. Litters of 12–14 pups were adjusted to 12 pups for each dam while maintaining as close to a 1: 1 sex ratio as possible. Four groups were established: CC in which dams that received the control diet during pregnancy continued to be fed the control diet during lactation; RR in which dams that had received the restricted diet during pregnancy continued to receive the restricted diet during lactation; CR in which dams that received the control diet during pregnancy received the restricted diet during lactation; and RC in which dams that received the restricted diet during pregnancy were provided with the control diet during lactation. After weaning (postnatal day 21) all pups were fed with control (20% casein) diet ad libitum. Pups continued to be weighed daily.
Measurement of food intake after weaning
Rats from the same experimental group were housed two of the same sex to a cage. Food was provided in the form of large flat biscuits, which were retained behind a grill through which the rats nibbled the food. The amount of food provided each day was weighed as was the amount remaining after 24 h. The amount consumed was averaged between the two rats.
Glucose tolerance test at 110 days postnatal life in the F1 generation
One or two F1 female rats from each litter were fasted overnight. One gram per kilogram body weight d-glucose was administered i.p. at 09.00 h. Blood was taken by retro- orbital puncture as approved by the American Veterinary Medical Association at time 0 min, 30 min, 60 min, and 120 min. Blood was collected into polyethylene tubes and allowed to clot at 4°C for 1 h. The blood samples were centrifuged at 1500 g for 15 min at 4°C. Serum samples were kept at −20°C until assayed. Where two females from one litter were studied their data were averaged.
Breeding of the F1 female offspring
Following the glucose tolerance test at 110 days postnatal life, F1 females were bred to proven males from outside the experiment to produce the F2 offspring. During the pregnancy and for the rest of the study, all F1 females were fed the control diet. All F1 females underwent spontaneous vaginal delivery. Timing of delivery of the F2 pups, F2 litter size and pup weight were recorded at birth. Ano-genital distance was measured at birth to enable determination of sex. At birth, the F2 litters were adjusted to 12 pups per dam while maintaining as close to a 1: 1 sex ratio as possible. At weaning pups were divided by sex and placed into separate group cages.
Glucose tolerance test in the F2 offspring at postnatal day 110d
One F2 male and one F2 female from each F1 female were chosen at random, fasted overnight and studied in a glucose tolerance test at 110 days of postnatal life. The glucose tolerance test was conducted as described above.
Biochemical analyses
Blood glucose measurement
Serum glucose concentrations were determined spectrophotometrically using the enzymatic hexokinase method (Beckman Coulter, Co., Fullerton, CA, USA).
Insulin radioimmunoassay
Serum insulin concentrations were determined by RIA using commercial rat kits from Linco Research, Inc., Cat. no. RI-13K. The intra- and interassay coefficients of variations were < 4% and < 6%.
Statistical analysis
For the glucose tolerance tests, area under curve was calculated using SigmaPlot 7. Insulin resistance index (IRI) was calculated with the baseline values from the formula IRI = Glucose × Insulin/22.5 (Nandhini et al. 2005). All data are presented as means ± s.e.m. Differences between groups for F1 and F2 females and F2 males were compared using multiple analysis of variance (ANOVA) followed by Dunnett's test. Student's unpaired t test was used to compare male and female F2 data for baseline values and areas under curve for the same variable and IRI. A χ2 test was used to determine differences in the duration of pregnancy and timing of delivery; P≤ 0.05 was considered significant.
Results
Timing of delivery of F1 pups by F0 mothers, litter size and sex ratio of the F1 pups and growth profile of F1 females
At birth F1 female pups of F0 mothers fed the control diet weighed 6.1 ± 0.08 g while those of F0 restricted mothers weighed 5.7 ± 0.1 g (P < 0.05). There were no differences in the time of delivery, litter size and litter sex distribution between control and restricted F0 mothers (Table 2A). Table 2B provides the data on body weight at 21, 100 and 270 days and at 1 year of postnatal life of the females that grew up to be the F1 mothers. The females who did not become pregnant were not included in these groups. When compared with CC, weights of female offspring of the RR and CR groups were reduced by 41 and 29% at 21 days, by 22 and 19% at 100 days, by 19 and 18% at 270 days and by 19 and 18% at 1 year (P < 0.01). The weights of the female RR group offspring were less than the RC group at all postnatal ages and the CR group was less than RC at 21 days, 270 days and 1 year (P < 0.05).
Table 2.
Results from F1 female offspring
| C | R | |||
|---|---|---|---|---|
| A. Timing of delivery by the F0 mothers | ||||
| Pups delivered before noon (%) | 75 (16) | 53 (22) | ||
| Pups delivered after noon (%) | 25 (16) | 47 (22) | ||
| Litter size | 14.4 ± 0.37 (16) | 13.9 ± 0.35 (22) | ||
| Litter sex distribution M: F | 1.06 ± 0.16 (16) | 1.35 ± 0.28 (22) | ||
| Birth weight (g) | 6.1 ± 0.08a (16) | 5.7 ± 0.10b (22) | ||
| CC | RR | CR | RC | |
| B. F1 female gth data (g) | ||||
| 21 days | 38.7 ± 1.93a (5) | 22.9 ± 0.69b (5) | 27.3 ± 0.36b (6) | 42.9 ± 1.04a (6) |
| 100 days | 319.2 ± 9.96a (5) | 248.7 ± 16.21b (5) | 259.7 ± 10.4b,c (6) | 311.0 ± 25.10a,c (6) |
| 270 days | 406.8 ± 16.89a (4) | 328.9 ± 14.27b (4) | 331.9 ± 8.15b (5) | 396.5 ± 47.37a (5) |
| 1 year | 400.4 ± 15.33a (4) | 322.1 ± 15.45b (4) | 326.9 ± 6.52b (5) | 493.3 ± 43.72a (5) |
| C. F1 female food intake at 100 days | ||||
| Food intake (g day−1) | 22.8 ± 1.03a (5) | 19.5 ± 0.19b (5) | 19.5 ± 0.40b (6) | 20.1 ± 2.20a,b (6) |
| Food intake (g (g body wt)−1) | 0.07 ± 0.003 (5) | 0.08 ± 0.005 (5) | 0.08 ± 0.003 (6) | 0.06 ± 0.002 (6) |
Data are means ± s.e.m. with number of litters shown in parentheses. A, timing of delivery of the F1 pups by the F0 mothers (%), litter size, sex ratio and birth weights (g) from the C (control −20% casein) and R (restricted −10% casein) group. B, gth profile (g) of rats fed with control (C) or restricted (R) diet during pregnancy (first letter) and lactation (second letter) of F1 female pups at 21, 100, 270 days and 1 year. C, food intake in adult life of female F1 offspring. P > 0.05 for data with at least one letter in common.
Food intake in adult life of female F1 offspring
Total food intake of the RR and CR female F1 offspring groups was reduced by 15 and 14% at 100 days when compared with the CC group (P < 0.05; Table 2C). However when expressed per unit body weight, total food intake was not different between groups.
Glucose tolerance tests in F1 females
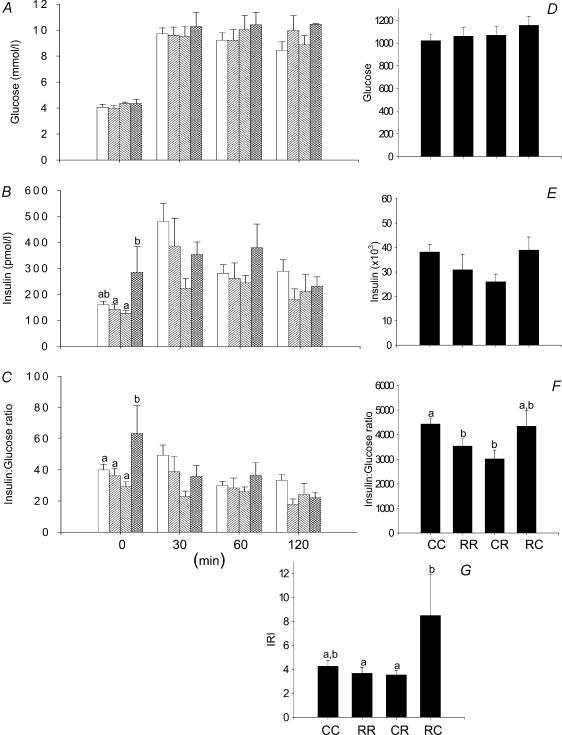
There were no differences in the glucose responses to the i.p. glucose tolerance test in the four groups of F1 female offspring represented either as glucose values at different times (Fig. 1A) or as the area under the curve for glucose (Fig. 1D). Resting insulin was higher in the RC group than CR and RR (Fig. 1B; P < 0.05). The insulin: glucose ratio baseline was higher in RC offspring than the three other groups (Fig. 1C; P < 0.05). The area under the curve for the insulin: glucose ratio was less in RR and CR than CC (Fig. 1F; P < 0.05). The IRI was higher in RC than RR and CR (Fig. 1G; P < 0.05).
Figure 1. Results from the glucose tolerance tests performed in the F1 females at 110 days of postnatal life.
A, serum glucose; B, serum insulin; C, insulin to glucose ratio; D, area under curve for serum glucose during glucose tolerance tests; E, area under curve for insulin; F, area under curve for insulin: glucose ratio; G, insulin resistance index calculated as (glucose × insulin concentration)/22.5. Data represented as means ± s.e.m. from 5 CC litters, 5 RR litters, 6 CR litters, and 6 RC litters. Diets of F0 mothers are denoted as control (C) or restricted (R) during pregnancy (first letter), followed by lactation (second letter). CC: control–control  ; RR: restricted–restricted
; RR: restricted–restricted  ; CR: control–restricted
; CR: control–restricted  ; RC: restricted–control
; RC: restricted–control  . P > 0.05 for data with at least one letter in common.
. P > 0.05 for data with at least one letter in common.
Timing of delivery of the F2 pups by the F1 mothers, litter size, sex distribution and F2 birth weights and growth of the F2 offspring
There was no difference in the timing of delivery, litter size and sex distribution between the litters of the groups of F1 mothers (Table 3A). Birth weights of F2 female offspring of F1 pups from the original RC mothers were heavier than the F2 offspring of F1 pups from the RR mothers (P < 0.05). The F2 female RC offspring were heavier than the F2 female CR offspring at 100 and 270 days (Table 3B; P < 0.05).
Table 3.
Results from F2 female and male offspring
| CC | RR | CR | RC | |
|---|---|---|---|---|
| A. F1 delivery data | ||||
| Pups delivered before noon (%) | 81.8 (6) | 60 (6) | 73.68 (6) | 75 (4) |
| Pups delivered after noon (%) | 18.2 (6) | 40 (6) | 26.31 (6) | 25 (4) |
| Litter size | 14.1 ± 0.91 (6) | 13.4 ± 1.13 (6) | 12.2 ± 0.58 (6) | 11.8 ± 1.24 (4) |
| Litter sex distribution M:F | 1.13 ± 0.19 (6) | 1.30 ± 0.24 (6) | 0.83 ± 0.11 (6) | 0.98 ± 0.10 (4) |
| B. F2 female gth data (g) | ||||
| Birth | 5.9 ± 0.14a,b (6) | 5.6 ± 0.13a (6) | 6.0 ± 0.13a,b (6) | 6.3 ± 0.16b (4) |
| 21 days | 45.0 ± 0.86 (6) | 43.6 ± 0.86 (6) | 45.0 ± 0.76 (6) | 45.0 ± 2.18 (4) |
| 100 days | 292.2 ± 8.24a,b (6) | 298.1 ± 10.16a,b (6) | 284.6 ± 6.06a (6) | 317.3 ± 6.15b (4) |
| 270 days | 332.6 ± 10.87a,b (5) | 320.2 ± 15.42a,b (5) | 308.6 ± 5.69a (5) | 346.0 ± 10.41b (4) |
| 1 year | 381.7 ± 26.06 (5) | 347.6 ± 18.58 (5) | 367.2 ± 14.99 (5) | 403.0 ± 26.18 (4) |
| C. F2 Female Food Intake at 100 days | ||||
| Food intake (g day−1) | 19.7 ± 0.71 (6) | 18.9 ± 0.78 (6) | 19.5 ± 0.46 (6) | 19.0 ± 0.34 (4) |
| Food intake (g (g body wt)−1) | 0.068 ± 0.002 (6) | 0.064 ± 0.003 (6) | 0.065 ± 0.003 (6) | 0.060 ± 0.003 (4) |
| D. F2 male gth data | ||||
| Birth (g) | 6.21 ± 0.18 (6) | 6.00 ± 0.16 (6) | 6.29 ± 0.11 (6) | 6.53 ± 0.12 (4) |
| 21 days (g) | 48.35 ± 0.66a (6) | 45.41 ± 0.63b (6) | 46.24 ± 0.88a,b (6) | 44.85 ± 1.73b (4) |
| 100 days (g) | 489.63 ± 6.70a (6) | 459.14 ± 8.84b (6) | 460.21 ± 8.30b (6) | 483.86 ± 11.65a,b (4) |
| 270 days (g) | 559.65 ± 7.65a,b (5) | 542.37 ± 10.86a,b (5) | 522.59 ± 9.85a (5) | 570 ± 7.05b (4) |
| E. F2 Male Food Intake at 100 days | ||||
| Food intake (g day−1) | 32.41 ± 0.87a (6) | 27.89 ± 1.22b (6) | 27.99 ± 0.87b (6) | 31.98 ± 0.80a (4) |
| Food intake (g (g body wt)−1) | 0.066 ± 0.0020 (6) | 0.062 ± 0.0022 (6) | 0.062 ± 0.0015 (6) | 0.066 ± 0.0018 (4) |
Data are means ± s.e.m. with number of litters shown in parentheses. A, timing of delivery of the F2 pups by the F1 mothers (%), litter size, sex ratio and birth weights (g) from the C (control −20% casein) and R (restricted −10% casein) group. B, gth profile (g) of rats fed with control (C) or restricted (R) diet during pregnancy (first letter) and lactation (second letter) of F2 female pups at 21, 100, 270 days and 1 year. C, food intake in adult life of F2 female offspring. D, gth profile (g) of rats fed with control (C) or restricted (R) diet during pregnancy (first letter) and lactation (second letter) of F2 male pups at 21, 100, 270 days. E, food intake in adult life of F2 male offspring. P > 0.05 for data with at least one letter in common.
There were no differences in birth weight among the groups of F2 males. F2 male body weights at 100 days postnatal life were lower in the F2 RR and CR offspring than the F2 CC offspring (Table 3D; P < 0.05). The F2 male RC offspring were heavier than the F2 male CR offspring at 270 days (Table 3D; P < 0.05).
Food intake in adult life of female F2 offspring
There were no differences in either absolute food intake or intake per gram body weight at 100 days of age between the four groups of F2 female offspring (Table 3C).
Food intake in adult life of male F2 offspring
F2 male total food intake at 100 days postnatal life were lower in the F2 RR and CR offspring (P < 0.05). However there were no differences between groups in food intake per unit body weight (Table 3E).
Glucose tolerance tests in the F2 females at 110 days of postnatal life
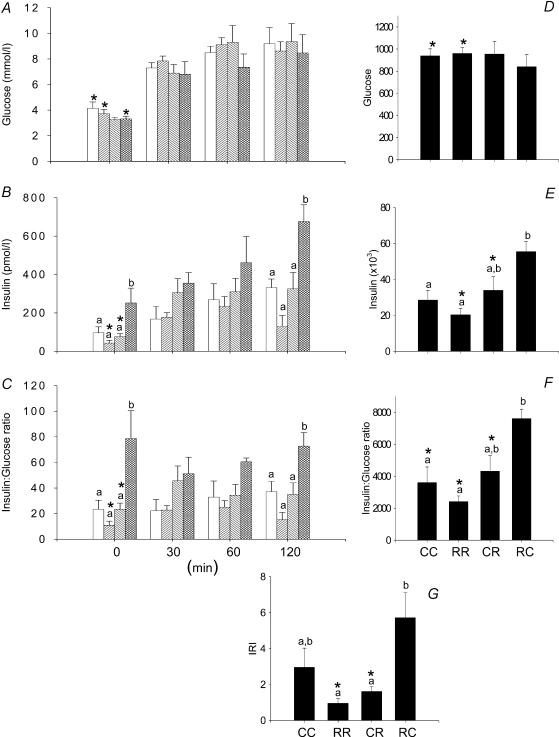
There were no differences in the glucose responses to the i.p. glucose tolerance test in the four groups of F2 female offspring represented either as glucose values at different times (Fig. 2A) or as the area under the curve for glucose (Fig. 2D). Baseline, 120 min insulin and insulin area under curve were all elevated in the RC F2 females compared with CC group (Fig. 2B and E; P < 0.05). Insulin: glucose ratio was increased in female RC offspring at baseline and 120 min compared to all other groups. Insulin: glucose ratio area under curve was increased in RC compared to CC and RR (P < 0.05; Fig. 2C and F). The IRI was increased in the RC group compared with both the RR and CR (Fig. 2G).
Figure 2. Results from the glucose tolerance tests performed in the F2 females at 110 days of postnatal life.
A, serum glucose; B, serum insulin; C, insulin to glucose ratio; D, area under curve for serum glucose during glucose tolerance tests; E, area under curve for insulin; F, area under curve for insulin:glucose ratio; G, insulin resistance index calculated as (glucose × insulin concentration)/22.5. Data represented as means ± s.e.m. from 5 to 6 CC litters, 5–6 RR litters, 6 CR litters, and 4–6 RC litters. Diets of F0 mothers are denoted as control (C) or restricted (R) during pregnancy (first letter), followed by lactation (second letter). CC: control–control  ; RR: restricted–restricted
; RR: restricted–restricted  ; CR: control–restricted
; CR: control–restricted  ; RC: restricted–control
; RC: restricted–control  . P > 0.05 for data with at least one letter in common. *P < 0.05 versus male.
. P > 0.05 for data with at least one letter in common. *P < 0.05 versus male.
Glucose tolerance tests in F2 males at 110 days of postnatal life
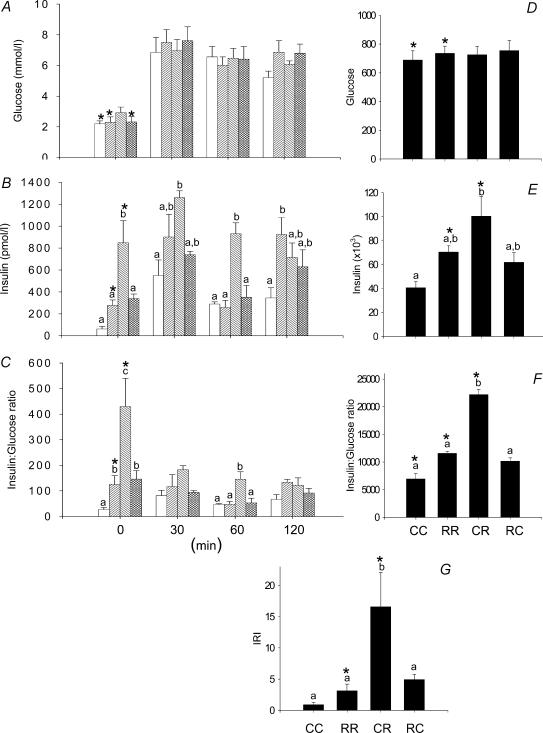
In male F2 offspring there was no difference between groups in glucose concentrations (Fig. 3A). Resting insulin was elevated in male CR F2 offspring compared with all other groups (Fig. 3B). The area under curve for both insulin and the insulin: glucose ratio was only significantly elevated in CR above the CC group (Fig. 3E and F). The IRI was significantly higher in CR compared with all other groups (Fig. 3G).
Figure 3. Results from the glucose tolerance tests performed in the F2 males at 110 days of postnatal life.
A, serum glucose; B, serum insulin; C, insulin to glucose ratio; D, area under curve for serum glucose during glucose tolerance tests; E, area under curve for insulin; F, area under curve for insulin:glucose ratio; G, insulin resistance index calculated as (glucose × insulin concentration)/22.5. Data represented as means ± s.e.m. from 5 CC litters, 5 RR litters, 6 CR litters, and 6 RC litters. Diets of F0 mothers are denoted as control (C) or restricted (R) during pregnancy (first letter), followed by lactation (second letter). CC: control–control  ; RR: restricted–restricted
; RR: restricted–restricted  ; CR: control–restricted
; CR: control–restricted  ; RC: restricted–control
; RC: restricted–control  . P > 0.05 for data with at least one letter in common. *P < 0.05 versus female.
. P > 0.05 for data with at least one letter in common. *P < 0.05 versus female.
Differences between F2 males and females
All significant sex based differences indicate increased insulin sensitivity in the females compared with males even in the presence of significantly higher baseline glucose in CC, RR and RC females compared with the same male groups (see Figs 2 and 3). In F2 females of CC and RR mothers, the area under curve for glucose was greater than the corresponding F2 males. Baseline and area under curve for insulin were lower in female RR and CR than in the male groups. Baseline insulin: glucose ratio was lower in RR and CR females compared with the corresponding male groups, while the area under curve for the insulin: glucose ratio area was lower in CC, RR and CR females compared with corresponding male groups. Area under curve for IRI was lower in RR and CR females compared with males. The difference in the baseline glucose was the only sex differences in any of the variables between the RC groups.
Discussion
Several previous studies in rats have shown that altered maternal carbohydrate and protein metabolism during an initial pregnancy (F0) due to maternal diabetes (Van Assche et al. 2001), maternal glucocorticoid administration (Drake & Walker, 2004; Drake et al. 2005), maternal low protein diets (Reusens & Remacle, 2001b), global caloric restriction (Garofano et al. 1997) or bilateral uterine artery ligation in late pregnancy (Simmons et al. 2001) can result in altered carbohydrate metabolism in F1 offspring as well as F2 offspring of F1 females. In the protein restricted model the effects on F2 of maternal F0 protein restriction have so far only been shown during fetal life (Reusens & Remacle, 2001a). No data exist for postnatal consequences in the F2 generation. Our study focused on the effects of protein restriction and was designed to address three related, previously unaddressed questions in adult F2 offspring of protein restricted mothers. Firstly, do the impaired structural effects reported in the F2 fetal pancreas result in disordered in vivo insulin function in adult life? Secondly are there differences in the outcomes in male and female F2 progeny? Finally can the transgenerational effects in F2 pups be related to the window of development, fetal life or during lactation, in which the developing female F1 rat pup is exposed to protein restriction?
To enable conclusions to be drawn from the F1 females' pregnancies, we felt it necessary to characterize carbohydrate metabolism as the F1 mothers grew up. As expected, birth weight was lower in female offspring of F0 mothers exposed to the low protein diet during pregnancy. Although offspring exposed to protein restriction during both pregnancy and lactation weighed 16% less at weaning than offspring exposed to protein restriction during lactation alone, this difference was not significant. The postnatal growth data for F1 offspring at all four ages studied up to 1 year of postnatal age clearly show that the effects of prenatal protein restriction, while marked at birth, can be recuperated by feeding the control diet during lactation. The major negative effects on post-weaning weight resulted from restriction of maternal protein intake during lactation.
Several studies demonstrate that fetal exposure to nutrient restriction adversely affects the development of the fetal pancreas (Snoeck et al. 1990; Sener et al. 1996; Petrik et al. 1999). However, our data support the view proposed by others that nutrient restriction of pups during lactation has the major effect on impaired pancreatic function in adult life (Garofano et al. 1998a, b).
Maternal F0 nutrient restriction during lactation had a more pronounced inhibitory effect on offspring growth in the F1 females and the F2 males than restriction during pregnancy. This result also shows that the nutrient restriction to which the F1 mothers were themselves exposed as breast-feeding pups before they were themselves weaned during lactation is transferred across the generations to their own F2 male offspring but not F2 female offspring. In the F1 females a comparison of the RR and RC groups and the CC and RC groups indicates that the effects of nutrient delivery during pregnancy can be restored during lactation.
The finding of increased insulin sensitivity as indicated by the decreased insulin: glucose ratio in the 110-day-old female F1 offspring of the RR and CR F0 mothers is similar to the observations by others that offspring of Sprague-Dawley dams fed an 8% protein diet demonstrated better glucose regulation than controls early in life but eventually develop glucose intolerance by 1 year of age (Petry et al. 1997; Shepherd et al. 1997). Enhanced insulin sensitivity as demonstrated by glucose uptake from isolated skeletal muscle has been demonstrated in 3-month-old offspring following maternal protein deprivation (Ozanne et al. 1996). By 15 months the offspring of protein deprived rats demonstrated insulin resistance (Ozanne et al. 2003).
Increased ability to deliver glucose and amino acids to cells in early life may well be a predictive adaptation of the offspring of protein restricted mothers. According to this view, exposure to protein restriction during development leads to a thrifty phenotype that enables the organism to conserve available food and store it against the likelihood that they may continue to experience similar periods of nutrient restriction after weaning. In our studies the offspring of mothers who were protein restricted during lactation had food available ad libitum after weaning. Thus their preparation for postweaning life was inappropriate to the level of food available. When offspring of protein restricted rats are maintained on that diet throughout life they have enhanced insulin sensitivity (Holness & Sugden, 1999). When these rats are given a fat rich diet they develop greater insulin resistance than control animals fed the fat diet. This study supports the view that the programmed predisposition to dealing more effectively with poor postnatal nutrition renders the offspring vulnerable to a high calorie postnatal diet.
The increased IRI in the F1 female RC offspring when compared with RR and CR offspring also supports the view that glucose regulatory mechanisms are at least to some extent programmed by the level of nutrient availability prenatally. According to this view the availability of plentiful food postnatally to F1 offspring that had developed prenatally in a deprived nutrient environment increases insulin resistance when compared with offspring restricted prenatally that continue to be restricted after birth. It is noteworthy that IRI is not increased when the reverse situation occurs, namely postnatal nutrition is impaired compared with the prenatal level.
We did not obtain glucose tolerance data on these F1 females during their own pregnancies. However, Dahri et al. (1995) have shown that female offspring of dams fed an 8% protein diet had low insulin secretion during a glucose tolerance test at 18.5 days of gestation of their own pregnancies. These findings would support the hypothesis that the second generation (F2) grow up to demonstrate insulin dysfunction as a result of a poor metabolic response to the challenge of pregnancy by their F1 mothers. Further studies are needed to determine the role of altered maternal F1 insulin metabolism in conjunction with other consequences of protein restriction prior to the time they themselves were weaned such as smaller maternal size (and hence presumably uterine size) and alterations in mammary gland function.
We were successful in our first aim, to demonstrate altered insulin responses in the F2 generation in early adulthood. Evidence of reduced insulin sensitivity was observed in one group of males (CR) and one group of females (RC) compared with the F2 offspring derived from the original CC F0 mothers who were fed the 20% casein in pregnancy and lactation. This finding of impaired in vivo function in F2 offspring at 110 days postnatal life extends the observations of Reusens & Remacle (2001a, b) who showed that F2 fetuses of mothers themselves exposed to low protein during fetal life exhibited high glucose levels and lower insulin levels with a decreased pancreatic insulin content and β-cell volume.
The F2 findings mentioned above indicate the existence of sex differences in the F2 offspring. Strikingly, baseline glucose levels were higher in females than males in two of the four F2 groups. Male F2 CR and female F2 RC offspring showed decreased insulin sensitivity. The mechanistic explanation for this sex difference remains to be determined. These findings indicate that adverse second generational effects can follow protein restriction in both pregnancy and lactation. Sex differences have been observed in other examples of developmental programming. For example, the adult hypertension that results from feeding pregnant rats a low protein diet during pregnancy is dependent on fetal glucocorticoid exposure in the male offspring but not in female offspring (McMullen & Langley-Evans, 2005). Thus it is important for future studies to evaluate changes in both sexes.
We know of no other study in which an attempt has been made to isolate the critical window of exposure that results in the disordered insulin function in either F1 or F2 offspring. Many of the maturational processes that occur during the period of lactation in the rat are completed in the final trimester of human development. Epidemiological data from the Dutch hunger winter indicate that exposure to maternal nutrient restriction during the third trimester predisposes the offspring to diabetes (Ravelli et al. 1998). Although it is customary to equate the stages of development in late human gestation with the lactation period in rats, caution in interpretation is necessary when extrapolating between stages of development in altricial rodents and precocial mammals such as humans. Interactions between nutrient state that existed during different stages of development and an organism's exposures in the mature adult state have been proposed as part of the concept of predictive adaptation (Gluckman & Hanson, 2004). In the present study it appeared that maternal nutrient restriction had a greater effect on F1 female offspring when restriction occurred during pregnancy. As discussed above, increased insulin resistance was observed in the RC group in F2 females and the CR group of F2 males.
Several mechanisms have been proposed to explain transgenerational consequences of developmental programming. Both DNA based and epigenetic effects should be considered. One DNA based candidate is an alteration to the mitochondrial DNA of the developing female fetus resulting from the challenge experienced during development. If such mitochondrial DNA changes occur in the female gametes, they would be passed on to the offspring. Several epigenetic causes could stem from the altered conditions experienced by the developing F2 fetus within the F1 uterus. The F1 mother's experience during her own intrauterine life may alter many aspects of the intrauterine environment. For example, we have unpublished data indicating that F1 CR offspring have high testosterone levels in the adult non-pregnant state. If elevated maternal androgen levels persist during pregnancy it would lead to varying fetal hormone exposures in mothers with different F1 phenotypes. These differences could yield a variety of F2 outcomes as a result of developmental programming during the F1 mother's pregnancy and lactation. It has been demonstrated that F1 offspring of nutrient restricted mothers have a tendency to become diabetic during pregnancy (Fernandez-Twinn et al. 2003) but the influence of different degrees and types of nutrient restriction during the F1 mother's own development on the degree and extent of any diabetic state when she herself becomes pregnant have not been evaluated. Since maternal nutrient restriction alters offspring peripheral cardiovascular development, it is also possible that differing degrees of altered uterine perfusion during the F1 pregnancy would produce differing F2 phenotypes. Thus impaired vasodilatory responses have been shown in mesenteric resistance arteries of F1 rat offspring whose mothers were protein restricted during pregnancy (Torrens et al. 2003). Further work is clearly necessary to address these various possibilities.
Finally, in the process of conducting these studies we became aware of many possible confounds that have been ignored to date. We have preliminary unpublished data that, surprisingly, milk yield and composition are adversely affected to a greater extent in CR than RR. The lactating F0 mothers in the CR group lost more weight than those in the RR group. We have no explanation for these findings but they sound a cautionary note against too rapid conclusions that one period of development exhibits a greater sensitivity to a specified challenge than another. It may be that the same challenge imposed in two different groups at one developmental stage during an experiment yields both qualitatively and quantitatively different results because of the different prior history of each group.
Conclusion
In conclusion, we confirmed the findings in several previous reports that the F1 female (Fernandez-Twinn et al. 2005) offspring of pregnant F0 rats provided with an isocaloric, low protein diet during lactation have evidence of increased insulin sensitivity compared with controls. We have shown for the first time that an isocaloric low protein diet adversely affects the glucose and insulin metabolism of adult male and female F2 offspring. In addition, male and female offspring are affected differently. Finally, the effects on F2 offspring differ according to the critical window of exposure of the F0 females to the low protein diet.
Acknowledgments
This work was partially supported by CONACyT-138259 (Mexico) and the NIH HD21350. M. Deás was a recipient of a grant from PLACIRH, Mexico.
References
- Aerts L, Holemans K, Van Assche FA. Maternal diabetes during pregnancy: consequences for the offspring. Diabetes Metab Rev. 1990;6:147–167. doi: 10.1002/dmr.5610060303. [DOI] [PubMed] [Google Scholar]
- Aerts L, Van Assche FA. Islet transplantation in diabetic pregnant rats normalizes glucose homeostasis in their offspring. J Dev Physiol. 1992;17:283–287. [PubMed] [Google Scholar]
- Barker DJ. The fetal and infant origins of disease. Eur J Clin Invest. 1995a;25:457–463. doi: 10.1111/j.1365-2362.1995.tb01730.x. [DOI] [PubMed] [Google Scholar]
- Barker DJ. Intrauterine programming of adult disease. Mol Med Today. 1995b;1:418–423. doi: 10.1016/s1357-4310(95)90793-9. [DOI] [PubMed] [Google Scholar]
- Dahri S, Reusens B, Remacle C, Hoet JJ. Nutritional influences on pancreatic development and potential links with non-insulin-dependent diabetes. Proc Nutr Soc. 1995;54:345–356. doi: 10.1079/pns19950003. [DOI] [PubMed] [Google Scholar]
- Dahri S, Snoeck A, Reusens-Billen B, Remacle C, Hoet JJ. Islet function in offspring of mothers on low-protein diet during gestation. Diabetes. 1991;40(Suppl. 2):115–120. doi: 10.2337/diab.40.2.s115. [DOI] [PubMed] [Google Scholar]
- Drake AJ, Walker BR. The intergenerational effects of fetal programming: non-genomic mechanisms for the inheritance of low birth weight and cardiovascular risk. J Endocrinol. 2004;180:1–16. doi: 10.1677/joe.0.1800001. [DOI] [PubMed] [Google Scholar]
- Drake AJ, Walker BR, Seckl JR. Intergenerational consequences of fetal programming by in utero exposure to glucocorticoids in rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R34–R38. doi: 10.1152/ajpregu.00106.2004. [DOI] [PubMed] [Google Scholar]
- Fernandez-Twinn DS, Ozanne SE, Ekizoglou S, Doherty C, James L, Gusterson B, et al. The maternal endocrine environment in the low-protein model of intra-uterine growth restriction. Br J Nutr. 2003;90:815–822. doi: 10.1079/bjn2003967. [DOI] [PubMed] [Google Scholar]
- Fernandez-Twinn DS, Wayman A, Ekizoglou S, Martin MS, Hales CN, Ozanne SE. Maternal protein restriction leads to hyperinsulinemia and reduced insulin-signaling protein expression in 21-mo-old female rat offspring. Am J Physiol Regul Integr Comp Physiol. 2005;288:R368–R373. doi: 10.1152/ajpregu.00206.2004. [DOI] [PubMed] [Google Scholar]
- Garofano A, Czernichow P, Breant B. In utero undernutrition impairs rat beta-cell development. Diabetologia. 1997;40:1231–1234. doi: 10.1007/s001250050812. [DOI] [PubMed] [Google Scholar]
- Garofano A, Czernichow P, Breant B. Beta-cell mass and proliferation following late fetal and early postnatal malnutrition in the rat. Diabetologia. 1998a;41:1114–1120. doi: 10.1007/s001250051038. [DOI] [PubMed] [Google Scholar]
- Garofano A, Czernichow P, Breant B. Postnatal somatic growth and insulin contents in moderate or severe intrauterine growth retardation in the rat. Biol Neonate. 1998b;73:89–98. doi: 10.1159/000013964. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- Holemans K, Aerts L, Van Assche FA. Lifetime consequences of abnormal fetal pancreatic development. J Physiol. 2003;547:11–20. doi: 10.1113/jphysiol.2002.036582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holemans K, Van Bree R, Verhaeghe J, Meurrens K, Van Assche FA. Maternal semistarvation and streptozotocin-diabetes in rats have different effects on the in vivo glucose uptake by peripheral tissues in their female adult offspring. J Nutr. 1997;127:1371–1376. doi: 10.1093/jn/127.7.1371. [DOI] [PubMed] [Google Scholar]
- Holness MJ, Sugden MC. Antecedent protein restriction exacerbates development of impaired insulin action after high-fat feeding. Am J Physiol. 1999;276:E85–E93. doi: 10.1152/ajpendo.1999.276.1.E85. [DOI] [PubMed] [Google Scholar]
- Kind KL, Clifton PM, Grant PA, Owens PC, Sohlstrom A, Roberts CT, et al. Effect of maternal feed restriction during pregnancy on glucose tolerance in the adult guinea pig. Am J Physiol Regul Integr Comp Physiol. 2003;284:R140–R152. doi: 10.1152/ajpregu.00587.2001. [DOI] [PubMed] [Google Scholar]
- McMullen S, Langley-Evans SC. Maternal low protein diet in rat pregnancy programmes blood pressure through sex-specific mechanisms. Am J Physiol Regul Integr Comp Physiol. 2005;288:R85–R90. doi: 10.1152/ajpregu.00435.2004. [DOI] [PubMed] [Google Scholar]
- Nandhini AT, Thirunavukkarasu V, Ravichandran MK, Anuradha CV. Effect of taurine on biomarkers of oxidative stress in tissues of fructose-fed insulin-resistant rats. Singapore Med J. 2005;46:82–87. [PubMed] [Google Scholar]
- Nyirenda MJ, Welberg LA, Seckl JR. Programming hyperglycaemia in the rat through prenatal exposure to glucocorticoids – fetal effect or maternal influence? J Endocrinol. 2001;170:653–660. doi: 10.1677/joe.0.1700653. [DOI] [PubMed] [Google Scholar]
- Oh W, Gelardi NL, Cha CJ. The cross-generation effect of neonatal macrosomia in rat pups of streptozotocin-induced diabetes. Pediatr Res. 1991;29:606–610. doi: 10.1203/00006450-199106010-00016. [DOI] [PubMed] [Google Scholar]
- Ozanne SE, Olsen GS, Hansen LL, Tingey KJ, Nave BT, Wang CL, et al. Early growth restriction leads to down regulation of protein kinase C zeta and insulin resistance in skeletal muscle. J Endocrinol. 2003;177:235–241. doi: 10.1677/joe.0.1770235. [DOI] [PubMed] [Google Scholar]
- Ozanne SE, Wang CL, Coleman N, Smith GD. Altered muscle insulin sensitivity in the male offspring of protein-malnourished rats. Am J Physiol. 1996;271:E1128–E1134. doi: 10.1152/ajpendo.1996.271.6.E1128. [DOI] [PubMed] [Google Scholar]
- Petrik J, Reusens B, Arany E, Remacle C, Coelho C, Hoet JJ, et al. A low protein diet alters the balance of islet cell replication and apoptosis in the fetal and neonatal rat and is associated with a reduced pancreatic expression of insulin-like growth factor-II. Endocrinology. 1999;140:4861–4873. doi: 10.1210/endo.140.10.7042. [DOI] [PubMed] [Google Scholar]
- Petry CJ, Hales CN. Long-term effects on offspring of intrauterine exposure to deficits in nutrition. Hum Reprod Update. 2000;6:578–586. doi: 10.1093/humupd/6.6.578. [DOI] [PubMed] [Google Scholar]
- Petry CJ, Ozanne SE, Wang CL, Hales CN. Early protein restriction and obesity independently induce hypertension in 1-year-old rats. Clin Sci (Lond) 1997;93:147–152. doi: 10.1042/cs0930147. [DOI] [PubMed] [Google Scholar]
- Petry CJ, Ozanne SE, Wang CL, Hales CN. Effects of early protein restriction and adult obesity on rat pancreatic hormone content and glucose tolerance. Horm Metab Res. 2000;32:233–239. doi: 10.1055/s-2007-978627. [DOI] [PubMed] [Google Scholar]
- Ravelli AC, van der Meulen JH, Michels RP, Osmond C, Barker DJ, Hales CN, et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet. 1998;351:173–177. doi: 10.1016/s0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- Ravelli AC, van Der Meulen JH, Osmond C, Barker DJ, Bleker OP. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr. 1999;70:811–816. doi: 10.1093/ajcn/70.5.811. [DOI] [PubMed] [Google Scholar]
- Reusens B, Remacle C. Intergenerational effect of an adverse intrauterine environment on perturbation of glucose metabolism. Twin Res. 2001a;4:406–411. doi: 10.1375/1369052012597. [DOI] [PubMed] [Google Scholar]
- Reusens B, Remacle C. Effects of maternal nutrition and metabolism on the developing endocrine pancreas. In: Barker DJP, editor. Fetal Origins of Cardiovascular and Lung Disease. New York: Marcel Dekker; 2001b. pp. 339–358. [Google Scholar]
- Roseboom TJ. The fetal origins hypothesis. Twin Res. 2001;4:iii. [PubMed] [Google Scholar]
- Roseboom TJ, van der Meulen JH, Ravelli AC, Osmond C, Barker DJ, Bleker OP. Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Twin Res. 2001;4:293–298. doi: 10.1375/1369052012605. [DOI] [PubMed] [Google Scholar]
- Sener A, Reusens B, Remacle C, Hoet JJ, Malaisse WJ. Nutrient metabolism in pancreatic islets from protein malnourished rats. Biochem Mol Med. 1996;59:62–67. doi: 10.1006/bmme.1996.0066. [DOI] [PubMed] [Google Scholar]
- Shepherd PR, Crowther NJ, Desai M, Hales CN, Ozanne SE. Altered adipocyte properties in the offspring of protein malnourished rats. Br J Nutr. 1997;78:121–129. doi: 10.1079/bjn19970124. [DOI] [PubMed] [Google Scholar]
- Simmons RA, Templeton LJ, Gertz SJ. Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes. 2001;50:2279–2286. doi: 10.2337/diabetes.50.10.2279. [DOI] [PubMed] [Google Scholar]
- Snoeck A, Remacle C, Reusens B, Hoet JJ. Effect of a low protein diet during pregnancy on the fetal rat endocrine pancreas. Biol Neonate. 1990;57:107–118. doi: 10.1159/000243170. [DOI] [PubMed] [Google Scholar]
- Stewart RJ, Preece RF, Sheppard HG. Twelve generations of marginal protein deficiency. Br J Nutr. 1975;33:233–253. doi: 10.1079/bjn19750027. [DOI] [PubMed] [Google Scholar]
- Torrens C, Brawley L, Barker AC, Itoh S, Poston L, Hanson MA. Maternal protein restriction in the rat impairs resistance artery but not conduit artery function in pregnant offspring. J Physiol. 2003;547:77–84. doi: 10.1113/jphysiol.2002.026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Assche FA, Holemans K, Aerts L. Long-term consequences for offspring of diabetes during pregnancy. Br Med Bull. 2001;60:173–182. doi: 10.1093/bmb/60.1.173. [DOI] [PubMed] [Google Scholar]
- Zambrano E, Rodriguez-Gonzalez GL, Guzman C, Garcia-Becerra R, Boeck L, Diaz L, et al. A maternal low protein diet during pregnancy and lactation in the rat impairs male reproductive development. J Physiol. 2005;563:275–284. doi: 10.1113/jphysiol.2004.078543. [DOI] [PMC free article] [PubMed] [Google Scholar]