Abstract
A series of recent studies have indicated that ensembles of neurones, distributed within the neural structures that form the primary thalamocortical loop (TCL) of the trigeminal component of the rat somatosensory system, change the way they respond to similar tactile stimuli, according to both the behavioural strategy employed by animals to gather information and the animal's internal brain states. These findings suggest that top-down influences, which are more likely to play a role during active discrimination than during passive whisker stimulation, may alter the pattern of neuronal firing within both the distinct layers of the primary somatosensory cortex (S1) and the ventral posterior medial nucleus (VPM). We propose that through this physiological process, which involves concurrent dynamic modulations at both cellular and circuit levels in the TCL, rats can either optimize the detection of novel or hard to sense stimuli or they can analyse complex patterns of multiwhisker stimulation, during natural exploration of their surrounding environment.
The trigeminal component of the rat somatosensory system is widely recognized as a versatile and invaluable experimental model to investigate the principles of development (Rice, 1995), anatomical organization (Woolsey & Van der Loos, 1970), physiological properties (Chapin & Woodward, 1981; Simons, 1985; Connors & Gutnick, 1990; Silva et al. 1991; Nicolelis & Chapin, 1994; Ghazanfar & Nicolelis, 1999), coding strategy (Simons & Carvell, 1989; Ahissar et al. 1997; Fee et al. 1997; Ghazanfar et al. 2000), and plastic potential (Nicolelis et al. 1993; Castro-Alamancos et al. 1995; Polley et al. 1999a,b) of sensory systems in mammals. As a result, this sensory system has been scrutinized by a variety of powerful techniques, such as in vivo and in vitro patch clamp recordings (Zhu & Connors, 1999; Petersen & Sakmann, 2000), intra- and extracellular recording (Markram et al. 1995; Nicolelis et al. 1997; Markram, 1997), and optical imaging (Masino & Frostig, 1996; Kleinfeld & Delaney, 1996; Sheth et al. 1998; Polley et al. 1999a, b). More recently, chronic multisite, multielectrode recordings in freely behaving animals have been employed to characterize, for the first time, the simultaneous activity of distinct populations of neurones that define the main thalamocortical circuit of the rat somatosensory system (Fanselow & Nicolelis, 1999). This new experimental paradigm has provided a unique opportunity to correlate the physiological properties of thalamocortical neural ensembles with the main behaviours employed by rats to extract tactile information from their surrounding environment.
By employing multielectrode recordings in freely behaving rats we have recently obtained physiological and behavioural data supporting the hypothesis that the thalamocortical loop dynamically adjusts its physiological mode of operation, at both cellular and circuit levels, in accordance with internal brain states and the specific behaviours used by rats to explore their surrounding environment. Here, we briefly review the evidence that supports this hypothesis. A series of papers published elsewhere summarizes this argument in greater detail (Nicolelis & Fanselow, 2002; Krupa et al. 2004a; Gervasoni et al. 2004).
The rat somatosensory thalamocortical circuit
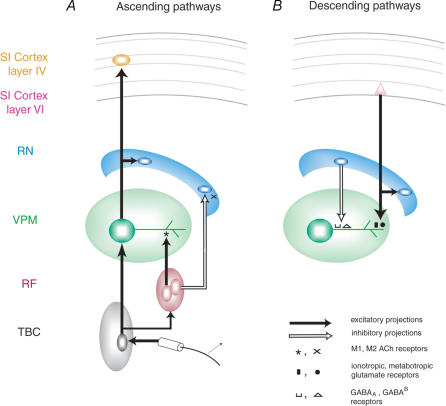
Figure 1 summarizes the circuit that defines the main thalamocortical loop of the trigeminal component of the rat somatosensory system (Fig. 1A). Ascending action potentials resulting from mechanical stimulation of the whiskers travel to the trigeminal brainstem complex and the reticular formation. Projections from the trigeminal brainstem complex terminate on neurones in the VPM thalamus. In the rat, the VPM thalamic nucleus contains only one type of neurone, excitatory cells that project mainly to layer IV (CTX IV) of the primary somatosensory cortex (SI). On the way to the cortex, the axons of VPM also give off a projection to the thalamic reticular nucleus (RT). VPM and RT neurones also receive dense ascending cholinergic projections from the brainstem reticular formation (RF) (Hallanger et al. 1987). These projections can excite VPM cells through nicotinic and M1-type muscarinic receptors (Zhu & Uhlrich, 1998; Plummer et al. 1999), but inhibit RT cells via M2-type receptors (Carden & Bickford, 1999).
Figure 1. Schematic diagram of the main rat thalamocortical loop.
A, diagram of the main ascending pathways. B, diagram of the main descending pathways. From Nicolelis & Fanselow, 2002. Reproduced with permission (http://www.nature.com.nn).
A major source of descending projections in the thalamocortical loop originates primarily in layer VI of SI cortex (CTX VI) (Fig. 1B). The distal dendrites of VPM neurones are densely innervated by these projections, which activate both ionotropic and metabotropic glutamate receptors. On their way to VPM, corticothalamic projections also give off branches to RT neurones. The RT consists exclusively of inhibitory neurones, which project either to the VPM nucleus, where they terminate near the cell bodies where they activate GABAA and GABAB receptors, or locally within RT. The rat VPM nucleus is unique in that, unlike other main thalamic nuclei in rats and in other species, it does not contain intrinsic inhibitory neurones. Due to this lack of inhibitory interneurones, RT neurones are the only source of GABAergic inhibition in the rat VPM (McCormick, 1992).
Although considerable anatomical and physiological data indicate that top-down inputs may have a significant effect on mechanisms of tactile information processing (Mignard & Malpeli, 1991; Roelfsema et al. 1998; Hupe et al. 1998), the nature of these descending influences even on the primary thalamocortical loop of the rat somatosensory system remains poorly understood. As a rat actively samples a tactile stimulus, particularly one involving salient associative memories, several higher-order processes that would not be activated by random passive stimuli, delivered in either anaesthetized or paralysed animals, will likely be engaged. For instance, the animal's state of attention, motivation, sensory-motor integration and reward expectation are all likely to influence the nature of the tactile responses generated by thalamocortical neurones. However, what effect these, or other, higher-order processes might have on mechanisms of tactile processing in rat SI remains largely unknown.
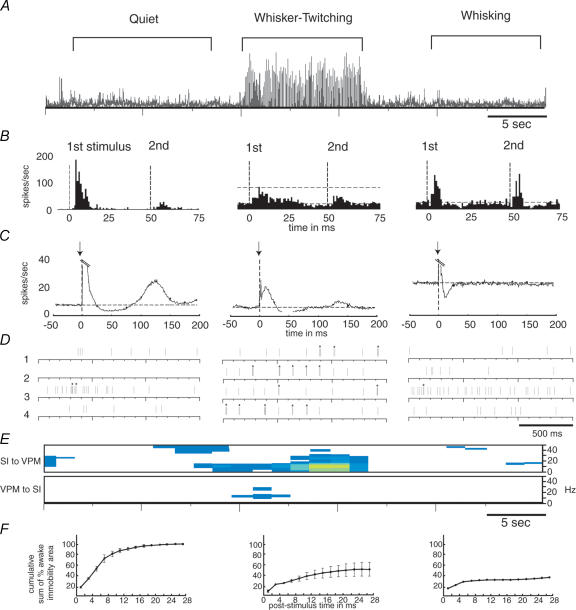
Over the past decade, our laboratory has performed a series of experiments in which chronic recordings of the simultaneous activity of ensembles of thalamocortical neurones were obtained in freely behaving rats engaged in a variety of tasks (for a review see Nicolelis & Fanselow, 2002). In our first studies, we measured how the tactile responses of ensembles of single neurones located in S1 and VPM of the thalamus varied according to three basic behaviours exhibited by rats. The first of these we refer to as the ‘quiet’ behaviour, in which rats are standing or sitting still and there is no movement of the whiskers (Fanselow & Nicolelis, 1999; Nicolelis et al. 1995). The second is known as the ‘whisker twitching’ behaviour. During this behaviour, rats are also standing or sitting still, but twitch their whiskers in very rhythmic, small amplitude movements at a rate of 7–12 Hz (Semba et al. 1980; Semba & Komisaruk, 1984). The third behaviour, referred to as ‘whisking’, occurs when rats move their whiskers back and forth in large-amplitude sweeps at a rate of ∼4–6 Hz. Rats use these whisking movements to repeatedly put their whiskers in contact with surfaces or objects so they can gather tactile information as they actively explore their environment (Carvell & Simons, 1990). During the quiet and whisking behaviours, there is no large-scale, coherent neural activity among the cells in either VPM or SI, and the activity is thus referred to as ‘desynchronized’. In contrast, the whisker twitching state is accompanied by a highly synchronous 7–12 Hz oscillatory neural activity (Semba et al. 1980; Semba & Komisaruk, 1984) (Fig. 2A), which appears first in the rat SI cortex and later in the VPM thalamus (Nicolelis et al. 1995; Fanselow et al. 2001). Shortly after the onset of this oscillatory neural activity in the thalamocortical loop, rats start producing the rhythmic, small amplitude whisker twitching movements characteristic of this behaviour, which are phase-locked to the neural oscillations (Semba & Komisaruk, 1984; Welker, 1964).
Figure 2. Neural activity in VPM thalamus during three behavioural states.
A, a continuous 50 s trace of the first principal component of neural ensemble activity in VPM across quiet, whisker twitching and whisking behaviours. B, responses of single VPM neurones to the presentation of two infraorbital nerve stimuli with an interstimulus interval of 50 ms (stimuli presented at bold dotted lines; horizontal dotted lines indicate baseline firing level). C, average peristimulus time histograms (PSTHs) of neural activity in VPM neurones before and after one stimulation of the infraorbital nerve (note that the peaks of the responses to the stimulus have been truncated so the lower-magnitude activity can easily be seen; stimuli presented at bold dotted lines; horizontal dotted lines indicate baseline firing level). D, rasters showing the activity of four single units in VPM during each behaviour. Bursting activity is identified by asterisks above each raster. E, amount of partial directed coherence observed during each of the three behaviours. The top panel is for partial directed coherence from SI to VPM, the bottom panel from VPM to SI. Colour indicates the intensity of the coherence, white indicating none and yellow indicating the highest level of coherence. F, cumulative sum of amount of cortical area activated by a single infraorbital nerve stimulus. Values are normalized to the maximum activated area in the quiet state. From Nicolelis & Fanselow, 2002. Reproduced with permission (http://www.nature.com.nn).
Overall, we observed that the tactile responses of both S1 and VPM neurones can vary significantly in several ways as a rat shifts between these three behavioural states (Fig. 2A and B). First, when a single tactile stimulus is presented, e.g. one brief deflection of a whisker, the probability of a neuronal response is largest during the quiet behaviour, smaller during whisking, and lowest during whisker twitching (Fig. 2B). Second, following the stimulus, there is a robust inhibitory period lasting ∼75 ms during the quiet behaviour. This poststimulus inhibitory period is shorter during whisker twitching, relative to the quiet behaviour, and is substantially shorter during whisking (Fig. 2C). Finally, when pairs of stimuli are presented, the ability of a neurone to fire in response to the second stimulus in the pair is dependent on the interstimulus interval and the animal's behavioural state. During the quiet and whisker twitching behaviours, if the interstimulus interval is less than 75 ms, the probability of VPM and SI neurones responding to the second stimulus in a pair will be very small (Fig. 2B). However, during whisking, the probability of a response to the second stimulus of a pair is only reduced if the interstimulus interval is 25 ms or less. Thus, the ability of VPM and SI neurones to respond reliably to rapidly repeated stimuli is correlated with the duration of the poststimulus inhibitory period, which differs according to behavioural state.
Analysis of the firing properties of thalamocortical cells (Fanselow et al. 2001) in these behaving rats has shown that during the 7–12 Hz oscillations observed in the whisker twitching behaviour, VPM and S1 neurones fire bursts of action potentials substantially more frequently (average of once every 7.2 s) than during the quiet (average of once every 45.5 s) or whisking (average of once every 28.6 s) behaviours (Fig. 2D). Moreover, signals directed from SI to VPM are significantly more coherent during whisker twitching episodes than during the other two behavioural states (Fig. 2E). In addition, inactivation of the SI cortex via local infusion of the GABAA agonist muscimol, abolished whisker twitching movements, 7–12 Hz oscillations and bursting activity in the VPM. These results suggest that during whisker twitching the SI cortex exerts a powerful rhythmic influence on VPM neurones and that these descending cortical signals are required for the emergence of 7–12 Hz oscillations, the bursting activity observed in VPM during these oscillations, and the genesis of the whisker twitching behaviour.
These results led us to propose that the active use of whiskers, in multiple types of whisker movements, is integral to processing tactile stimuli in rats. Indeed, our hypothesis proposes that two distinct types of whisker movements serve as differential dynamic ‘filters’ to process specific types of incoming tactile information that result from whisker stimulation. The second global operating principle we proposed is that, as with the determination of receptive field properties and maps in SI and VPM (Krupa et al. 1999; Ghazanfar et al. 2001), the asynchronous convergence of ascending and descending projections in the thalamus is critical for generating the animal's range of sensory processing strategies. When this circuit arrangement is combined with the range of intrinsic cellular properties of cells in the thalamocortical loop, a complex and dynamic system emerges, which is capable of quickly shifting its physiological properties in order to maximize the type of tactile information sampled by a particular active exploratory behaviour. Finally, we hypothesized that internal changes in brain state would significantly impact on the way ensembles of thalamocortical neurones respond to incoming tactile stimuli.
Together, these principles illustrate that the rat somatosensory system does not merely play the role of a ‘passive observer’ of the environment. Instead, it can choose from multiple functional modes in order to actively examine and analyse tactile inputs from the world, based on expectations built throughout a life of whisking.
Layer specific tactile responses in the rat S1 during active tactile discrimination
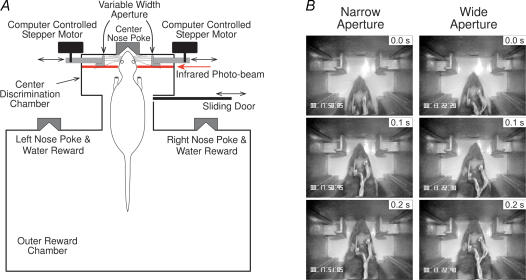
A recent series of experiments further highlighted the profound differences in tactile information processing that exist across the layers of the S1 cortex when rats engage themselves in active whisker discrimination, versus serving as passive recipients of comparable tactile stimuli (Krupa et al. 2004a). In these experiments, Krupa et al. recorded the activity of ensembles of single neurones through different layers of the barrel region of S1 in five rats that were trained to perform a whisker-dependent tactile discrimination task (Krupa et al. 2001b, 2004b). The task required rats to actively sample a variable-width aperture with their large facial whiskers, and then signal whether the aperture was ‘narrow’ or ‘wide’ (Fig. 3A). Video analysis showed that rats approached and sampled the aperture in a very repeatable, stereotypical manner, using only their large facial whiskers to contact the aperture (Fig. 3B). Single-unit activity was recorded through all layers of SI with chronically implanted, movable arrays of high impedance microwire electrodes. Electrodes were orientated perpendicular to the cortical surface so that recordings along an individual electrode track were from the same cortical column. This allowed neural activity recorded at different depths through individual cortical columns to be compared.
Figure 3. Active tactile discrimination task.
A, schematic diagram of the behavioural apparatus. Trials begin when the sliding door opens. Rats enter the centre discrimination chamber and sample the variable width aperture with their facial whiskers. Rats then poke their nose into either the left or right reward nose poke to receive a water reward: left nose poke if the aperture was narrow (60 mm), right nose poke if wide (68 mm). Immediately after, the sliding door closes and the aperture is randomly reset to wide or narrow. The next trial begins 30 s later. B, video frame captures showing a rat approaching and sampling the Narrow and Wide aperture. The 0.0 s frame (top-most frames) shows the rat breaking the infrared photobeam; the middle frame (0.1 s later) shows the whiskers initially contacting the aperture; bottom frame (0.2 s) shows the whiskers fully contacting the aperture. From Krupa et al. (2004). Reproduced with permission.
A total of 317 units were recorded bilaterally in the barrel region of S1 while the rats performed the active tactile discrimination: 114 units in supragranular layers; 105 in layer IV; and 98 in infragranular layers. Sixty-seven per cent (212) of these units displayed significant modulations in firing rate as rats performed the tactile discrimination: 37% (78) showed significant increases in firing (excitatory responses); 26% (56) decreased firing (inhibitory responses); and 37% (78) had multiphasic responses consisting of combinations of increases and decreases. The overall percentage of responsive units per layer did not differ significantly (64% in supragranular layers, 70% in layer IV, and 66% in infragranular layers).
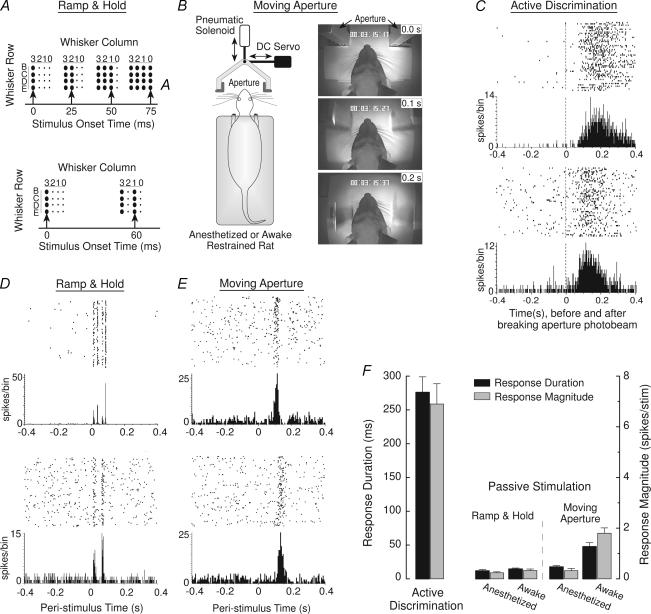
These responses were compared with the activity of 244 units recorded in the S1 of 10 additional rats that received several different forms of passive whisker stimulation designed to simulate the spatio-temporal dynamics of whisker deflections that occurred during the active discrimination. Three of the rats received patterned ramp-and-hold stimulation of 16 individual whiskers with a multichannel whisker stimulator (Krupa et al. 2001a, 2004b) while lightly anaesthetized (Fig. 4A). Three rats were habituated to calm, head-fixed restraint and received similar, patterned ramp-and-hold stimuli (Fig. 4A) while fully awake (Wiest & Nicolelis, 2003; Krupa et al. 2004b). Four rats received bilateral whisker stimulation with a movable aperture while lightly anaesthetized or restrained and fully awake (Fig. 4B). This moving aperture (same size as the aperture in the active discrimination) was accelerated across the facial whiskers at velocities and trajectories that replicated the whisker deflection dynamics that occurred during active discrimination (Krupa et al. 2004b).
Figure 4. Passive stimulations and neural response properties.
A, upper schematic diagram: pattern of multiwhisker ramp-and-hold passive stimuli delivered to anaesthetized rats. Large black dots represent stimulation of a particular whisker. Upward arrows show stimulation onsets. Lower schematic diagram: stimulation pattern of the awake, restrained rats. B, left, schematic diagram of the moving aperture stimulus. Aperture is accelerated across the facial whiskers (with variable onsets and velocities) by the pneumatic solenoid and also simultaneously deflected laterally in varying amounts by the DC servo in order to accurately replicate the range of whisker deflection dynamics that occurred during active discrimination. Right, video frame captures showing an example of the aperture moving caudally across the whiskers of an awake restrained rat while simultaneously deflecting laterally 5 mm (to the right) over a 200 ms interval. C, representative single-unit responses showing long-duration, tonic activation during active discrimination. Upper portion of each panel is a raster plot where each line represents a consecutive trial in a recording session and each dot is a unit spike; lower portion of each panel shows summed activity for all trials in 5 ms bins. The 0 time-point represents the moment that rats disrupted the aperture photobeam (Fig. 1). D, representative single-unit responses evoked by passive ramp-and-hold stimulation of 16 whiskers in lightly anaesthetized rats (upper panel) and by passive stimulation of 8 whiskers in awake, restrained rats (lower). The 0 time-point represents stimulus onset. E, representative single-unit responses evoked by moving aperture stimulation of awake, restrained rats (0 time-point represents onset of aperture movement). F, mean (+ s.e.m.) excitatory response duration and magnitude evoked during the active discrimination and by the different passive stimuli delivered to anaesthetized or awake restrained rats. From Krupa et al. (2004). Reproduced with permission.
Excitatory responses displayed a distinct shift from phasic activation during passive stimulation to tonic activation during active discrimination. This tonic activation (Fig. 4C) consisted of sustained increases in firing-rate with a mean duration (Krupa et al. 2004b) that was significantly different across cortical layers: supragranular layers, 282 ± 29 ms (mean ± s.e.m.); layer IV, 207 ± 16 ms; and infragranular layers, 339 ± 19 ms; (F2,54 = 8.8, P < 0.001). The magnitude of tonic responses (Krupa et al. 2004b) during active discrimination also varied significantly across layers: supragranular layers, 7.0 ± 0.7 spikes per trial; layer IV, 4.3 ± 0.5 spikes per trial; and infragranular layers, 9.5 ± 1.1 spikes per trial; (F2,54 = 10.6, P < 0.0005).
In contrast, passive ramp-and-hold whisker stimulation (in either anaesthetized or awake, restrained rats) or moving aperture stimulation (in anaesthetized or awake rats) evoked excitatory responses that consisted of relatively brief, transient increases in activity (Fig. 4D and E). The mean excitatory response durations and magnitudes evoked by these passive stimuli are summarized in Fig. 4F. Response duration during active discrimination was highly significantly different from each of the passive stimulation conditions (F4,85 = 59.2, all P < 0.0005; Tukey's HSD. The same was true of the response magnitude measure (F4,85 = 29.9, all P < 0.00001, Tukey's HSD. In short, excitatory activation of S1 by passive whisker stimulation was fundamentally different in nature than S1 excitation evoked by the active discrimination. Several additional lines of evidence indicate that this shift from phasic to tonic activation during passive and active stimulation (as well as several other functional differences, described below) could not have resulted solely from variations in whisker deflection dynamics during passive and active stimulation (Krupa et al. 2004b).
Another functional difference between active and passive stimulation was a significant shift in the relative balance between excitatory and inhibitory responses. Only 7% of S1 units responded to the ramp-and-hold or moving aperture passive stimulation with either purely inhibitory responses or inhibitory followed by excitatory activation; the remaining 92% responded with either purely excitatory or excitatory followed by inhibitory activity, results consistent with earlier studies (Simons, 1978; Swadlow, 1989; Brumberg et al. 1999; Sachdev et al. 2000). Moreover, there was no significant difference in the percentage of inhibitory responses evoked by the different passive stimuli delivered to either anaesthetized or awake, restrained rats. In contrast, nearly half (49%) of S1 units responded during the active discrimination with either a purely inhibitory response (26%) or a response that was initially inhibitory followed by an excitatory phase (23%). The mean duration of inhibitory responses during the active discrimination (246 ± 21 ms) was significantly longer than the duration of inhibitory responses evoked by the passive stimuli (12.4 ± 1.8 ms); (t(12) = 9.9, P < 0.0001). During active discrimination, purely inhibitory responses were evenly distributed across layers: 33% in supragranular layers, 36% in layer IV and 31% in infragranular; inhibitory–excitatory responses were asymmetrically concentrated in the granular layer: 23% in supragranular, 54% in layer IV, and 23% in infragranular. Collectively, these different inhibitory responses indicate that the activation dynamics of S1 following passive whisker stimulation are fundamentally different from during active discrimination. Passive multiwhisker stimulation causes a predominately excitatory deviation from prestimulus baseline activity, whereas similar stimulation during active discrimination evokes almost equally balanced excitatory and inhibitory shifts from baseline.
The functional nature of these actively evoked inhibitory responses was examined using an artificial neural network, based on the learning vector quantization (LVQ) algorithm (Krupa et al. 2004b). Results revealed that information about different aperture widths is encoded simultaneously by excitatory and inhibitory responses in S1 (see LVQ Based Analyses in Krupa et al. 2004b). Further, LVQ-analysis of ensemble activity in different layers indicates that layer-specific tactile coding mechanisms may be engaged during active discrimination. For instance, single-trial prediction of wide and narrow apertures by infragranular ensembles was significantly more accurate than supragranular ensembles (Krupa et al. 2004b).
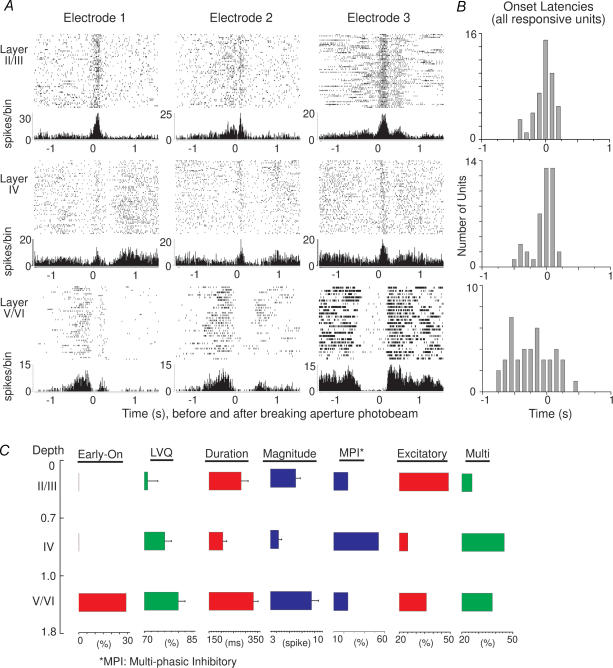
Finally, cortical columns displayed significantly different functional properties during active discrimination and passive stimulation. S1 recordings in monkeys, cats, and rats show that responses within a column evoked by passive stimulation share similar functional properties of place (all units have a common receptive field locus), and mode (all units respond to similar stimulus modalities) (Mountcastle, 1957; Powell & Mountcastle, 1959; Simons, 1978; Chapin, 1986; Armstrong-James et al. 1992; Brumberg et al. 1999). In contrast, during the active discrimination described here, 36% of infragranular units began responding significantly before the rats' whiskers contacted the aperture during active discrimination. More importantly, these units began responding significantly earlier than units recorded directly above them in supragranular or granular layers (Fig. 5A). Onset latencies between supragranular and granular layers did not differ significantly (Fig. 5B). In contrast, onset latencies of infragranular units were significantly earlier than units in the more superficial layers (Tukey's HSD, P < 0.0005).
Figure 5. Neural response properties during active discrimination.
A, examples of single-unit responses recorded at different depths along 3 different electrode tracks during the active discrimination (wide aperture). Units recorded in infragranular layers respond significantly earlier than units in more superficial layers and before whiskers contact the aperture. B, distribution of onset-latencies for all responsive cells recorded in the different layers during active discrimination. C, summary diagram showing significant major effects across layers during active discrimination. The Early On column shows the percentage of units showing early onsets per layer. LVQ: mean (+ s.e.m.) performance of the LVQ for populations recorded at the different depths. Duration: mean (+ s.e.m.) excitatory response duration. Magnitude: mean (+ s.e.m.) magnitude of excitatory responses. MPI: distribution of units with multiphasic responses that began with an inhibitory phase. Excitatory: percentage of units with excitatory responses. Multi: percentage of multiphasic units. From Krupa et al. (2004). Reproduced with permission.
These early responsive units in infragranular layers appear to represent a functionally different class of neurones. First, early responses were not seen in layer IV, indicating that the afferent source(s) of these responses was not ascending thalamic input. Second, video analysis of rats performing the task shows that these responses occurred as the rats were moving towards the aperture, although no distinct tactile stimuli appeared to contact the whiskers. Third, the duration of these early responses was significantly longer than responses of other infragranular units that responded only when the whiskers contacted the aperture (see Krupa et al. 2004a). Finally, the whiskers on one side of the face of one rat were cut prior to a behavioural recording session. Early onset responses were still observed in the infragranular layers contralateral to the whisker cut. Together, these results indicate that the early onset units are not activated by whisker stimulation directly. As such, these early onset responses do not appear to share the same functional properties of place and modality as units recorded more superficially in the same cortical column.
In summary, numerous functionally significant differences in S1 activity were observed in different cortical layers as rats performed an active tactile discrimination (Fig. 5C). Moreover, fundamental differences in the functional nature of S1 activity were observed during active discrimination and passive whisker stimulation. These results suggest that S1 receives significantly different afferent input during actively acquired and passively delivered stimuli. These differences do not appear to result exclusively from changes in bottom-up ascending input to S1. Instead, during active discrimination, top-down influences may also affect tactile processing in S1. For instance, the early onset units were only observed in infragranular layers and not layer IV, indicating that these responses did not arise from ascending thalamic input. Because motor cortex (M1) sends a significant projection to S1 infragranular layers (Miyashita et al. 1994; Zhang & Deschenes, 1998), these early onset responses might represent modulation from MI as rats initiate the discrimination. Also, excitatory response durations and magnitudes in supra- and infragranular layers were substantially greater than those in layer IV during active discrimination, indicating that the non-granular laminae received additional excitatory input from sources other than ascending thalamocortical input. Possible sources of this input include the secondary somatosensory cortex (Koralek et al. 1990; Jackson & Cauller, 1998) and the contralateral SI (Olavarria et al. 1984; Koralek et al. 1990; Shuler et al. 2001; Shuler et al. 2002), both of which innervate the non-granular laminae.
Conclusions
Evidence obtained under a variety of experimental conditions indicates that tactile responses produced by ensembles of S1 and VPM vary according to the animal's behavioural strategy and internal brain state. Functional differences observed in the rat thalamocortical loop during active versus passive stimulation also indicate that passively evoked tactile responses constitute a relatively poor predictor of the processing mechanisms operating in the mammalian somatosensory system during active discrimination.
References
- Ahissar E, Haidarliu S, Zacksenhouse M. Decoding temporally encoded sensory input by cortical oscillations and thalamic phase comparators. Proc Natl Acad Sci U S A. 1997;94:11633–11638. doi: 10.1073/pnas.94.21.11633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong-James M, Fox K, Das-Gupta A. Flow of excitation within rat barrel cortex on striking a single vibrissa. J Neurophysiol. 1992;68:1345–1358. doi: 10.1152/jn.1992.68.4.1345. [DOI] [PubMed] [Google Scholar]
- Brumberg JC, Pinto DJ, Simons DJ. Cortical columnar processing in the rat whisker-to-barrel system. J Neurophysiol. 1999;82:1808–1817. doi: 10.1152/jn.1999.82.4.1808. [DOI] [PubMed] [Google Scholar]
- Carden WB, Bickford ME. Location of muscarinic type 2 receptors within the synaptic circuitry of the cat visual thalamus. J Comp Neurol. 1999;410:431–443. [PubMed] [Google Scholar]
- Carvell GE, Simons DJ. Biometric analyses of vibrissal tactile discrimination in the rat. J Neurosci. 1990;10:2638–2648. doi: 10.1523/JNEUROSCI.10-08-02638.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Donoghue JP, Connors BW. Different forms of synaptic plasticity in somatosensory and motor areas of the neocortex. J Neurosci. 1995;15:5324–5333. doi: 10.1523/JNEUROSCI.15-07-05324.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin JK. Laminar differences in sizes, shapes, and response profiles of cutaneous receptive fields in the rat SI cortex. Exp Brain Res. 1986;62:549–559. doi: 10.1007/BF00236033. [DOI] [PubMed] [Google Scholar]
- Chapin JK, Woodward DJ. Modulation of sensory responsiveness of single somatosensory cortical cells during movement and arousal behaviors. Exp Neurol. 1981;72:164–178. doi: 10.1016/0014-4886(81)90135-7. [DOI] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 1990;13:99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- Fanselow EE, Nicolelis MAL. Behavioral modulation of tactile responses in the rat somatosensory system. J Neurosci. 1999;19:7603–7616. doi: 10.1523/JNEUROSCI.19-17-07603.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow EF, Sameshima K, Baccala LA, Nicolelis MAL. Thalamic bursting in rats during different awake behavioral states. Proc Natl Acad Sci U S A. 2001;98:15330–15335. doi: 10.1073/pnas.261273898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fee MS, Mitra PP, Kleinfeld D. Central versus peripheral determinants of patterned spike activity in rat vibrissa cortex during whisking. J Neurophysiol. 1997;78:1144–1149. doi: 10.1152/jn.1997.78.2.1144. [DOI] [PubMed] [Google Scholar]
- Gervasoni D, Lin S-C, Ribeiro S, Soares ES, Pantoja J, Nicolelis MAL. Global forebrain dynamics predict rat behavioral states and their transitions. J Neurosci. 2004;24:11137–11147. doi: 10.1523/JNEUROSCI.3524-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazanfar AA, Krupa DJ, Nicolelis MAL. Role of corticothalamic feedback in processing of simple and complex tactile signals. Exp Brain Res. 2001;141:88–100. doi: 10.1007/s002210100849. [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA, Nicolelis MAL. Spatiotemporal properties of layer V neurons in the rat primary somatosensory cortex. Cereb Cortex. 1999;9:348–361. doi: 10.1093/cercor/9.4.348. [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA, Stambaugh CR, Nicolelis MAL. Encoding of tactile stimulus location by somatosensory thalamocortical ensembles. J Neurosci. 2000;20:3761–3775. doi: 10.1523/JNEUROSCI.20-10-03761.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallanger AE, Levey AI, Lee HJ, Rye DB, Wainer BH. The origins of cholinergic and other subcortical afferents to the thalamus in the rat. J Comp Neurol. 1987;262:105–124. doi: 10.1002/cne.902620109. [DOI] [PubMed] [Google Scholar]
- Hupe JM, James AC, Payne BR, Lomber SG, Girard P, Bullier J. Cortical feedback improves discrimination between figure and background by V1, V2 and V3 neurons. Nature. 1998;394:784–787. doi: 10.1038/29537. [DOI] [PubMed] [Google Scholar]
- Jackson ME, Cauller LJ. Neural activity in SII modifies sensory evoked potentials in SI in awake rats. Neuroreport. 1998;9:3379–3382. doi: 10.1097/00001756-199810260-00008. [DOI] [PubMed] [Google Scholar]
- Kleinfeld D, Delaney KR. Distributed representation of vibrissa movement in the upper layers of somatosensory cortex revealed with voltage-sensitive dyes [published erratum appears in J Comp Neurol 378, 594] J Comp Neuro. 1996;375:89–108. doi: 10.1002/(SICI)1096-9861(19961104)375:1<89::AID-CNE6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Koralek KA, Olavarria J, Killackey HP. Area 1 and laminar organization of corticocortical projections in the rat somatosensory cortex. J Comp Neurol. 1990;299:133–150. doi: 10.1002/cne.902990202. [DOI] [PubMed] [Google Scholar]
- Krupa DJ, Brisben AJ, Nicolelis MA. A multi-channel whisker stimulator for producing spatiotemporally complex tactile stimuli. J Neurosci Meth. 2001a;104:199–208. doi: 10.1016/s0165-0270(00)00345-9. [DOI] [PubMed] [Google Scholar]
- Krupa DJ, Ghazanfar AA, Nicolelis MAL. Immediate thalamic sensory plasticity depends on corticothalamic feedback. Proc Natl Acad Sci U S A. 1999;96:8200–8205. doi: 10.1073/pnas.96.14.8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupa DJ, Matell MS, Brisben AJ, Oliveira LM, Nicolelis MA. Behavioral properties of the trigeminal somatosensory system in rats performing whisker-dependent tactile discriminations. J Neurosci. 2001b;21:5752–5763. doi: 10.1523/JNEUROSCI.21-15-05752.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupa DJ, Wiest MC, Shuler MG, Laubach M, Nicolelis MAL. Layer-specific somatosensory cortical activation during active tactile discrimination. Science. 2004a;304:1989–1992. doi: 10.1126/science.1093318. [DOI] [PubMed] [Google Scholar]
- Krupa DJ, Wiest MC, Shuler MG, Laubach M, Nicolelis MAL. Layer-specific somatosensory cortical activation during active tactile discrimination. 2004b doi: 10.1126/science.1093318. Materials and methods in Supporting online material. http://www.sciencemag.org/cgi/data/304/5679/1989/DC1/1. [DOI] [PubMed]
- Markram H. A network of tufted layer 5 pyramidal neurons. Cereb Cortex. 1997;7:523–533. doi: 10.1093/cercor/7.6.523. [DOI] [PubMed] [Google Scholar]
- Markram H, Helm PJ, Sakmann B. Dendritic calcium transients evoked by single back-propagating action potentials in rat neocortical pyramidal neurons. J Physiol. 1995;485:1–20. doi: 10.1113/jphysiol.1995.sp020708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masino SA, Frostig RD. Quantitative long-term imaging of the functional representation of a whisker in rat barrel cortex. Proc Natl Acad Sci U S A. 1996;93:4942–4947. doi: 10.1073/pnas.93.10.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA. Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog Neurobiol. 1992;39:337–388. doi: 10.1016/0301-0082(92)90012-4. [DOI] [PubMed] [Google Scholar]
- Mignard M, Malpeli JG. Paths of information flow through visual cortex. Science. 1991;251:1249–1251. doi: 10.1126/science.1848727. [DOI] [PubMed] [Google Scholar]
- Miyashita E, Keller A, Asanuma H. Input-output organization of the rat vibrissae motor cortex. Exp Brain Res. 1994;99:223–232. doi: 10.1007/BF00239589. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB. Modality and topographic properties of single neurons of cat's somatic sensory cortex. J Neurophysiol. 1957;20:408–434. doi: 10.1152/jn.1957.20.4.408. [DOI] [PubMed] [Google Scholar]
- Nicolelis MAL, Ghazanfar AA, Faggin B, Votaw S, Oliveira LMO. Reconstructing the engram: simultaneous, multisite, many single neuron recordings. Neuron. 1997;18:529–537. doi: 10.1016/s0896-6273(00)80295-0. [DOI] [PubMed] [Google Scholar]
- Nicolelis MAL, Baccala LA, Lin RC, Chapin JK. Sensorimotor encoding by synchronous neural ensemble activity at multiple levels of the somatosensory system. Science. 1995;268:1353–1358. doi: 10.1126/science.7761855. [DOI] [PubMed] [Google Scholar]
- Nicolelis MAL, Chapin JK. The spatiotemporal structure of somatosensory responses of many-neuron ensembles in the rat ventral posterior medial nucleus of the thalamus. J Neurosci. 1994;14:3511–3532. doi: 10.1523/JNEUROSCI.14-06-03511.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolelis MAL, Fanselow EF. Thalamocortical optimization of tactile processing according to behavioral state. Nature Neurosci. 2002;5:517–523. doi: 10.1038/nn0602-517. [DOI] [PubMed] [Google Scholar]
- Nicolelis MAL, Lin RCS, Woodward DJ, Chapin JK. Induction of immediate spatiotemporal changes in thalamic networks by peripheral block of ascending cutaneous information. Nature. 1993;361:533–536. doi: 10.1038/361533a0. [DOI] [PubMed] [Google Scholar]
- Olavarria J, Van Sluyters RC, Killackey HP. Evidence for the complementary organization of callosal and thalamic connections within rat somatosensory cortex. Brain Res. 1984;291:364–368. doi: 10.1016/0006-8993(84)91270-8. [DOI] [PubMed] [Google Scholar]
- Petersen CC, Sakmann B. The excitatory neuronal network of rat layer 4 barrel cortex. J Neurosci. 2000;20:7579–7586. doi: 10.1523/JNEUROSCI.20-20-07579.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer KL, Manning KA, Levey AI, Rees HD, Uhlrich DJ. Muscarinic receptor subtypes in the lateral geniculate nucleus: a light and electron microscopic analysis. J Comp Neurol. 1999;404:408–425. doi: 10.1002/(sici)1096-9861(19990215)404:3<408::aid-cne9>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Polley DB, Chen-Bee CH, Frostig RD. Varying the degree of single-whisker stimulation differentially affects phases of intrinsic signals in rat barrel cortex. J Neurophysiol. 1999a;81:692–701. doi: 10.1152/jn.1999.81.2.692. [DOI] [PubMed] [Google Scholar]
- Polley DB, Chen-Bee CH, Frostig RD. Two directions of plasticity in the sensory-deprived adult cortex. Neuron. 1999b;24:623–637. doi: 10.1016/s0896-6273(00)81117-4. [DOI] [PubMed] [Google Scholar]
- Powell TP, Mountcastle VB. Some aspects of the functional organization of the cortex of the postcentral gyrus of the monkey. A correlation of findings obtained in a single unit analysis with cyto-architecture. Bull Johns Hopkins Hosp. 1959;105:133–162. [PubMed] [Google Scholar]
- Rice F. Comparative aspects of barrel structure and development. In: Jones E, Diamond I, editors. The Barrel Cortex of Rodents. New York: Plenum Press; 1995. pp. 1–76. [Google Scholar]
- Roelfsema PR, Lamme VA, Spekreijse H. Object-based attention in the primary visual cortex of the macaque monkey. Nature. 1998;395:376–381. doi: 10.1038/26475. [DOI] [PubMed] [Google Scholar]
- Sachdev RN, Sellien H, Ebner FF. Direct inhibition evoked by whisker stimulation in somatic sensory (si) barrel field cortex of the awake rat. J Neurophysiol. 2000;84:1497–1504. doi: 10.1152/jn.2000.84.3.1497. [DOI] [PubMed] [Google Scholar]
- Semba K, Komisaruk BR. Neural substrates of two different rhythmical vibrissal movements in the rat. Neuroscience. 1984;12:761–774. doi: 10.1016/0306-4522(84)90168-4. [DOI] [PubMed] [Google Scholar]
- Semba K, Szechtman H, Komisaruk BR. Synchrony among rhythmical facial tremor, neocortical ‘alpha’ waves, and thalamic non-sensory neuronal bursts in intact awake rats. Brain Res. 1980;195:281–298. doi: 10.1016/0006-8993(80)90065-7. [DOI] [PubMed] [Google Scholar]
- Sheth BR, Moore CI, Sur M. Temporal modulation of spatial borders in rat barrel cortex. J Neurophysiol. 1998;79:464–470. doi: 10.1152/jn.1998.79.1.464. [DOI] [PubMed] [Google Scholar]
- Shuler MG, Krupa DJ, Nicolelis MA. Bilateral integration of whisker information in the primary somatosensory cortex of rats. J Neurosci. 2001;21:5251–5261. doi: 10.1523/JNEUROSCI.21-14-05251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuler MG, Krupa DJ, Nicolelis MA. Integration of bilateral whisker stimuli in rats: role of the whisker barrel cortices. Cereb Cortex. 2002;12:86–97. doi: 10.1093/cercor/12.1.86. [DOI] [PubMed] [Google Scholar]
- Silva LR, Amitai Y, Connors BW. Intrinsic oscillations of neocortex generated by layer 5 pyramidal neurons. Science. 1991;251:432–435. doi: 10.1126/science.1824881. [DOI] [PubMed] [Google Scholar]
- Simons DJ. Response properties of vibrissa units in rat SI somatosensory neocortex. J Neurophysiol. 1978;41:798–820. doi: 10.1152/jn.1978.41.3.798. [DOI] [PubMed] [Google Scholar]
- Simons DJ. Temporal and spatial integration in the rat SI vibrissa cortex. J Neurophysiol. 1985;54:615–635. doi: 10.1152/jn.1985.54.3.615. [DOI] [PubMed] [Google Scholar]
- Simons DJ, Carvell GE. Thalamocortical response transformation in the rat vibrissa/barrel system. J Neurophysiol. 1989;61:311–330. doi: 10.1152/jn.1989.61.2.311. [DOI] [PubMed] [Google Scholar]
- Swadlow HA. Efferent neurons and suspected interneurons in S-1 vibrissa cortex of the awake rabbit: receptive fields and axonal properties. J Neurophysiol. 1989;62:288–308. doi: 10.1152/jn.1989.62.1.288. [DOI] [PubMed] [Google Scholar]
- Welker WI. Analysis of sniffing of the albino rat. Behavior. 1964;22:223–244. [Google Scholar]
- Wiest MC, Nicolelis MA. Behavioral detection of tactile stimuli during 7–12 Hz cortical oscillations in awake rats. Nat Neurosci. 2003;6:913–914. doi: 10.1038/nn1107. [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- Zhang ZW, Deschenes M. Projections to layer VI of the posteromedial barrel field in the rat: a reappraisal of the role of corticothalamic pathways. Cereb Cortex. 1998;8:428–436. doi: 10.1093/cercor/8.5.428. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Connors BW. Intrinsic firing patterns and whisker-evoked synaptic responses of neurons in the rat barrel cortex. J Neurophysiol. 1999;81:1171–1183. doi: 10.1152/jn.1999.81.3.1171. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Uhlrich DJ. Cellular mechanisms underlying two muscarinic receptor-mediated depolarizing responses in relay cells of the rat lateral geniculate nucleus. Neuroscience. 1998;87:767–781. doi: 10.1016/s0306-4522(98)00209-7. [DOI] [PubMed] [Google Scholar]