Abstract
In the mammalian visual system the output of the retina reaches the cerebral cortex by means of a synaptic link within the thalamus, the dorsal lateral geniculate nucleus (dLGN). In higher mammals this structure is visibly laminated, such that input from the two eyes remains segregated, binocular responses in essence being seen first in the cerebral cortex. In the rat this segregation is less obvious. With only around 3–10% of retinal ganglion cells projecting axons to the ipsilateral dLGN, the dLGN may be considered basically monocular; however, these ipsilaterally projecting axons contact cells in a region described as the ‘hidden lamina’, whose physiological properties have not been well described. In the anatomical literature, there is some debate as to the possibility of cross-over between the terminations of the two eyes. Here, a population of cells physiologically receiving input from the ipsilateral eye is described – surprisingly, the majority (63%) had powerful, excitatory input from both eyes, suggesting a simple form of binocular integration at a stage earlier than previously described for other, more ‘visually developed’ species, in which thalamic binocular integration is complex.
The traditional view of the mammalian visual system sees the output of each retina faithfully transported to the visual cortex without binocular mixing. The thalamus retains this monocular structure in the visible lamination seen most obviously in the most widely used visual model animals, the cat and primate (for recent reviews see for example Chalupa & Werner, 2003). Rodents, traditionally animals of choice for laboratory studies, have been much less favoured for work on the visual system, despite the wealth of available information on the anatomy and chemistry of their central nervous system (for a comprehensive review of the rat nervous system, see Paxinos, 2004). With its relatively low visual acuity, and its exceptional somatosensory and olfactory abilities, the rat is not often considered as a suitable model for visual studies. Nevertheless the rat has a well developed visual system, which contains all the elements seen in higher species. Thus the output of the retina, while projecting heavily to the superior colliculus, also directly projects to the dorsal lateral geniculate nucleus of the thalamus (dLGN) and from there to the primary visual cortex (Reese & Cowey, 1983; Reese & Jeffery, 1983; Reese, 1984; Sefton et al. 2004). The dLGN of the rat has no visible lamination: at least 90% and perhaps as much as 97% of the output of the retinal ganglion cell axons cross at the optic chiasm to reach the dLGN of the opposite hemisphere (Polyak, 1957; Jeffery, 1984). However, the remaining ∼3% of axons course ipsilaterally and are known to make contact with cells in the ipsilateral dLGN. Within the dLGN this ‘hidden lamina’ (Reese, 1988) of ipsilateral input represents some 30–60 deg of frontal visual space (Sefton et al. 2004). This study reports an unusual aspect of the visual response properties of the cells in this region of the rat dLGN. Surprisingly, the majority of these cells appear to respond in a straightforward excitatory fashion to stimulation of either eye, making this binocular integration in the rat visual system unlike that in higher species such as the cat, where ‘non-dominant’ eye responses are often a complex and much weaker mixture of excitation masked by overlying inhibition (Murphy & Sillito, 1989).
Methods
Experiments were carried out on 32 adult rats of either sex, weighing between 210 and 510 g. Animals were anaesthetized with halothane (2–5% for induction and surgery, 0.7–1% for maintenance) in N2O and O2 (70: 30). While the use of neuromuscular block in this and similar species has been questioned, on the basis of the available evidence (Reese, 1982) all animals were neuromuscularly blocked with gallamine triethiodide (15 mg as an initial dose, then 20 mg kg−1 h−1, determined empirically). ECG, expired CO2 and temperature were monitored and maintained continuously to ensure that anaesthetic levels were altered as appropriate to maintain an adequate state of anaesthesia. Changes in parameters that indicated a decrease in anaesthetic levels (e.g. changes in intersystolic interval, rise in end-tidal CO2, normal range 3.8–4.2%) were immediately counteracted by increasing halothane levels accordingly. Lidocaine (lignocaine) gel was applied to the ear bars of the stereotaxic frame. The eyes were protected by zero power contact lenses but were otherwise untreated, and ancillary lenses were not used (Hughes 1977, 1979). At the end of the experiments all of the animals were killed by anaesthetic overdose. All procedures were carried out to the standards laid down in the Animals (Scientific Procedures) Act 1986, under UK Home Office Licence.
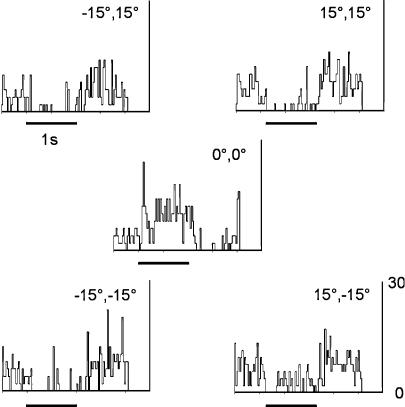
Single units were recorded in the dLGN of the right side only using tungsten in glass microelectrodes (Merrill & Ainsworth, 1972). The extracellular waveform was continuously monitored on a Gould 6000 series storage oscilloscope (see http://www.lds-group.com), and units were discriminated conventionally using amplifier, filter and spike trigger units from Neurolog (Digitimer Ltd, Welwyn Garden City, UK). Initially cells were driven qualitatively by hand-controlled stimuli using an ophthalmoscope or overhead projector. Quantitative visual stimuli comprised flashing spots and drifting gratings, and were delivered monocularly to the appropriate eye, under computer control, displayed on a flat screen monitor mounted on a custom fabricated arm which permitted accurate placement of the monitor in space relative to the rat's eye. The occluder placed over the unstimulated eye was constructed from a curved aluminium spoon, 2 mm in thickness, shaped to fit the facial contours of the rat. Stimuli were presented and responses recorded using the freely available Cortex software (http://www.cortex.salk.edu). The display was placed between 14 and 28 cm from the eye, and the angular size of stimulus calculated by the software. Once the receptive field was located by hand the display was centred on the receptive field using a protocol of a flashed stimulus or patch of drifting grating located in 1 of 5 (in some cases 9) stimulus locations onscreen to determine the final position, the process being repeated iteratively. This process is best illustrated by the example of Fig. 1. In this case, a 10 deg diameter white stimulus was flashed in one of five locations, four at ± 15 deg XY from the centre, and the centre itself. It should be clear that the stimulus produced a significant ‘off’-only response at each of the peripheral locations, but a clear ‘on’ response at the centre, indicating a well positioned location, the stimulus occupying the centre of the field only at the centre of the display. This location was then utilized for further testing. Off-line analysis was carried out using Matlab (http://www.mathworks.com) and Matoff (http://speed.nimh.nih.gov/matoff/matoff.html). Since this species lacks a fovea or area centralis, the locations of the receptive fields are measured in degrees from the vertical mid-line of the animal, centred on the horizontal (stereotaxic) projection of the mid-point of the eyes. Some of this work has been reported previously (Grieve & Sassaroli, 2004).
Figure 1. Peri-stimulus time histograms (PSTHs) documenting the visual responses of a cell in the rat dLGN to a 10 deg spot of light, flashed for 1 s at each of 5 spatial locations.
The stimulus was presented monocularly to the left (contralateral) eye. The screen was initially placed such that the centre was in correspondence with the location of the field as measured qualitatively and moved until the best response position was found using the quantitative stimuli (see Methods). The 4 peripheral locations, at ± 15 deg in XY coordinates from the centre of the screen, elicited only OFF type responses – the centre location showed a clear ON response with OFF inhibition, indicating a well centred display. Bin size 25 ms and the Y axis represent the firing rate in hertz.
Results
From a total of 32 animals, 51 cells were examined. Of these 30 were recorded from for long enough to carry out the appropriate tests. These included both ON and OFF subtypes. Receptive fields mapped with flashing spots generally showed centre–surround antagonism, although the power of the suppressive surround varied between cells (see below). No cells showing ON/OFF properties were found in this sample.
Contralaterally driven cells
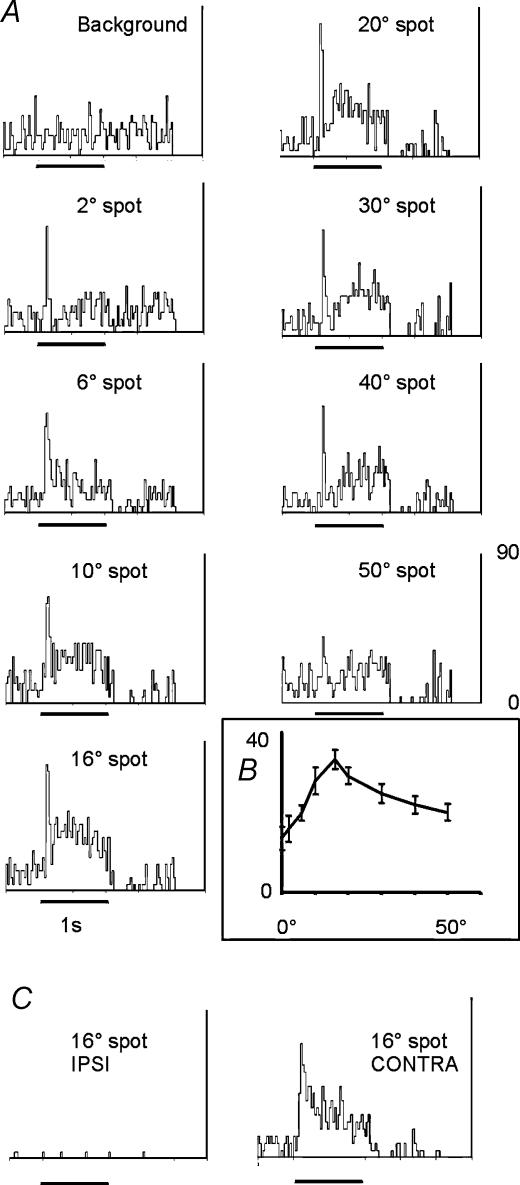
The standard protocol is illustrated in Figs 1 and 2 for a cell driven exclusively by the contralateral eye. Figure 1 shows the responses to a flashed spot of 10 deg in diameter, whose size was first established qualitatively. The PSTHs represent the responses to the spot in five positions on the monitor ranging from −15 deg, 15 deg (upper left) to 15 deg, −15 deg (lower right) with the centre PSTH derived from a stimulus centred on the middle of the screen (see Methods). Centred on this position, the size of the receptive field was then determined quantitatively as shown in Fig. 2. Circular ‘spots’ of light ranging between 2 and 50 deg were flashed for 1 s (black bar beneath each PSTH). It is clear that this cell responded increasingly up to a peak value after which responses declined in magnitude – typical of a cell which has a centre–surround antagonistic field. The inset to the figure (Fig. 2B) illustrates the strength of this surround, showing the magnitude of the response with respect to spot size. Clearly spots greater than 16–20 deg in diameter invoked increased inhibition, reducing response magnitude significantly. Notably, as seen in Fig. 1, it is possible to see pure surround responses from this cell with relatively large stimulus sizes (10 deg). More importantly, Fig. 2C shows the response of the cell firstly to a spot of 16 deg diameter flashed with the ipsilateral eye uncovered (it is interesting to note that the spontaneous activity of this cell was markedly reduced when the contralateral eye was covered) – no response could be obtained; this was followed by a second stimulation using the contralateral eye – at this point the cell was clearly still responsive. This is typical of the majority of the sample reported here – cells in the dLGN exclusively driven by the contralateral eye, as expected; 22/30 cells were of this type. Within the population of 22 cells, 14 were of the ON subtype and 8 of the OFF subtype.
Figure 2. PSTHs representing a ‘classic’ monocular receptive field.
A, PSTHs showing the responses of an exclusively contralaterally driven cell to flashed spots of light of increasing diameter. Bin size 25 ms. This cell showed an initial transient response, followed by a more sustained component, most obvious with larger stimulus sizes. Peak responses were seen to stimuli of the order of 16–20 deg in diameter. This is most evident in the inset (B), a histogram of the response magnitudes to the various stimulus sizes. Values are the total number of spikes fired ± 1 s.e.m.C, activity of this cell during visual stimulation via the ipsilateral eye, left, and repeated contralateral stimulation using a 16 deg diameter stimulus, right.
Ipsilaterally driven cells
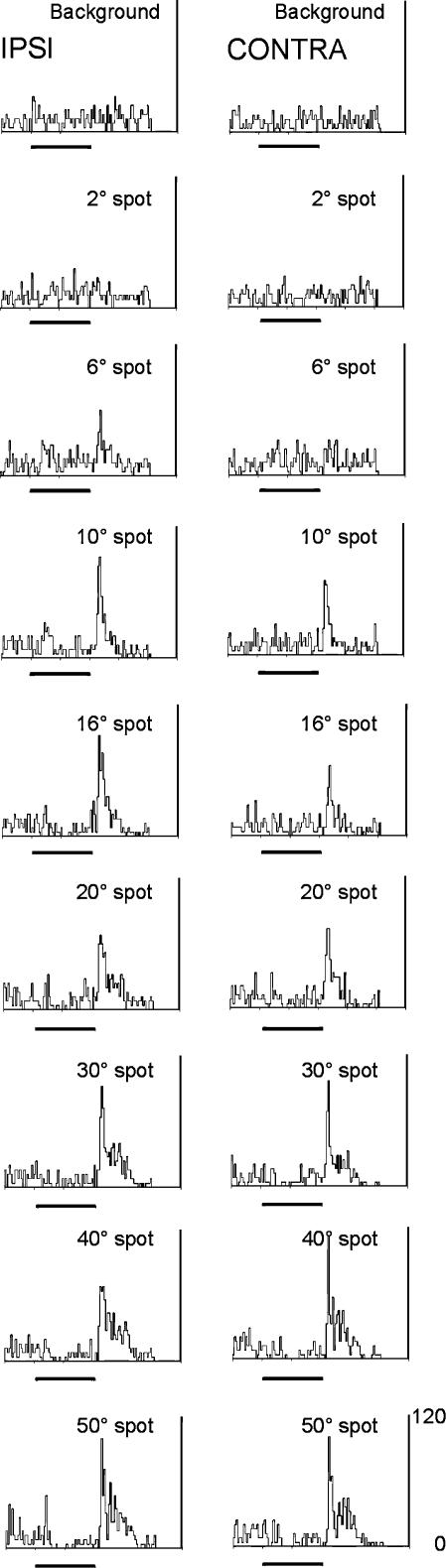
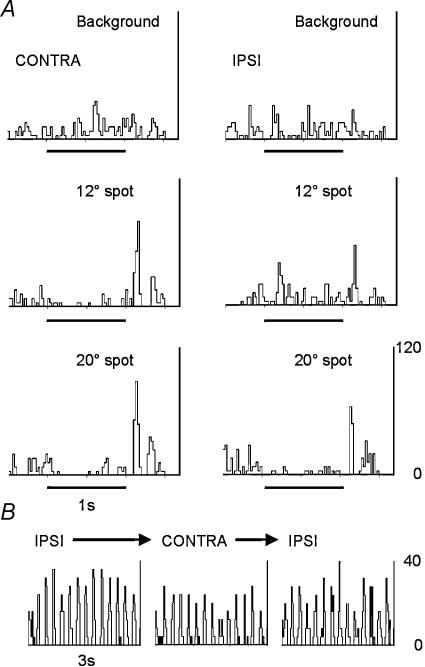
Eight of 30 cells showed visual responses driven by the ipsilateral eye. While initially this was taken to be unsurprising, given the known anatomy of the rat dLGN, the example shown in Fig. 3 was surprising in that the cell was equally responsive to either eye. The left column of PSTHs illustrates the responses of this cell to spots of different diameter displayed monocularly to the ipsilateral eye. Unlike the cell shown in Figs 1 and 2, this was an OFF centre cell. In this case the size of the receptive was large, probably exceeding the maximum spot diameter displayed, 50 deg. The right column shows responses when only the contralateral eye was stimulated. In this case, in all respects, the spontaneous activity, response polarity and magnitude are similar to the ipsilaterally driven responses; in other words, it has the same receptive field characteristics. Purists may pick out the observation that the receptive field measured through the contralateral eye seems ‘larger’, with no response to the 6 deg diameter stimulus. Two points of protocol should be noted – the contralaterally driven responses were recorded following the ipsilateral in time – that is to say, while the spot sizes were appropriately interleaved within each set, the two sets were recorded sequentially, so that time elapsed between the left and right columns. Secondly, time did not permit the remapping of the ‘second’ field – the display was unmoved between contra- and ipsilateral stimulation. With these caveats accepted, the receptive fields measured through the two eyes are remarkably similar. A second example of this type is shown in Fig. 4A; the left and right columns show the responses of a cell to stimulation through the contralateral (left) and ipsilateral (right) eyes to a range of spot sizes (taken from a full range as illustrated in Figs 2 and 3). Again, the receptive field measured through either eye is very similar (note the secondary discharge following the main responses, typical of this cell). A third example is briefly illustrated in Fig. 4B. Here, an ON centre cell is stimulated with a drifting sinusoidal grating, first through the ipsilateral eye, then the contra-, and again through the ipsilateral eye. The cell responded equally well to stimulation through either eye. Of the population of eight cells, five were responsive to stimulation through either eye. Of the remaining three cells, critically, all, recorded in the early phase of the study, were not tested for responses to stimulation of the contralateral eye. Thus it remains possible that all cells responding to ipsilateral stimulation in this study would be equally responsive to stimulation of the contralateral eye, and would therefore be regarded as binocular. Within the population of eight cells, five were ON centre, three OFF centre. This proportion is similar to the proportion in the monocularly driven cell population (see above). The average receptive field size, measured in the ipsilateral eye, for this population of eight cells was 25 deg (± 1.7 deg), while the 22 cells driven exclusively by the contralateral eye only averaged 22 deg (± 0.8 deg), values which are not significantly different. Cells exclusively driven by the contralateral eye had receptive field locations up to 48 deg lateral from the animals mid-line in the horizontal plane (range, 25 deg into ipsilateral space to 48 deg contralateral space, average 19.6 ± 2.9 deg), while cells driven by the ipsilateral eye all had fields less than 30 deg from the mid-line (range,10 deg into ipsilateral space to 30 deg contralateral space, average 10.4 ± 3.2 deg), confirming that these cells had receptive fields in that region of space typically considered to be the region of binocular overlap in this species (Sefton et al. 2004).
Figure 3. Contra- and ipsilateral eye input to a single dLGN cell.
Left column: PSTHs documenting the responses of an OFF centre cell to spots of light of increasing diameter, stimulated through the ipsilateral (right) eye. The dark bar below each PSTH shows the duration of the flashed light spot. Right column: responses of the same cell when tested via the contralateral (left) eye. Bin size 25 ms, Y axis = firing rate in hertz.
Figure 4. Further examples of excitatory contra- and ipsilateral inputs.
A, representative PSTHs taken form the full range of spot sizes tested, showing the responses of a second OFF centre cell to contralateral (left column) and subsequent ipsilateral (right column) stimulation. Note the similarity in response pattern with a characteristic secondary burst of activity, seen with stimulation of both contra- and ipsilateral eyes. B, responses of an ON centre cell to a patch of drifting grating of 0.01 cycle deg−1 at ∼4 Hz, repeated 10 times for each PSTH. The stimulus was first shown to the ipsilateral eye then in rapid sequence to the contralateral and repeated to the ipsilateral eye. Bin size 25 ms, Y axis = firing rate in hertz.
Discussion
The data presented above represent the first demonstration of simple, powerful excitatory responses to stimulation of each eye in single cells in the mammalian dLGN. The simplest hypothesis to account for these data is that the majority of cells in the rat dLGN which receive input from the ipsilateral eye also receive direct excitatory input from the contralateral eye. This situation is radically different from that of other mammals such as the cat and monkey. In the cat, the situation is complex. Cells are found in distinct laminae within the dLGN, and are powerfully driven by the dominant eye for each lamina. Coupled with this, however, there are much smaller responses driven by the ‘non-dominant’ eye, whose fields appear to be in spatial register and include both inhibitory and excitatory components. Excitatory responses may be powerfully suppressed by local inhibition such that they can only be revealed by removal of the overlying inhibition by pharmacological means (Murphy & Sillito, 1989), although this is a matter of some debate (see Guido et al. 1989). In the data reported here, while many cells respond well to stimulation of the contralateral eye and appear unresponsive to the ‘non-dominant’ ipsilateral eye (as would be expected form the lateral positioning of the eyes in the skull), some single units respond equally well to stimulation of either eye, with receptive field organization similar for each eye. This type of response has not been reported previously in any mammalian species. The cell shown in Figs 1 and 2 was recorded some 200 μm below a similar, but binocular, cell (therefore in the same animal, same penetration, with the same electrode). The dLGN of the rat is known to contain a specialized region receiving input from the ipsilateral retina, the so-called ‘hidden lamina’ (Reese, 1988). This uncrossed component of the optic input may be as small as 3% of the fibres, but the volume of dLGN contact is much larger and a degree of mixing has been suggested (Hayhow et al. 1962; Reese, 1988), and the extent of the binocular representation within the primary visual cortex is also significantly larger (Fagiolini et al. 1994). The presence of similar contra- and ipsilaterally driven responses at the level of single cells in the dLGN is extremely unusual and suggests that the majority, if not all, of the input to the binocular segment of the rat visual cortex is already binocular. Interestingly, this obviates the need for ocular dominance columns within the cortex and is in keeping with their suggested absence in this species (Peters, 1985; Fagiolini et al. 1994).
Evidence from the developing rat dLGN also supports the presence of binocular input to some cells – although in the main supporting the view that dLGN cells received binocular inhibitory input, Ziburkus et al. (2003) also concluded that excitatory input ‘were encountered far less frequently’ in the older animals, but were presumably still present. Given the small number of fibres carrying the input, and the technical differences in study based upon in vitro brain slices, these data are not inconsistent with the data presented here.
The retina of the rat is largely unspecialized, unlike the foveal development of higher species. Nevertheless, there are suggestions that the region of retina which provides this ipsilateral input may be different, containing a significantly higher proportion of the larger retinal ganglion cells (Dreher et al. 1991). However, at least as judged by the sorts of responses seen here (ON versus OFF, RF size, spontaneous activity, etc.), the receptive field properties of cells responding to the ipsilateral eye (or, more accurately, to both eyes) were not significantly different from those responding to contralateral alone, even when the retinal location of the contralaterally driven field occupied exclusively monocular space. The small eye and lack of a fovea in this species has made it difficult to compare directly the exact retinal positions of ipsi- and contralateral fields in these binocular cells. Indeed, the animals were both anaesthetized and neuromuscularly blocked, and the effects of neuromuscular block on ocular position in this species is not known. Nevertheless, since the binocular cells were responsive to the same frontal region of space in each case, it seems likely that this was due at least to substantial overlap, and the nature of the interaction between the two eyes at this level is currently under study.
Interestingly, it is known that, in the rat (albino and pigmented), a small number of retinal ganglion cells have bifurcating axons which project to both ipsi- and contralateral dLGN (Jeffrey et al. 1981; Kondo et al. 1993). While this may be the source of the responses reported in this study, there is as yet no direct available evidence to confirm this. Indeed, it remains possible that at least some binocular input to this nucleus is not of direct retinal origins, since the dLGN of the rat receives significant input from other brain structures, such as the parabigeminal nucleus (Turlejski et al. 1994; reviewed in Sefton et al. 2004). Nevertheless, there is significant binocular retinal overlap in this species, and a significant representation of this space in the visual cortex. Given the known optical properties of the rat eye (low acuity, small pupil, large depth of field, etc.; Wiesenfield & Branchek, 1976; Hughes, 1977, 1979), it would seem unlikely that high resolution binocular vision is used for stereopsis of the type seen in higher mammals. The data presented here suggest that, for whatever purpose, binocular integration in the primary visual pathway begins in this species at the level of the dLGN and these data represent the first demonstration of such binocular input in the mammalian dLGN.
References
- Chalupa LM, Werner JS. The Visual Neurosciences. I and II. Cambridge, MA, USA: MIT Press; 2003. [Google Scholar]
- Dreher B, Sefton AJ, Ni SY, Nisbett G. The morphology, number, distribution and central projections of Class I retinal ganglion cells in albino and hooded rats. Brain Behav Evol. 1991;26:10–48. doi: 10.1159/000118764. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Izzorusso P, Berardi N, Domenici L, Maffei L. Functional postnatal development of the rat primary visual cortex and the role of visual experience: dark rearing and monocular deprivation. Vis Res. 1994;34:709–720. doi: 10.1016/0042-6989(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Grieve KL, Sassaroli P. Receptive field size and retinotopic location in the rat dLGN. Soc Neurosci Abstract. 2004;34:409.3. [Google Scholar]
- Guido W, Tumosa N, Spear PD. Binocular interactions in the cat's dorsal lateral geniculate nucleus. I. Spatial-frequency analysis of responses of X, Y, and W cells to nondominant-eye stimulation. J Neurophysiol. 1989;62:543. doi: 10.1152/jn.1989.62.2.526. [DOI] [PubMed] [Google Scholar]
- Hayhow WR, Sefton A, Webb C. Primary optic centres of the rat in relation to the terminal distribution of the crossed and uncrossed fibres. J Comp Neurol. 1962;112:295–307. doi: 10.1002/cne.901180303. [DOI] [PubMed] [Google Scholar]
- Hughes A. The refractive state of the rat eye. Vis Res. 1977;17:927–939. doi: 10.1016/0042-6989(77)90068-2. [DOI] [PubMed] [Google Scholar]
- Hughes A. A schematic eye for the rat. Vis Res. 1979;19:569–588. doi: 10.1016/0042-6989(79)90143-3. [DOI] [PubMed] [Google Scholar]
- Jeffery G. Retinal ganglion cell death and terminal field retraction in the developing rodent visual system. Dev Brain Res. 1984;13:81–96. doi: 10.1016/0165-3806(84)90079-8. [DOI] [PubMed] [Google Scholar]
- Jeffrey G, Cowey A, Kuypers H. Bifurcating retinal ganglion cell axons in the rat, demonstrated by retrograde double labelling. Exp Brain Res. 1981;44:34–40. doi: 10.1007/BF00238747. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Tanaka M, Honda Y, Mizuno N. Bilateral projections of single retinal ganglion cells to the lateral geniculate nuclei and superior colliculi in the albino rat. Brain Res. 1993;608:204–215. doi: 10.1016/0006-8993(93)91460-a. [DOI] [PubMed] [Google Scholar]
- Merrill EG, Ainsworth A. Glass-coated platinum-plated tungsten microelectrodes. Med Biol Eng. 1972;10:662–672. doi: 10.1007/BF02476084. [DOI] [PubMed] [Google Scholar]
- Murphy PC, Sillito AM. The binocular input to cells in the feline dorsal lateral geniculate nucleus (dLGN) J Physiol. 1989;415:393–408. doi: 10.1113/jphysiol.1989.sp017727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G. The Rat Nervous System. Burlington, MA: Academic Press; 2004. [Google Scholar]
- Peters A. The visual cortex of the rat. In: Peters A, Jones EG, editors. Cerebral Cortex. Vol. 3. New York: Plenum Press; 1985. pp. 19–80. [Google Scholar]
- Polyak S. The Vertebrate Visual System. Chicago: University of Chicago Press; 1957. [Google Scholar]
- Reese BE. Restricting eye movements in the anesthetized rat: a comparison of four techniques. Physiol Behav. 1982;29:995–998. doi: 10.1016/0031-9384(82)90289-x. [DOI] [PubMed] [Google Scholar]
- Reese BE. The projection from the superior colliculus to the dorsal lateral geniculate nucleus in the rat. Brain Res. 1984;305:162–168. doi: 10.1016/0006-8993(84)91133-8. [DOI] [PubMed] [Google Scholar]
- Reese BE. ‘Hidden lamination’ in the dorsal lateral geniculate nucleus: the functional organization of this thalamic region in the rat. Brain Res. 1988;472:119–137. doi: 10.1016/0165-0173(88)90017-3. [DOI] [PubMed] [Google Scholar]
- Reese BE, Cowey A. Projection lines and the ipsilateral retino-geniculate pathway in the hooded rat. Neurosci. 1983;10:1233–1247. doi: 10.1016/0306-4522(83)90110-0. [DOI] [PubMed] [Google Scholar]
- Reese BE, Jeffery G. Crossed and uncrossed visual topography in dorsal lateral geniculate nucleus of the pigmented rat. J Neurophysiol. 1983;49:877–885. doi: 10.1152/jn.1983.49.4.877. [DOI] [PubMed] [Google Scholar]
- Sefton SJ, Dreher B, Harvey A. Visual system. In: Paxinos G, editor. The Rat Nervous System. 3. Burlington, MA, USA: Academic Press; 2004. pp. 1083–1141. [Google Scholar]
- Turlejski K, Djavadian RL, Dreher B. Parabigeminal, pretectal and hypothalamic afferents to rat's dorsal lateral geniculate nucleus. Comparison between albino and pigmented strains. Neurosci Lett. 1994;160:225–231. doi: 10.1016/0304-3940(93)90419-l. [DOI] [PubMed] [Google Scholar]
- Wiesenfield Z, Branchek T. Refractive state and visual acuity of the hooded rat. Vis Res. 1976;16:823–827. doi: 10.1016/0042-6989(76)90142-5. [DOI] [PubMed] [Google Scholar]
- Ziburkus J, Lo FS, Guido W. Nature of inhibitory postsynaptic activity in developing relay cells of the lateral geniculate nucleus. J Neurophysiol. 2003;90:1063–1070. doi: 10.1152/jn.00178.2003. [DOI] [PubMed] [Google Scholar]