Abstract
CerebRal blood flow is known to increase in response to hypoxia and to decrease with hypocapnia. It is not known, however, whether these responses are altered in high-altitude dwellers who are not only chronically hypoxic and hypocapnic, but also polycythaemic. Here we examined cerebral blood flow responses to hypoxia and hypocapnia, separately and together, in Andean high-altitude dwellers, including some with chronic mountain sickness (CMS), which is characterized by excessive polycythaemia. Studies were carried out at high altitude (Cerro de Pasco (CP), Peru; barometric pressure (PB) 450 mmHg) and repeated, following relief of the hypoxia, on the day following arrival at sea level (Lima, Peru; PB 755 mmHg). We compared these results with those from eight sea-level residents studied at sea level. In nine high-altitude normal subjects (HA) and nine CMS patients, we recorded middle cerebral artery mean blood flow velocity (MCAVm) using transcranial Doppler ultrasonography, and expressed responses as changes from baseline. MCAVm responses to hypoxia were determined by changing end-tidal partial pressure of oxygen (PET,O2) from 100 to 50 mmHg, with end-tidal partial pressure of carbon dioxide clamped. MCAVm responses to hypocapnia were studied by voluntary hyperventilation with (PET,O2) clamped at 100 and 50 mmHg. There were no significant differences between the cerebrovascular responses of the two groups to any of the interventions at either location. In both groups, the MCAVm responses to hypoxia were significantly greater at Lima than at CP (HA, 12.1 ± 1.3 and 6.1 ± 1.0%; CMS, 12.5 ± 0.8 and 5.6 ± 1.2%; P < 0.01 both groups). The responses at Lima were similar to those in the sea-level subjects (13.6 ± 2.3%). The responses to normoxic hypocapnia in the altitude subjects were also similar at both locations and greater than those in sea-level residents. During hypoxia, both high-altitude groups showed responses to hypocapnia that were significantly smaller at Lima than at CP (HA, 2.17 ± 0.23 and 3.29 ± 0.34% mmHg−1, P < 0.05; CMS, 1.87 ± 0.16 and 3.23 ± 0.24% mmHg−1; P < 0.01). The similarity of the results from the two groups of altitude dwellers suggests that haematocrit is unlikely to greatly affect cerebrovascular reactivity to hypoxia and hypocapnia. The smaller vasodilatation to hypoxia and larger vasoconstriction to hypoxic hypocapnia at high altitude suggest that cerebrovascular responses may be impaired at the high altitude, i.e. a maladaptation. The changes in the responses within less than 24 h at sea level indicate that this impairment is rapidly reversible.
Exposure to high altitude results in hypobaric hypoxia, due to low ambient air pressure. This results in hypoxaemia, which stimulates chemoreceptors inducing hyperventilation and subsequent hypocapnia (Chiodi, 1957; Otis et al. 1989). Hypoxia and hypocapnia have opposing effects on cerebral blood flow. Cerebral blood flow increases in response to hypoxia (Lassen & Christensen, 1976; Poulin et al. 2002; Van Mil et al. 2002), and decreases in response to hypocapnia (Poulin et al. 2002). In addition, the cerebral circulation is much less sensitive to hypoxia than to hypocapnia, when expressed as percentage change per millimetre of mercury change in respiratory gas tension (Lassen & Christensen, 1976; Poulin et al. 2002).
Chronic high-altitude exposure is associated with polycythaemia, although the extent of this varies between populations. The increase in haematocrit is particularly pronounced in Andean high-altitude dwellers (Beall et al. 2002), and even more so in those who suffer from chronic mountain sickness (CMS) (Milledge & Sorensen, 1972). CMS is characterized by haematocrits greater than 60% and various symptoms including fatigue and cognitive impairment (Leon-Velarde et al. 1994). Ultimately it leads to heart failure (Penaloza & Sime, 1971) and death.
Many of the symptoms of CMS are thought to be caused by cerebral hypoxia (Sorensen et al. 1974; Arregui et al. 1991), and this may be exacerbated by the low cerebral blood flow caused by the high viscosity of the blood (Massik et al. 1987).
There have been a number of studies which have investigated cerebrovascular control in Andean dwellers, and these have indicated an impaired vasodilatation to hypercapnia (Appenzeller et al. 2004), and to combined hypoxia and hypercapnia during sleep apnoea (Sun et al. 1996), but it is not known whether the vasodilatation to hypoxia alone, as well as the vasconstriction to hypocapnia, are also impaired. Cerebral autoregulation may also be impaired (Arregui et al. 1991; Sun et al. 1996), and this would cause cerebral blood flow to become deficient during decreases in blood pressure, for example during postural changes. The reason for these apparently abnormal cerebrovascular responses is unknown, but one possibility is that they may be related to the high haematocrit, as Andeans, who are both hypoxic and polycythaemic, have impaired cerebrovascular responses to changes in oxygen and carbon dioxide levels (Sun et al. 1996), whereas Tibetans, who are also hypoxic but less polycythaemic, have apparently normal responses (Jansen et al. 2000). These are not the only factors that may affect cerebrovascular control in these high-altitude dwellers and, in addition to possible effects of chronic hypoxia and high haematocrit levels, the different populations would have quite different genetic constitutions, and the possible influence of this should not be ignored. There may also be other environmental differences.
The present study was undertaken to try to determine the importance of chronic altitude-induced hypoxia and polycythaemia in influencing the chemical control of the cerebral circulation in Andeans. We studied responses of the cerebral circulation to hypoxia and hypocapnia, both separately and combined, in a high-altitude Andean population of presumed genetic similarity. Some of the subjects suffered from CMS and had haematocrits in excess of 60%, and this would thus allow the influence (amongst possibly other things) of haematocrit to be examined. To examine the possible effects of relief of the hypoxia, all subjects were taken to sea level, and studies of cerebrovascular responses were repeated within a day of arrival. We compared results from both high-altitude groups with those from sea-level residents studied at sea level to determine whether cerebrovascular control was different in these chronically hypoxic subjects.
Methods
The study was carried out in Cerro de Pasco (CP), Peru (altitude 4338 m; barometric pressure (PB) 450 mmHg), and in Lima, Peru, at sea level (PB 755 mmHg) on the day following arrival. Additional control studies were carried out at Leeds, UK (PB 765 mmHg). The Research Ethics Committees of the United Leeds Teaching Hospitals NHS Trust, UK, and the Universidad Peruana Cayetano Heredia, Peru, approved the experiments, and all subjects gave written informed consent.
Subjects
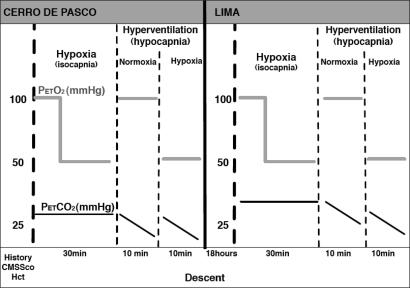
Eighteen male high-altitude dwellers were recruited; all had been born and had lived all their lives in CP. Their previous visit to low altitude had been at least 6 months prior to the study. None had worked as a miner. The subjects were recruited from those who had taken part in our recently reported studies (Claydon et al. 2004, 2005). Nine of the subjects were known to suffer from CMS. On arrival at the laboratory in CP, subjects gave a detailed clinical history and had all test procedures explained. Peripheral haematocrit (Hct) was measured, and CMS scores (CMSSco) were calculated using the scoring system based on the absence or severity of the 10 most common clinical symptoms and signs of CMS (Leon-Velarde et al. 2003). The nine normal subjects had Hct <60% and were categorized as high-altitude normals (HA). The nine CMS subjects all had Hct >65% as well as high CMS scores. Eight sea-level male control subjects (age 33 ± 5 years) were also recruited from the University of Leeds. All were healthy normotensive volunteers and were assumed to have normal haematocrits. Figure 1 illustrates the time course of the procedures.
Figure 1. Timeline of the study.
CMSSco, chronic mountain sickness score; Hct, haematocrit; (PET,O2), end-tidal partial pressure of oxygen (mmHg); (PET,CO2), end-tidal partial pressure of carbon dioxide (mmHg). Studies began at Cerro de Pasco (CP). Subjects gave a clinical history and CMSSco were calculated. Resting end-tidal gases, blood pressure and heart rate were measured. Peripheral haematocrit was determined. Cerebrovascular responses to hypoxia (alone) were determined during a step decrease in (PET,O2) (100 to 50 mmHg). Cerebrovascular responses to hypocapnia (alone) were determined during voluntary hyperventilation with (PET,O2) clamped at 100 mmHg. Cerebrovascular responses to combined hypoxia and hypercapnia during hyperventilation with (PET,O2) clamped at 50 mmHg. Between each trial, subjects were given sufficient time to rest, approximately 10 min, to ensure that there was no carryover effect from the previous trial. Subjects descended to Lima. Within 18 h all studies were repeated.
All subjects first rested in a seated position for 5 min to allow measurements of initial end-tidal partial pressure of carbon dioxide (PET,CO2) and end-tidal partial pressure of oxygen (PET,O2) using a small nasal catheter, and arterial oxygen saturation using finger oximetry. Characteristics of all subjects are summarized in Table 1.
Table 1.
Characteristics of high-altitude subjects breathing ambient air at Cerro de Pasco
| Age (years) | Height (m) | Weight kg) | Hct (%) | CMSSco (points) | SaO2 (%) | PET,CO2 (mmHg) | PET,CO2 (mmHg) | |
|---|---|---|---|---|---|---|---|---|
| HA | 39.0 ± 2.3 | 1.60 ± 0.01 | 65.3 ± 2.0 | 52.8 ± 1.3 | 7.0 ± 4.0 | 84.7 ± 0.8 | 26.2 ± 0.4 | 50.4 ± 1.2 |
| CMS | 42.2 ± 1.6 | 1.63 ± 0.02 | 62.5 ± 2.4 | 67.4 ± 2.5* | 19.0 ± 5.0* | 82.3 ± 1.4 | 30.5 ± 0.4* | 45.5 ± 1.0† |
Values are means ± s.e.m. Hct, haematocrit; CMSSco, chronic mountain sickness score; SaO2, finger or ear capillary arterial oxygen saturation; (PET,CO2), end-tidal partial pressure of carbon dioxide; (PET,O2), end-tidal partial pressure of oxygen; HA, high-altitude normal subjects; CMS, chronic mountain sickness subjects.
Student's unpaired t test, denotes significant difference from corresponding value in HA group (P < 0.001);
Student's unpaired t test, denotes significant difference from corresponding value in HA group (P < 0.002).
Measurement of cerebral blood flow velocity
Transcranial Doppler ultrasonography was used to measure changes in middle cerebral artery mean blood flow velocity (MCAVm), from which changes in cerebral blood flow were deduced in accordance with previous validation studies (Bishop et al. 1986; Aaslid et al. 1989). A 2 mHz pulsed-wave Doppler probe (Multi-Dop T2, TCD-8.01; DWL Elektronische System GmbH, Sipplingen, Germany) was mounted on a headband over the transcranial window. The middle cerebral artery (MCA) wave form was located at the optimal depth and the ultrasound probe fixed into position. Beat-to-beat values of MCAVm were recorded and analysed off-line after completion of the study. Blood pressure (autoinflating sphygmomanometer), heart rate (ECG) and arterial oxygen saturation (finger oximetry) were also measured throughout the study (Hewlett Packard 78325C, Boebringen, Germany).
Protocol 1: cerebrovascular responses to hypoxia
The protocol was designed to determine cerebrovascular responses to isocapnic hypoxia. Subjects were seated and breathed through a mouthpiece for a minimum of 10 min whilst recordings were stabilized. Dynamic end-tidal forcing was used to manipulate end-tidal gases (Robbins et al. 1982), which were assumed to be representative of arterial blood gases. The technique is a fast-flow breath-by-breath method using voltage-gated valves to alter the flow of inspiratory gases in order to clamp or forcibly change the end-tidal gases. Throughout all procedures, brachial blood pressure and arterial oxygen saturation were measured using the autoinflating sphygmomanometer and finger oximeter, respectively.
The subject's (PET,CO2) was held close to predetermined resting levels throughout the procedure. (PET,O2) was held at 100 mmHg for 10 min to establish a steady state. It was then dropped in a single step to 50 mmHg for 20 min, and recordings of cerebral blood velocity were taken throughout. Cerebrovascular responses to hypoxia were taken as the percentage changes in MCA velocity that occurred following the step decrease in (PET,O2) during the steady state, from minute 8 to minute 20.
Protocol 2: cerebrovascular responses to hypocapnia
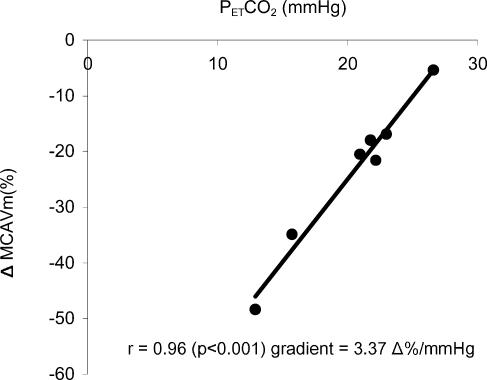
The protocol was designed to determine cerebrovascular reactivity to hypocapnia with end-tidal oxygen clamped constant at two levels: normoxia (PET,O2 100 mmHg) and hypoxia (PET,O2 50 mmHg). Subjects breathed either normoxic or hypoxic gases with carbon dioxide unclamped until in a steady state. (PET,CO2) was then decreased over a range of (PET,CO2) levels by instructing the subjects to hyperventilate by varying amounts. Cerebral responses to hypocapnia were calculated using linear regression of the relationship between (PET,CO2) and change in MCA velocity (e.g. Fig. 2). Data points were only included in the linear range and where correlation coefficients were statistically significant. The gradient of the relationship was used to measure the vascular reactivity of the cerebral circulation.
Figure 2. Determination of cerebrovascular responses to hypocapnia.
Example of response of a high-altitude normal subject (HA) at CP during hypoxia. ΔMCAVm (%), percentage change in middle cerebral artery blood flow velocity; r, correlation coefficient (P < 0.001); the gradient of the relationship was taken as a measure of cerebral reactivity to carbon dioxide.
Statistical analysis
All data are expressed as means ± standard errors of the means (s.e.m.). Spearman ranked correlation coefficients were used to assess correlations between variables. Unpaired t tests were used to compare the HA and CMS groups. Kolmogorov and Smirnov assumption methods were used to examine data for normal distribution, and on the basis of these findings the appropriate parametric or nonparametric ANOVA test was used to compare differences in responses of groups at CP, Lima and Leeds to the various procedures. GraphPad Instat version 3.00 for Macintosh (Graph Pad software, San Diego, CA, USA) was used to perform all statistical analyses. P < 0.05 was considered significant.
Results
Cardiovascular and blood variables during normoxia
During normoxia (PET,O2 100 mmHg), blood pressure and heart rate were not significantly different between the two altitude groups or at the two locations (Table 2). During normoxia (PET,O2 100 mmHg), diastolic blood pressure was significantly lower in the sea-level controls compared with HA and CMS at CP (both P < 0.01). Heart rate was significantly higher in the sea-level controls compared with high-altitude subjects at CP and Lima (all P < 0.05).
Table 2.
Values of cardiovascular variables, oxygen saturation and end-tidal gases in CMS and HA during normoxia (PET,O2 clamped at 100 mmHg) at Cerro de Pasco and Lima, and in sea-level controls at Leeds
| HA | CMS | ||||
|---|---|---|---|---|---|
| CP | Lima | CP | Lima | SL Leeds | |
| SAP (mmHg) | 118 ± 4.7 | 116 ± 5.3 | 121 ± 3.0 | 113 ± 4.1 | 115 ± 4.7 |
| DAP (mmHg) | 81.3 ± 3.5* | 76.6 ± 3.5 | 84.8 ± 3.4* | 77.4 ± 2.3 | 68 ± 3.7 |
| MAP (mmHg) | 94.1 ± 3.8* | 88.7 ± 4.1* | 94.0 ± 5.0* | 89.1 ± 2.7* | 83.5 ± 3.5 |
| Heart rate (beats min−1) | 63.9 ± 2.0 | 58.9 ± 3.4 | 66.6 ± 2.0 | 64.1 ± 3.5 | 78.4 ± 4.7 |
| SaO2 (%) | 96.9 ± 0.3 | 96.8 ± 0.5 | 95.4 ± 0.5 | 94.9 ± 1.0 | 97.9 ± 0.6 |
| PET,CO2 (mmHg) | 31.4 ± 0.5* | 35.8 ± 0.6‡ | 31.3 ± 0.7*† | 35.6 ± 0.6‡ | 35.8 ± 0.7 |
Values are means ± s.e.m. CP, Cerro de Pasco; SAP, systolic arterial pressure; DAP, diastolic arterial pressure; MAP, mean arterial pressure.
Parametric ANOVA test, denotes values are significantly different from SL controls (P < 0.05).
Parametric ANOVA test, denotes values are significantly different from corresponding values in HA subjects (P < 0.01).
Parametric ANOVA test, denotes values are significantly different from those in the same subjects at Lima (P < 0.01).
Values of oxygen saturation were similar in both altitude groups at both locations. (PET,CO2) was higher in CMS than in the HA at CP. Carbon dioxide levels were higher in both groups at Lima than at CP. However, the magnitude of the increases was smaller in the CMS patients; at Lima there was no significant difference in (PET,CO2) between the two groups. Oxygen saturation was higher in the sea level controls compared to the CMS patients at Lima (P < 0.05). (PET,CO2) was higher in sea-level controls than in the HA and CMS at CP (both P < 0.05). These results are summarized in Table 2.
Cerebrovascular responses to hypoxia
Satisfactory data were obtained from eight HA subjects, nine CMS patients and eight sea-level controls. At CP, the decrease in inspired oxygen during the protocol reduced arterial oxygen saturation in HA from 96.8 ± 0.2 to 86.9 ± 0.3%, and in CMS from 95.3 ± 0.4 to 86.5 ± 0.4%. At Lima, hypoxia reduced oxygen saturation in HA from 96.6 ± 0.4 to 86.5 ± 0.9%, and in CMS from 94.6 ± 0.8 to 84.3 ± 0.9%. These changes were similar in both groups at both locations. Heart rate and blood pressure changes were similar in all tests (Table 3).
Table 3.
Change in cardiovascular variables and oxygen saturation in CMS and HA during hypoxia at Cerro de Pasco and Lima, and in sea-level controls at Leeds
| HA | CMS | ||||
|---|---|---|---|---|---|
| CP | Lima | CP | Lima | SL Leeds | |
| ΔSAP (mmHg) | 4.6 ± 1.3 | 4.0 ± 5.1 | 3.7 ± 5.7 | 3.7 ± 1.3 | 3.5 ± 1.3 |
| ΔDAP (mmHg) | 3.2 ± 1.4 | 1.5 ± 1.0 | 0.8 ± 1.3 | 1.5 ± 1.3 | 1.4 ± 1.1 |
| ΔMAP (mmHg) | 3.0 ± 1.0 | 2.0 ± 0.9 | 1.3 ± 0.9 | 2.5 ± 1.2 | 2.0 ± 1.0 |
| ΔHeart rate (beats min−1) | 8.9 ± 2.1 | 5.7 ± 1.4 | 6.6 ± 0.7 | 5.3 ± 0.7 | 6.9 ± 2.7 |
| ΔSaO2 (%) | −9.8 ± 0.4 | −10.1 ± 1.0 | −8.9 ± 0.4 | −10.3 ± 0.8 | −8.1 ± 0.7 |
Values are means ± s.e.m. Changes were similar in all tests. PET,CO2 was clamped constant through out the hypoxia protocol and unchanged from the values given in Table 2.
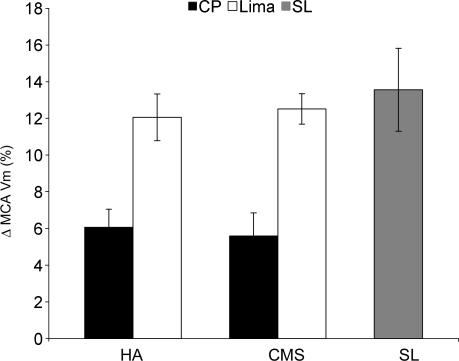
At CP, hypoxia increased cerebral blood velocity in HA and CMS by 6.1 ± 1.0 and 5.6 ± 1.2%, respectively. At Lima, responses were significantly greater in both groups (12.1 ± 1.3 and 12.5 ± 0.8%; P < 0.01). In sea-level subjects, hypoxia reduced oxygen saturation from 98.9 ± 0.4 to 90.6 ± 0.9%. This was similar to the change seen in HA and CMS groups at both locations. Hypoxia increased cerebral blood velocity by 13.6 ± 2.2%. This was greater than the change seen in HA and CMS patients at CP (both P < 0.01), but similar to the changes seen in both groups at Lima. The effects of hypoxia on cerebral blood velocity are shown in Fig. 3.
Figure 3. Cerebrovascular responses to hypoxia.
HA, high-altitude normal subjects; CMS, chronic mountain sickness patients; SL, sea-level controls. Both high-altitude groups have significantly greater responses to hypoxia at Lima compared to CP (both P < 0.01). Responses were lower in CMS and HA at CP compared to SL controls (both parametric ANOVA test, P < 0.01).
Cerebrovascular responses to hypocapnia
Complete data from CP and Lima, with significant correlations between MCAVm and (PET,CO2) (e.g. Fig. 2), were obtained from eight HA subjects and eight CMS patients. Data were unsatisfactory from two subjects due to poor-quality ultrasound signals resulting in insignificant correlation coefficients of the (PET,CO2)–MCAVm relationships. All remaining (PET,CO2)–MCAVm gradients were significantly correlated (mean correlation coefficient 0.89 ± 0.1). The range of changes in carbon dioxide were similar in all studies: at CP during hypoxia, HA changed from 28.0 ± 0.50 to 12.4 ± 0.4 mmHg, CMS from 31.4 ± 0.7 to 13.7 ± 0.9 mmHg; during normoxia, HA changed from 31.4 ± 2.2 to 14.5 ± 1.28 mmHg, CMS from 31.3 ± 0.7 to 15.1 ± 1.2 mmHg. At Lima during hypoxia, HA changed from 32.8 ± 0.8 to 15.0 ± 0.6 mmHg, CMS from 31.4 ± 0.7 to 15.6 ± 0.9 mmHg; during normoxia, HA changed from 35.8 ± 0.6 to 19.3 ± 1.5 mmHg, CMS from 35.6 ± 0.6 to 19.8 ± 0.5 mmHg). The range of change of carbon dioxide in sea-level controls was from 35.8 ± 1.0 to 18.8 ± 0.93 mmHg.
Comparison between results from all subjects revealed that baseline values of carbon dioxide at CP were lower in HA than in CMS (P < 0.01). They were also lower during normoxia in HA at CP than in the same subjects at Lima (P < 0.05), and lower than in sea-level controls (P < 0.001). Hyperventilation–hypocapnic values during normoxia were consequently lower in the HA group than in CMS at CP (P < 0.001) and lower than in the same group at Lima (P < 0.01). They were also lower than in sea-level controls (P < 0.01). The ranges of change of carbon dioxide induced by hyperventilation, however, were similar in all tests, and adequate to allow us to construct hypocapnic linear regression lines. MCAVm values were only included in the linear range.
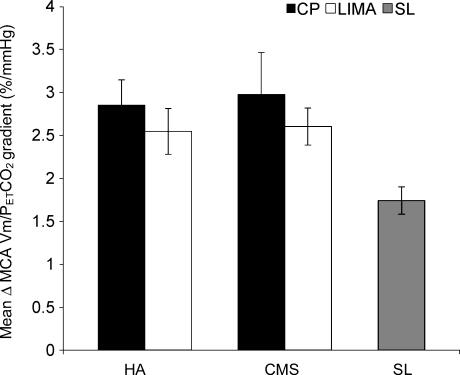
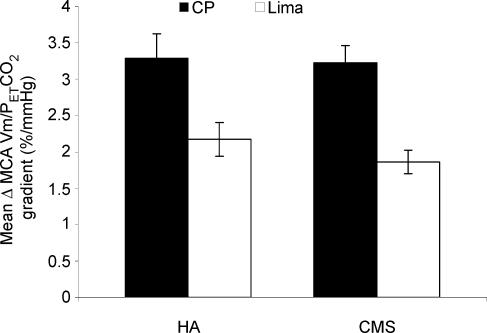
During normoxia, there was no significant difference between the sensitivities of the cerebral circulation to hypocapnia in the two groups and at both locations (Fig. 4). The changes in sensitivity at CP and Lima in the HA subjects were 2.85 ± 0.29 and 2.55 ± 0.27% mmHg−1, and in CMS the corresponding values were 2.97 ± 0.49 and 2.60 ± 0.22% mmHg−1. During hypoxia, the sensitivities of the cerebral velocities were again similar in both groups. However in both groups, sensitivity was significantly less in Lima (Fig. 5). In HA it decreased from 3.29 ± 0.34 to 2.17 ± 0.23% mmHg−1 (P < 0.05), and in CMS from 3.23 ± 0.24 to 1.86 ± 0.16% mmHg−1 (P < 0.01).
Figure 4. Cerebrovascular reactivity to hypocapnia during normoxia.
ΔMCAVm/(PET,CO2) gradient, change in middle cerebral artery velocity/(PET,CO2) gradient. There were no significant differences in cerebrovascular reactivity to hypocapnia in the two high-altitude groups at both locations. Responses were higher in CMS and HA at CP compared to SL controls (both non parametric ANOVA test, P < 0.05).
Figure 5. Cerebrovascular reactivity to hypocapnia during hypoxia.
Both groups had a lower cerebrovascular reactivity to hypocapnia at Lima (HA subjects P < 0.05; CMS P < 0.01, both parametric ANOVA test).
Cerebrovascular reactivity to hypocapnia in sea-level controls during normoxia was 1.75 ± 0.16% mmHg−1. This was significantly lower than all tests carried out at CP (all P < 0.05), and lower than the reactivity to normoxic hypocapnia at Lima (P < 0.05). However, reactivity to normoxic hypocapnia in sea-level controls was similar to that in the altitude groups to hypoxic hypocapnia.
Discussion
To our knowledge, this is the first study in which cerebrovascular responses to hypoxia and hypocapnia have been determined in high-altitude dwellers, both at high altitude and soon after descent to sea level, and then compared with results from sea-level residents. The techniques were entirely noninvasive, and changes in cerebral blood flow were estimated from changes in cerebral blood velocity determined by transcranial Doppler ultrasonography of the middle cerebral artery insonated through the temporal ‘window’. This technique is now well established for both clinical and research purposes. It is critically dependent on both the position of the transducer and the angle of insonation remaining constant, and it assumes that the diameter of the vessel does not change. With careful positioning and fixing of the transducer, these assumptions seem reasonable, and previous studies have indicated that the diameters of large cerebral arteries do not change measurably (Aaslid et al. 1989; Serrador et al. 2000). The technique has also been validated by comparisons with longer established 133xenon-washout methods (Bishop et al. 1986). Nevertheless we do not regard the method as adequate for measuring absolute values of flow, or even velocity. Whilst we can be sure that the angle of insonation was constant during the protocols, we can not be sure of identical positioning at all other times, therefore all data are given as percentage changes from appropriate steady-state control values.
The first main finding of this study was that there was no difference in the responses of the cerebral circulation to hypoxia or hypocapnia, separately or combined, between high-altitude residents with and without CMS, either determined at high altitude or within 24 h of arriving at sea level. The other main finding was that upon arrival at sea level, responses in both groups to hypoxia increased and, during hypoxia, the responses to hypocapnia were smaller. The responses to hypoxia in the high-altitude dwellers at sea level were similar to those in normal sea-level residents. Responses to normoxic hypocapnia in high-altitude dwellers, however, were greater than those in sea-level residents.
There has been a previous report which has suggested that CMS patients may have different cerebrovascular responses compared with high-altitude normals (Sun et al. 1996), and this would initially seem to be at variance with our results. The basis for the suggestion of different reactivities was that, during extreme hypoxia caused by sleep apnoea, cerebral flow decreased in CMS patients and increased in HA normals. However, the degree of hypoxia would have been considerably greater, particularly in the CMS patients, and very different from our moderate hypoxic stimulus. Interestingly, a recent report has indicated that hypoxia increases cerebral blood flow when subjects are awake, but decreases it during sleep (Meadows et al. 2004)
The mechanisms that cause the cerebrovascular response to hypoxia to be blunted at altitude are not known. One possibility is that it may be related to the release of vasoactive mediators, such as nitric oxide (NO). Acute hypoxia induces cerebral vasodilatation mainly through the release of NO (Van Mil et al. 2002). However, chronic hypoxia leads to a decline in nitric oxide synthase (NOS) levels (Mbaku et al. 2003), and it is possible that during chronic hypoxia at high altitude, the release of NO in response to acute hypoxia would be blunted. It is also possible that the sensitivity of the cerebral circulation to NO may be affected by chronic hypoxia and its alleviation. This suggestion is supported by a recent finding that NO donation causes a greater increase in cerebral flow in high-altitude dwellers after establishment of normoxia at sea level (Appenzeller et al. 2004). Another factor to be taken into consideration in our study is that carbon dioxide was higher at sea level during the hypoxia protocol. This may have had an effect on the sensitivity of the cerebral vessels to hypoxia. Whatever the mechanism responsible for the changes in reactivity, it does seem to take effect very rapidly because, within less than 24 h after arrival at sea level, responses were altered.
The results of the carbon dioxide reactivity tests are particularly intriguing. Although baseline carbon dioxide values were lower during concomitant hypoxia in the HA group at CP, this is unlikely to explain the difference in reactivity because reactivity was studied over a wide and overlapping linear range. Furthermore, in CMS patients, baseline values at CP were similar to those at Lima and cerebrovascular reactivity to hypocapnia showed the same change as in the HA subjects. We can only speculate on the possible reasons why the response of the cerebral blood flow velocity to hypocapnia were smaller at Lima that at CP, but only during concomitant hypoxia. At Lima, subjects had been normoxic and relatively normocapnic for between 12 and 18 h, and these changes in blood gas composition may have been responsible for the altered cerebrovascular reactivity to hypocapnia during hypoxia. Although the mechanism is uncertain, it is possible that NO might also have been involved in these changes. NO donation has been shown to blunt hypocapnic cerebral vasoconstriction (Lavi et al. 2003). Acute hypoxia increases flow and increases NO release. So, following the relief of the chronic hypoxia by descent to Lima when NOS increases, the acute hypoxia causes a greater release of NO and this would explain the reduction in the response to hypocapnia.
The altered cerebral blood flow responses at sea level implies that the adaptation to hypobaric hypoxia results in alteration of cerebrovascular reactivity in two ways: the vasodilatation induced by hypoxia is decreased, and the vasoconstriction induced by hypocapnia is exaggerated. In both cases this would lead to a reduction in cerebral blood flow and could be regarded as a potential maladaptation. However, by restricting blood flow it may have the benefit of protecting against cerebral oedema. Interestingly, bringing the subjects to sea level quickly alters cerebrovascular responses, suggesting that the change in cerebral control is rapidly reversible. This is supported by comparison of data with sea-level controls. Responses in high-altitude subjects to hypoxia were smaller at CP but similar at Lima to those in sea-level residents. Responses to normoxic hypocapnia, however, did not change and remained greater than those in sea level subjects.
One of the principal aims of this research was to try to determine whether cerebrovascular responses to changes in oxygen and carbon dioxide differed in subjects with different haematocrit values. The finding of identical cerebrovascular responses in subjects with varying degrees of polycythaemia suggests that haematocrit does not alter cerebrovascular control. Although we examined responses in subjects of presumed similar genetic constitutions in HA and CMS groups, we should perhaps insert a word of caution, as there may be other relevant differences between the CMS patients and the healthy Andeans. Furthermore, when relating the results of Andeans to sea-level subjects, the possible influences of both genetic and environmental differences must be taken into consideration. The enhanced vasodilatation in response to hypoxia, and decreased vasoconstriction to hypocapnia during hypoxia following sea-level descent, suggest that cerebrovascular control is impaired during chronic hypobaric hypoxia. However, this impairment is temporary as it is rapidly reversible within less than a day after arrival at low altitude. The changes in cerebrovascular reactivity have been clearly established, although we can only speculate on the likely mechanisms. What seems to be surprising is the speed with which these changes occurred, and it would be of interest to examine whether the changes could be detected within an even shorter period of time.
Acknowledgments
L.J.N. was a British Heart Foundation Student.
References
- Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Stroke. 1989;20:45–52. doi: 10.1161/01.str.20.1.45. [DOI] [PubMed] [Google Scholar]
- Appenzeller O, Passino C, Roach R, Gamboa J, Gamboa A, Bernardi L, Bonfichi M, Malcovati L. Cerebral vasoreactivity in Andeans and headache at sea level. J Neurol Sci. 2004;219:101–106. doi: 10.1016/j.jns.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Arregui A, Cabrera J, Leon-Velarde F, Paredes S, Viscarra D, Arbaiza D. High prevalence of migraine in a high-altitude population. Neurology. 1991;41:1668–1669. doi: 10.1212/wnl.41.10.1668. [DOI] [PubMed] [Google Scholar]
- Beall CM, Decker MJ, Brittenham GM, Kushner I, Gebremedhin A, Strohl KP. An Ethiopian pattern of human adaptation to high-altitude hypoxia. Proc Natl Acad Sci U S A. 2002;99:17215–17218. doi: 10.1073/pnas.252649199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop CC, Powell S, Rutt D, Browse NL. Transcranial Doppler measurement of middle cerebral artery blood flow velocity: a validation study. Stroke. 1986;17:913–915. doi: 10.1161/01.str.17.5.913. [DOI] [PubMed] [Google Scholar]
- Chiodi H. Respiratory adaptations to chronic high altitude hypoxia. J Appl Physiol. 1957;10:81–87. doi: 10.1152/jappl.1957.10.1.81. [DOI] [PubMed] [Google Scholar]
- Claydon VE, Norcliffe LJ, Moore JP, Rivera M, Leon-Velarde F, Appenzeller O, Hainsworth R. Cardiovascular responses to orthostatic stress in healthy altitude dwellers, and altitude residents with chronic mountain sickness. Exp Physiol. 2005;90:103–110. doi: 10.1113/expphysiol.2004.028399. [DOI] [PubMed] [Google Scholar]
- Claydon VE, Norcliffe LJ, Moore JP, Rivera ChM, Leon-Velarde F, Appenzeller O, Hainsworth R. Orthostatic tolerance and blood volumes in Andean high altitude dwellers. Exp Physiol. 2004;89:565–571. doi: 10.1113/expphysiol.2004.027698. [DOI] [PubMed] [Google Scholar]
- Jansen GF, Krins A, Basnyat B, Bosch A, Odoom JA. Cerebral autoregulation in subjects adapted and not adapted to high altitude. Stroke. 2000;31:2314–2318. doi: 10.1161/01.str.31.10.2314. [DOI] [PubMed] [Google Scholar]
- Lassen NA, Christensen MS. Physiology of cerebral blood flow. Br J Anaesth. 1976;48:719–734. doi: 10.1093/bja/48.8.719. [DOI] [PubMed] [Google Scholar]
- Lavi S, Egbarya R, Lavi R, Jacob G. Role of nitric oxide in the regulation of cerebral blood flow in humans: chemoregulation versus mechanoregulation. Circulation. 2003;107:1901–1905. doi: 10.1161/01.CIR.0000057973.99140.5A. [DOI] [PubMed] [Google Scholar]
- Leon-Velarde F, Arregui A, Vargas M, Huicho L, Acosta R. Chronic mountain sickness and chronic lower respiratory tract disorders. Chest. 1994;106:151–155. doi: 10.1378/chest.106.1.151. [DOI] [PubMed] [Google Scholar]
- Leon-Velarde F, McCullough RG, McCullough RE, Reeves JT. Proposal for scoring severity in chronic mountain sickness (CMS). Background and conclusions of the CMS Working Group. Adv Exp Med Biol. 2003;543:339–354. doi: 10.1007/978-1-4419-8997-0_24. [DOI] [PubMed] [Google Scholar]
- Massik J, Tang YL, Hudak ML, Koehler RC, Traystman RJ, Jones MD., Jr Effect of hematocrit on cerebral blood flow with induced polycythemia. J Appl Physiol. 1987;62:1090–1096. doi: 10.1152/jappl.1987.62.3.1090. [DOI] [PubMed] [Google Scholar]
- Mbaku EM, Zhang L, Pearce WJ, Duckles SP, Buchholz J. Chronic hypoxia alters the function of NOS nerves in cerebral arteries of near-term fetal and adult sheep. J Appl Physiol. 2003;94:724–732. doi: 10.1152/japplphysiol.00771.2002. [DOI] [PubMed] [Google Scholar]
- Meadows GE, O'Driscoll DM, Simonds AK, Morrell MJ, Corfield DR. Cerebral blood flow response to isocapnic hypoxia during slow-wave sleep and wakefulness. J Appl Physiol. 2004;97:1343–1348. doi: 10.1152/japplphysiol.01101.2003. [DOI] [PubMed] [Google Scholar]
- Milledge JS, Sorensen SC. Cerebral arteriovenous oxygen difference in man native to high altitude. J Appl Physiol. 1972;32:687–689. doi: 10.1152/jappl.1972.32.5.687. [DOI] [PubMed] [Google Scholar]
- Otis SM, Rossman ME, Schneider PA, Rush MP, Ringelstein EB. Relationship of cerebral blood flow regulation to acute mountain sickness. J Ultrasound Med. 1989;8:143–148. doi: 10.7863/jum.1989.8.3.143. [DOI] [PubMed] [Google Scholar]
- Penaloza D, Sime F. Chronic cor pulmonale due to loss of altitude acclimatization (chronic mountain sickness) Am J Med. 1971;50:728–743. doi: 10.1016/0002-9343(71)90181-1. [DOI] [PubMed] [Google Scholar]
- Poulin MJ, Fatemian M, Tansley JG, O'Connor DF, Robbins PA. Changes in cerebral blood flow during and after 48 h of both isocapnic and poikilocapnic hypoxia in humans. Exp Physiol. 2002;87:633–642. doi: 10.1113/eph8702437. [DOI] [PubMed] [Google Scholar]
- Robbins PA, Swanson GD, Micco AJ, Schubert WP. A fast gas-mixing system for breath-to-breath respiratory control studies. J Appl Physiol. 1982;52:1358–1362. doi: 10.1152/jappl.1982.52.5.1358. [DOI] [PubMed] [Google Scholar]
- Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke. 2000;31:1672–1678. doi: 10.1161/01.str.31.7.1672. [DOI] [PubMed] [Google Scholar]
- Sorensen SC, Lassen NA, Severinghaus JW, Coudert J, Zamora MP. Cerebral glucose metabolism and cerebral blood flow in high-altitude residents. J Appl Physiol. 1974;37:305–310. doi: 10.1152/jappl.1974.37.3.305. [DOI] [PubMed] [Google Scholar]
- Sun S, Oliver-Pickett C, Ping Y, Micco AJ, Droma T, Zamudio S, Zhuang J, Huang SY, McCullough RG, Cymerman A, Moore LG. Breathing and brain blood flow during sleep in patients with chronic mountain sickness. J Appl Physiol. 1996;81:611–618. doi: 10.1152/jappl.1996.81.2.611. [DOI] [PubMed] [Google Scholar]
- Van Mil AH, Spilt A, Van Buchem MA, Bollen EL, Teppema L, Westendorp RG, Blauw GJ. Nitric oxide mediates hypoxia-induced cerebral vasodilation in humans. J Appl Physiol. 2002;92:962–966. doi: 10.1152/japplphysiol.00616.2001. [DOI] [PubMed] [Google Scholar]