Abstract
Reductions in systemic and locomotor limb muscle blood flow and O2 delivery limit aerobic capacity in humans. To examine whether O2 delivery limits both aerobic power and capacity, we first measured systemic haemodynamics, O2 transport and O2 uptake  during incremental and constant (372 ± 11 W; 85% of peak power; mean ± s.e.m.) cycling exercise to exhaustion (n = 8) and then measured systemic and leg haemodynamics and
during incremental and constant (372 ± 11 W; 85% of peak power; mean ± s.e.m.) cycling exercise to exhaustion (n = 8) and then measured systemic and leg haemodynamics and  during incremental cycling and knee-extensor exercise in male subjects (n = 10). During incremental cycling, cardiac output
during incremental cycling and knee-extensor exercise in male subjects (n = 10). During incremental cycling, cardiac output  and systemic O2 delivery increased linearly to 80% of peak power (r2 = 0.998, P < 0.001) and then plateaued in parallel to a decline in stroke volume (SV) and an increase in central venous and mean arterial pressures (P < 0.05). In contrast, heart rate and
and systemic O2 delivery increased linearly to 80% of peak power (r2 = 0.998, P < 0.001) and then plateaued in parallel to a decline in stroke volume (SV) and an increase in central venous and mean arterial pressures (P < 0.05). In contrast, heart rate and  increased linearly until exhaustion (r2 = 0.993; P < 0.001) accompanying a rise in systemic O2 extraction to 84 ± 2%. In the exercising legs, blood flow and O2 delivery levelled off at 73–88% of peak power, blunting leg
increased linearly until exhaustion (r2 = 0.993; P < 0.001) accompanying a rise in systemic O2 extraction to 84 ± 2%. In the exercising legs, blood flow and O2 delivery levelled off at 73–88% of peak power, blunting leg  per unit of work despite increasing O2 extraction. When blood flow increased linearly during one-legged knee-extensor exercise,
per unit of work despite increasing O2 extraction. When blood flow increased linearly during one-legged knee-extensor exercise, 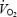 per unit of work was unaltered on fatigue. During constant cycling,
per unit of work was unaltered on fatigue. During constant cycling,  , SV, systemic O2 delivery and
, SV, systemic O2 delivery and  reached maximal values within ∼5 min, but dropped before exhaustion (P < 0.05) despite increasing or stable central venous and mean arterial pressures. In both types of maximal cycling, the impaired systemic O2 delivery was due to the decline or plateau in
reached maximal values within ∼5 min, but dropped before exhaustion (P < 0.05) despite increasing or stable central venous and mean arterial pressures. In both types of maximal cycling, the impaired systemic O2 delivery was due to the decline or plateau in  because arterial O2 content continued to increase. These results indicate that an inability of the circulatory system to sustain a linear increase in O2 delivery to the locomotor muscles restrains aerobic power. The similar impairment in SV and O2 delivery during incremental and constant load cycling provides evidence for a central limitation to aerobic power and capacity in humans.
because arterial O2 content continued to increase. These results indicate that an inability of the circulatory system to sustain a linear increase in O2 delivery to the locomotor muscles restrains aerobic power. The similar impairment in SV and O2 delivery during incremental and constant load cycling provides evidence for a central limitation to aerobic power and capacity in humans.
From rest to maximal exercise, the cardiovascular system must adjust to the increasing metabolic demand by ensuring the delivery of O2 and substrates to all body cells, in particular to the myocytes, without compromising arterial pressure. The prevailing theory is that the cardiovascular system responds to exercise of increasing intensity up to aerobic power (maximal oxygen uptake  ) by increasing systemic and contracting skeletal muscle O2 delivery in proportion to the rise in O2 demand, while increasing the perfusion pressure (Holmgren, 1956; Åstrand et al. 1964; Ekelund & Holmgren, 1967; Higginbotham et al. 1986; Reeves et al. 1990; Pawelczyk et al. 1992; Rowell, 1993; Rowell et al. 1996). However, recent findings during constant load maximal cycling exercise indicate that, following early adjustments that allow
) by increasing systemic and contracting skeletal muscle O2 delivery in proportion to the rise in O2 demand, while increasing the perfusion pressure (Holmgren, 1956; Åstrand et al. 1964; Ekelund & Holmgren, 1967; Higginbotham et al. 1986; Reeves et al. 1990; Pawelczyk et al. 1992; Rowell, 1993; Rowell et al. 1996). However, recent findings during constant load maximal cycling exercise indicate that, following early adjustments that allow  to be reached and maintained for 1–2 min, cardiac output
to be reached and maintained for 1–2 min, cardiac output 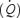 , leg blood flow (LBF), perfusion pressure and O2 delivery decline leading to reductions in
, leg blood flow (LBF), perfusion pressure and O2 delivery decline leading to reductions in  and leg
and leg  despite increases in O2 extraction (González-Alonso & Calbet, 2003; González-Alonso et al. 2004). These findings suggest that a reduction in systemic and locomotive skeletal muscle O2 delivery limits aerobic and maximal endurance capacity in trained individuals. The question then arises whether systemic and locomotor limb muscle O2 delivery is also impaired during incremental exercise and whether this could explain the plateau or decline in
despite increases in O2 extraction (González-Alonso & Calbet, 2003; González-Alonso et al. 2004). These findings suggest that a reduction in systemic and locomotive skeletal muscle O2 delivery limits aerobic and maximal endurance capacity in trained individuals. The question then arises whether systemic and locomotor limb muscle O2 delivery is also impaired during incremental exercise and whether this could explain the plateau or decline in 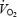 observed sometimes before exhaustion (Åstrand, 1952; Taylor et al. 1955; Mitchell et al. 1958; Froelicher et al. 1972; Pollock et al. 1976; Meyers et al. 1990; Knight et al. 1992; Day et al. 2003).
observed sometimes before exhaustion (Åstrand, 1952; Taylor et al. 1955; Mitchell et al. 1958; Froelicher et al. 1972; Pollock et al. 1976; Meyers et al. 1990; Knight et al. 1992; Day et al. 2003).
Indirect evidence indicates that O2 delivery might impose a limitation to  at intensities close to
at intensities close to  . Firstly, in humans the increase in
. Firstly, in humans the increase in 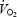 per unit of work is attenuated at high compared to low exercise intensities (Hill & Lupton, 1923; Åstrand & Saltin, 1961; Whipp & Wasserman, 1972). Secondly, the increase in
per unit of work is attenuated at high compared to low exercise intensities (Hill & Lupton, 1923; Åstrand & Saltin, 1961; Whipp & Wasserman, 1972). Secondly, the increase in  per litre increase in
per litre increase in  is attenuated at intensities above 40–70% of
is attenuated at intensities above 40–70% of  in humans, as in miniature swine (Saltin, 1964; Åstrand et al. 1964; Armstrong et al. 1987). These early observations lack conclusive support and are therefore not widely accepted. The general belief is that
in humans, as in miniature swine (Saltin, 1964; Åstrand et al. 1964; Armstrong et al. 1987). These early observations lack conclusive support and are therefore not widely accepted. The general belief is that  and O2 delivery increase linearly from rest to
and O2 delivery increase linearly from rest to  , implying that O2 delivery to locomotor limb muscle does not limit
, implying that O2 delivery to locomotor limb muscle does not limit  (Asmussen & Nielsen, 1952; Chapman et al. 1960; Bevegård et al. 1963; Rowell et al. 1964; Grimsby et al. 1966; Poliner et al. 1980; Higginbotham et al. 1986). This theory, however, is based on linear regression analysis of
(Asmussen & Nielsen, 1952; Chapman et al. 1960; Bevegård et al. 1963; Rowell et al. 1964; Grimsby et al. 1966; Poliner et al. 1980; Higginbotham et al. 1986). This theory, however, is based on linear regression analysis of  data that have not been normalized and on the assumption that systemic haemodynamics closely reflect skeletal muscle haemodynamics. At the skeletal muscle level, not only restrictions in the systemic supply of O2, but also limitations in diffusive O2 transport from the muscle capillary to the mitochondrial cytochrome and oxidative capacity of mitochondria could restrict
data that have not been normalized and on the assumption that systemic haemodynamics closely reflect skeletal muscle haemodynamics. At the skeletal muscle level, not only restrictions in the systemic supply of O2, but also limitations in diffusive O2 transport from the muscle capillary to the mitochondrial cytochrome and oxidative capacity of mitochondria could restrict  (Roca et al. 1989). In favour of a dominant O2 supply limitation, quadriceps muscle blood flow and
(Roca et al. 1989). In favour of a dominant O2 supply limitation, quadriceps muscle blood flow and  might reach ∼2.3 and ∼0.35 l kg−1 min−1 during maximal knee-extensor exercise, suggesting that only 10–15 kg of muscle needs to be recruited during whole body exercise to surpass the capacity of the human circulation to deliver O2 (Andersen & Saltin, 1985). Whether the O2 delivery to muscles and uptake of the quadriceps femoris are restricted during whole body compared to knee-extensor exercise remains unknown.
might reach ∼2.3 and ∼0.35 l kg−1 min−1 during maximal knee-extensor exercise, suggesting that only 10–15 kg of muscle needs to be recruited during whole body exercise to surpass the capacity of the human circulation to deliver O2 (Andersen & Saltin, 1985). Whether the O2 delivery to muscles and uptake of the quadriceps femoris are restricted during whole body compared to knee-extensor exercise remains unknown.
To investigate the contribution of the O2 transport system to 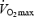 , we determined: (1) whether systemic O2 delivery imposes a limitation to aerobic power and capacity, (2) whether locomotor limb blood flow and O2 delivery are impaired during incremental exercise to exhaustion to the extent that they compromise limb
, we determined: (1) whether systemic O2 delivery imposes a limitation to aerobic power and capacity, (2) whether locomotor limb blood flow and O2 delivery are impaired during incremental exercise to exhaustion to the extent that they compromise limb  , and (3) whether quadriceps muscle blood flow and
, and (3) whether quadriceps muscle blood flow and  are lower during maximal exercise with a large compared to a small muscle mass. To accomplish these aims, we first measured systemic haemodynamics, O2 transport and
are lower during maximal exercise with a large compared to a small muscle mass. To accomplish these aims, we first measured systemic haemodynamics, O2 transport and 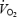 during incremental and constant cycling exercise to exhaustion in trained male subjects and then measured systemic and exercising leg haemodynamics, O2 transport and
during incremental and constant cycling exercise to exhaustion in trained male subjects and then measured systemic and exercising leg haemodynamics, O2 transport and  during incremental cycle and knee-extensor exercise to exhaustion in another group of active male subjects. We hypothesized that restrictions in O2 supply to locomotor limb muscles imposes a limitation to aerobic power and capacity in humans.
during incremental cycle and knee-extensor exercise to exhaustion in another group of active male subjects. We hypothesized that restrictions in O2 supply to locomotor limb muscles imposes a limitation to aerobic power and capacity in humans.
Methods
Eighteen endurance-trained or recreationally active male subjects participated in two studies. They had a mean (±s.d.) age of 27 ± 3 years, body weight of 81.3 ± 9.6 kg, height of 185 ± 9 cm, maximal heart rate (HR) of 192 ± 7 beats min−1 and  of 4.80 ± 0.46 l min−1. The subjects were informed of any risks and discomforts associated with the experiments before giving written consent to participate. The study was approved by the Ethics Committee of Copenhagen (KF 01-230/00) and conducted in accordance with the guidelines of the Declaration of Helsinki.
of 4.80 ± 0.46 l min−1. The subjects were informed of any risks and discomforts associated with the experiments before giving written consent to participate. The study was approved by the Ethics Committee of Copenhagen (KF 01-230/00) and conducted in accordance with the guidelines of the Declaration of Helsinki.
In the first study and on the first visit to the laboratory, eight endurance-trained subjects performed incremental exercise on a cycle ergometer (Excalibur, Lode, The Netherlands) to determine  , maximal HR and peak power. Thereafter, they completed four high-intensity training sessions on the cycle ergometer. During the last session, they carried out the same protocol as during the main experiment involving incremental (INC) and constant (CON) maximal exercise separated by one hour of recovery, while continuous measures of
, maximal HR and peak power. Thereafter, they completed four high-intensity training sessions on the cycle ergometer. During the last session, they carried out the same protocol as during the main experiment involving incremental (INC) and constant (CON) maximal exercise separated by one hour of recovery, while continuous measures of  , HR and oesophageal temperature were obtained. For the invasive experiment, the subjects arrived at the laboratory one hour prior to the experiment after a light breakfast. Catheters were placed into the brachial artery and an antecubital vein, with the latter catheter being advanced to the right atrium. Following 30 min of supine rest, the subjects completed INC and CON on the cycle ergometer preceded by a 15 min warm-up period (146 ± 6 W; <50%
, HR and oesophageal temperature were obtained. For the invasive experiment, the subjects arrived at the laboratory one hour prior to the experiment after a light breakfast. Catheters were placed into the brachial artery and an antecubital vein, with the latter catheter being advanced to the right atrium. Following 30 min of supine rest, the subjects completed INC and CON on the cycle ergometer preceded by a 15 min warm-up period (146 ± 6 W; <50%  ) and 3 min of rest, followed by 10 min of recovery. Throughout the protocol, the arms rested on aerobars simulating the position that cyclists adopt during a time-trial. During the resting and recovery periods, the subjects were allowed to move their legs (0 W). During INC, the workload was increased every minute using a computerized system to elicit 20, 40, 60, 80, 90, 95 and 100% of peak power. During CON, the intensity resulted in exhaustion within 5–7 min and
) and 3 min of rest, followed by 10 min of recovery. Throughout the protocol, the arms rested on aerobars simulating the position that cyclists adopt during a time-trial. During the resting and recovery periods, the subjects were allowed to move their legs (0 W). During INC, the workload was increased every minute using a computerized system to elicit 20, 40, 60, 80, 90, 95 and 100% of peak power. During CON, the intensity resulted in exhaustion within 5–7 min and  within 4–5 min (i.e. at 372 ± 11 W or at 85% of the 438 ± 13 W peak power of the initial incremental test). The order of the two trials was randomly assigned and counterbalanced across the subjects. Exercise was performed under thermoneutral conditions (∼20°C) with fans directed against the back and the side of the subjects.
within 4–5 min (i.e. at 372 ± 11 W or at 85% of the 438 ± 13 W peak power of the initial incremental test). The order of the two trials was randomly assigned and counterbalanced across the subjects. Exercise was performed under thermoneutral conditions (∼20°C) with fans directed against the back and the side of the subjects.
Ten subjects participated in the second investigation which was aimed at determining whether convective O2 transport and limb muscle  are compromised during incremental cycle exercise to exhaustion and whether quadriceps muscle blood flow and
are compromised during incremental cycle exercise to exhaustion and whether quadriceps muscle blood flow and  are lower during maximal cycle compared to maximal knee-extensor exercise. In these subjects, an additional catheter was inserted into the femoral vein 2 cm from the inguinal ligament to allow for blood sampling and measurements of LBF (Andersen & Saltin, 1985). During both types of incremental exercise, the workload was increased every 1.5 min to elicit 25, 50, 75, 90 and 100% of peak power.
are lower during maximal cycle compared to maximal knee-extensor exercise. In these subjects, an additional catheter was inserted into the femoral vein 2 cm from the inguinal ligament to allow for blood sampling and measurements of LBF (Andersen & Saltin, 1985). During both types of incremental exercise, the workload was increased every 1.5 min to elicit 25, 50, 75, 90 and 100% of peak power.
In study 1, blood samples (1–5 ml) were drawn simultaneously from the brachial artery and the right atrium at rest in the supine and upright positions, during the warm-up (4, 10 and 15 min), immediately before the start of maximal exercise, during maximal exercise and during the recovery (1, 3, 6 and 10 min). In INC, blood samples were drawn after 45 s of each workload and at exhaustion. In CON, they were drawn after 0.75, 1.5, 3, 4 and 5 min and before exhaustion. In study 2, blood samples were drawn simultaneously from the brachial artery, right atrium and the femoral vein after 45 s at each workload, and LBF was measured after 1 min.
Throughout the studies, pulmonary  was measured online (Medgraphics CPX/D, Saint Paul, MN, USA; study 1 and Cosmed Quark b2, Italy; study 2). During the invasive experiments, HR was obtained from an electrocardiogram while arterial and central venous pressures were monitored with transducers positioned at heart level (Pressure Monitoring Kit, Baxter). The LBF was measured by the constant-infusion thermodilution method (Andersen & Saltin, 1985; González-Alonso et al. 2000b), while
was measured online (Medgraphics CPX/D, Saint Paul, MN, USA; study 1 and Cosmed Quark b2, Italy; study 2). During the invasive experiments, HR was obtained from an electrocardiogram while arterial and central venous pressures were monitored with transducers positioned at heart level (Pressure Monitoring Kit, Baxter). The LBF was measured by the constant-infusion thermodilution method (Andersen & Saltin, 1985; González-Alonso et al. 2000b), while  was calculated using the Fick principle (
was calculated using the Fick principle ( (a–v) O2 difference), assuming negligible differences in blood oxygenation between the right atrium and the pulmonary artery (Barratt-Boyes & Wood, 1956). The
(a–v) O2 difference), assuming negligible differences in blood oxygenation between the right atrium and the pulmonary artery (Barratt-Boyes & Wood, 1956). The  data obtained in one subject using the direct Fick principle confirmed the
data obtained in one subject using the direct Fick principle confirmed the  results. Stroke volume (SV) was the quotient between
results. Stroke volume (SV) was the quotient between  and HR, and systemic and leg vascular conductance were the quotients between
and HR, and systemic and leg vascular conductance were the quotients between  and LBF and the perfusion pressure. Perfusion pressure was the difference between mean arterial (MAP) and central venous pressures and pulse pressure was that between the systolic and diastolic blood pressure. The left ventricular contractility index dP/dtmax was calculated as the peak systolic value of the first derivative of the arterial pressure curve over 20 cardiac cycles. For systemic O2 delivery,
and LBF and the perfusion pressure. Perfusion pressure was the difference between mean arterial (MAP) and central venous pressures and pulse pressure was that between the systolic and diastolic blood pressure. The left ventricular contractility index dP/dtmax was calculated as the peak systolic value of the first derivative of the arterial pressure curve over 20 cardiac cycles. For systemic O2 delivery,  was multiplied by the arterial O2 content whereas systemic O2 extraction was the ratio between the systemic a–v O2 difference and the arterial O2 content. Blood gases, haemoglobin, glucose and lactate concentrations were measured using an ABL700 analyser (Radiometer, Copenhagen, Denmark). Oesophageal temperature was measured with a thermocouple (MOV-A, Ellab, Copenhagen, Denmark) inserted through the nasal passage at a distance equal to one-fourth of the subject's standing height, and HR was measured with a Polar Sports Tester (Polar Electro). In study 1, blood gas variables were corrected for the temperature measured during the non-invasive trials, whereas in study 2 the correction was made from the femoral venous blood temperature. Leg muscle mass was calculated from the whole-body dual-energy X-ray absorptiometry scanning (Prodigy, General Electrics Medical Systems, WI, USA) as lean mass of the region. Quadriceps femoris muscle mass was calculated using the antropomethic method, as described by Anderson & Saltin, 1985.
was multiplied by the arterial O2 content whereas systemic O2 extraction was the ratio between the systemic a–v O2 difference and the arterial O2 content. Blood gases, haemoglobin, glucose and lactate concentrations were measured using an ABL700 analyser (Radiometer, Copenhagen, Denmark). Oesophageal temperature was measured with a thermocouple (MOV-A, Ellab, Copenhagen, Denmark) inserted through the nasal passage at a distance equal to one-fourth of the subject's standing height, and HR was measured with a Polar Sports Tester (Polar Electro). In study 1, blood gas variables were corrected for the temperature measured during the non-invasive trials, whereas in study 2 the correction was made from the femoral venous blood temperature. Leg muscle mass was calculated from the whole-body dual-energy X-ray absorptiometry scanning (Prodigy, General Electrics Medical Systems, WI, USA) as lean mass of the region. Quadriceps femoris muscle mass was calculated using the antropomethic method, as described by Anderson & Saltin, 1985.
Statistical analysis
A one-way repeated measures analysis of variance (ANOVA) was performed to test significance within and between the two trials. Following a significant F test, pair-wise differences were identified using Tukey's honestly significant difference (HSD) post hoc procedure. To determine whether exhaustion during the constant maximal exercise was preceded by reductions in  , SV and O2 delivery, final values were compared with peak values during exercise using one-way repeated measures ANOVA with Tukey's HSD post hoc procedure. The significance level was set at P < 0.05 and data are means ± s.e.m. unless indicated otherwise.
, SV and O2 delivery, final values were compared with peak values during exercise using one-way repeated measures ANOVA with Tukey's HSD post hoc procedure. The significance level was set at P < 0.05 and data are means ± s.e.m. unless indicated otherwise.
Results
Performance and maximal O2 uptake
No significant differences in endurance,  or peak power were observed between the non-invasive and the invasive experiments. In INC, time to fatigue was 6.45 ± 0.2 and 6.96 ± 0.1 min during the non-invasive and invasive experiments, respectively, accompanied by a similar
or peak power were observed between the non-invasive and the invasive experiments. In INC, time to fatigue was 6.45 ± 0.2 and 6.96 ± 0.1 min during the non-invasive and invasive experiments, respectively, accompanied by a similar  (4.81 ± 0.12 and 4.75 ± 0.15 l min−1, respectively) and peak power (440 ± 16 and 446 ± 13 W, respectively). Similarly, in CON, time to fatigue was 7.01 ± 0.23 and 6.87 ± 0.50 min during the non-invasive and invasive experiments, respectively, and
(4.81 ± 0.12 and 4.75 ± 0.15 l min−1, respectively) and peak power (440 ± 16 and 446 ± 13 W, respectively). Similarly, in CON, time to fatigue was 7.01 ± 0.23 and 6.87 ± 0.50 min during the non-invasive and invasive experiments, respectively, and  was 4.82 ± 0.12 versus 4.75 ± 0.13 l min−1. In INC the workload elicited 20 ± 0, 39 ± 0, 59 ± 1, 79 ± 1, 88 ± 15, 93 ± 1 and 100 ± 0% of peak power during the invasive experiment. Oesophageal temperature increased from ∼37.8°C at the onset of exercise to 39.5 ± 0.1 and 39.9 ± 0.1°C in INC and CON, respectively (Fig. 1).
was 4.82 ± 0.12 versus 4.75 ± 0.13 l min−1. In INC the workload elicited 20 ± 0, 39 ± 0, 59 ± 1, 79 ± 1, 88 ± 15, 93 ± 1 and 100 ± 0% of peak power during the invasive experiment. Oesophageal temperature increased from ∼37.8°C at the onset of exercise to 39.5 ± 0.1 and 39.9 ± 0.1°C in INC and CON, respectively (Fig. 1).
Figure 1. Core temperature during the constant and incremental protocols.
Oesophageal temperature at rest, during submaximal and maximal exercise, and during 10 min of recovery, in incremental (•) and constant (^) exercise protocols. Data are means ± s.e.m. for 8 subjects.
Systemic haemodynamics, O2 transport and O2 uptake
No differences in systemic haemodynamics, O2 transport or  were observed at rest or during the 15 min of submaximal exercise (Fig. 2). In INC,
were observed at rest or during the 15 min of submaximal exercise (Fig. 2). In INC,  increased linearly to 80% of peak power (r2 = 0.998; P < 0.001) and then plateaued (Figs 2, 3, 4). In CON,
increased linearly to 80% of peak power (r2 = 0.998; P < 0.001) and then plateaued (Figs 2, 3, 4). In CON,  increased during the first 1.5 min, reached a peak value of 27.1 ± 1.1 l min−1 after 4.6 ± 0.6 min (range 3–6 min) and then declined 1.9 ± 0.5 l min−1 before exhaustion (P < 0.05). The observed plateau in
increased during the first 1.5 min, reached a peak value of 27.1 ± 1.1 l min−1 after 4.6 ± 0.6 min (range 3–6 min) and then declined 1.9 ± 0.5 l min−1 before exhaustion (P < 0.05). The observed plateau in  above 80% of peak power in INC and the drop during CON were due to a fall in SV (20 ± 3 and 27 ± 6 ml beat−1 in INC and CON, respectively), because HR continued to increase to exhaustion (190 ± 2 and 192 ± 2 beats min−1, respectively). In both INC and CON, central venous pressure increased from −3 mmHg at rest to 2–5 mmHg at exhaustion. In INC, MAP increased from 94 ± 4 mmHg at the start of exercise to 136 ± 7 mmHg at exhaustion. In contrast, in CON, MAP stabilized at ∼128 mmHg after 1.5 min. In INC, perfusion pressure increased from 98 ± 3 to 131 ± 6 mmHg at exhaustion accompanying an increase in pulse pressure from 59 ± 4 to 144 ± 4 mmHg. In contrast, perfusion pressure in CON increased from 100 ± 4 to 127 ± 6 mmHg after 3 min and remained stable thereafter. Similarly, pulse pressure increased from 60 ± 3 to 137 ± 4 mmHg after 3 min. In INC, systemic vascular conductance increased to ∼80% of peak power (range 60–90%) and then declined (P < 0.05). In CON, vascular conductance reached a plateau (range 0.75–3 min) and then declined (P < 0.05). No difference in dP/dtmax was observed at peak SV and exhaustion in either INC (2287 ± 96 versus 2419 ± 77 mmHg s−1; P = 0.35) or CON (2119 ± 271 versus 2193 ± 230 mmHg s−1; P = 0.72).
above 80% of peak power in INC and the drop during CON were due to a fall in SV (20 ± 3 and 27 ± 6 ml beat−1 in INC and CON, respectively), because HR continued to increase to exhaustion (190 ± 2 and 192 ± 2 beats min−1, respectively). In both INC and CON, central venous pressure increased from −3 mmHg at rest to 2–5 mmHg at exhaustion. In INC, MAP increased from 94 ± 4 mmHg at the start of exercise to 136 ± 7 mmHg at exhaustion. In contrast, in CON, MAP stabilized at ∼128 mmHg after 1.5 min. In INC, perfusion pressure increased from 98 ± 3 to 131 ± 6 mmHg at exhaustion accompanying an increase in pulse pressure from 59 ± 4 to 144 ± 4 mmHg. In contrast, perfusion pressure in CON increased from 100 ± 4 to 127 ± 6 mmHg after 3 min and remained stable thereafter. Similarly, pulse pressure increased from 60 ± 3 to 137 ± 4 mmHg after 3 min. In INC, systemic vascular conductance increased to ∼80% of peak power (range 60–90%) and then declined (P < 0.05). In CON, vascular conductance reached a plateau (range 0.75–3 min) and then declined (P < 0.05). No difference in dP/dtmax was observed at peak SV and exhaustion in either INC (2287 ± 96 versus 2419 ± 77 mmHg s−1; P = 0.35) or CON (2119 ± 271 versus 2193 ± 230 mmHg s−1; P = 0.72).
Figure 2. Central haemodynamics during the constant and incremental protocols.
Cardiac output, heart rate, stroke volume, central venous and arterial pressure, systemic vascular conductance, arterial O2 content, systemic O2 delivery, a–v O2 difference and 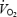 at rest, during submaximal and maximal exercise, and during 10 min of recovery, in incremental (•) and constant load (^) exercise. Data are means ± s.e.m. for 8 subjects, except arterial and central venous pressures and systemic vascular conductance for which data represent 7 subjects. * Lower than the value after 22 min when cycling at 80% of peak power, P < 0.05. ‡ Lower than the peak values observed after 20–24 min of constant load maximal cycling, P < 0.05.
at rest, during submaximal and maximal exercise, and during 10 min of recovery, in incremental (•) and constant load (^) exercise. Data are means ± s.e.m. for 8 subjects, except arterial and central venous pressures and systemic vascular conductance for which data represent 7 subjects. * Lower than the value after 22 min when cycling at 80% of peak power, P < 0.05. ‡ Lower than the peak values observed after 20–24 min of constant load maximal cycling, P < 0.05.
Figure 3. Central haemodynamics during incremental exercise to exhaustion.
Cardiac output, heart rate, stroke volume, arterial (•) and central venous (▾) pressure, systemic vascular conductance, arterial O2 content, systemic O2 delivery, systemic a–v O2 difference, systemic O2 extraction and pulmonary 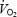 during incremental exercise to exhaustion plotted against the relative increase in power output. Data are means ± s.e.m. for 8 subjects. * Lower than 80% of peak power, P < 0.05.
during incremental exercise to exhaustion plotted against the relative increase in power output. Data are means ± s.e.m. for 8 subjects. * Lower than 80% of peak power, P < 0.05.
Figure 4. Relationship between cardiac output and systemic  during incremental exercise to exhaustion.
during incremental exercise to exhaustion.
Cardiac output, stroke volume and systemic O2 delivery plotted against the increases in 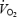 during incremental exercise. The plateau in cardiac output was due to a concomitant decline in stroke volume. Data are means ± s.e.m. for 8 subjects. * Lower than the stroke volume value observed at a systematic
during incremental exercise. The plateau in cardiac output was due to a concomitant decline in stroke volume. Data are means ± s.e.m. for 8 subjects. * Lower than the stroke volume value observed at a systematic  of 3.9 L min−1 when cycling at 80% of peak power, P < 0.05.
of 3.9 L min−1 when cycling at 80% of peak power, P < 0.05.
In both INC and CON, there was an increase in haemoglobin concentration, resulting in an elevated arterial O2 content, despite declining arterial O2 tension and saturation (Tables 1 and 2; Fig. 2). In INC, systemic O2 delivery increased linearly to 80% of peak power (r2 = 0.998; P < 0.001) and then levelled off (Figs 2, 3, 4). In CON, systemic O2 delivery increased to reach a maximal value of 5.7 ± 0.2 l min−1 after 4.4 ± 0.4 min (range 3–5 min) and then declined 0.35 ± 0.08 l min−1 before exhaustion (P < 0.05). During both INC and CON, systemic a–v O2 difference and O2 extraction increased until exhaustion. At exhaustion in INC and CON, systemic O2 extraction was 84 ± 2% and 87 ± 1%, respectively. In INC,  increased linearly to exhaustion and therefore
increased linearly to exhaustion and therefore  was reached during the last ∼0.5 min (r2 = 0.998; P < 0.001; Fig. 3). In CON,
was reached during the last ∼0.5 min (r2 = 0.998; P < 0.001; Fig. 3). In CON,  was reached within 4–6 min, maintained for 2.0 ± 0.3 min, but declined 0.14 ± 0.05 l min−1 before exhaustion (P < 0.05).
was reached within 4–6 min, maintained for 2.0 ± 0.3 min, but declined 0.14 ± 0.05 l min−1 before exhaustion (P < 0.05).
Table 1.
Blood variables at rest, during incremental exercise to exhaustion, and after 10 min of recovery
| Incremental exercise (% peak power) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rest | 20% | 40% | 60% | 80% | 90% | 95% | 100% | 10 min recovery | ||
| Haemoglobin (g l−1) | ||||||||||
| a | 150 ± 4 | 153 ± 3 | 155 ± 3 | 156 ± 2* | 157 ± 2* | 158 ± 3* | 160 ± 3* | 162 ± 3* | 156 ± 3* | |
| v | 151 ± 4 | 151 ± 4 | 152 ± 4 | 153 ± 4 | 158 ± 3* | 159 ± 3* | 161 ± 4* | 163 ± 4* | 154 ± 3 | |
| PO2 (mmHg) | ||||||||||
| a | 120 ± 4 | 101 ± 2* | 95 ± 2* | 92 ± 4* | 91 ± 3* | 93 ± 3* | 96 ± 3* | 102 ± 3* | 118 ± 2 | |
| v | 40 ± 2 | 30 ± 2* | 26 ± 1* | 22 ± 1* | 21 ± 1* | 20 ± 1* | 20 ± 1* | 21 ± 1* | 48 ± 2* | |
| O2 saturation (%) | ||||||||||
| a | 98.8 ± 0.1 | 98.0 ± 0.1 | 97.5 ± 0.2* | 97.1 ± 0.3* | 96.6 ± 0.3* | 96.2 ± 0.3* | 95.4 ± 0.5* | 95.0 ± 0.5* | 97.3 ± 0.2* | |
| v | 71.3 ± 2.3 | 50.4 ± 2.7* | 37.6 ± 3.0* | 30.4 ± 2.1* | 25.6 ± 2.1* | 20.9 ± 1.8* | 17.2 ± 1.7* | 14.8 ± 1.5* | 69.8 ± 1.2 | |
| O2 content (ml l−1) | ||||||||||
| a | 202 ± 5 | 203 ± 4 | 205 ± 3 | 205 ± 3 | 206 ± 3 | 206 ± 3 | 208 ± 4 | 210 ± 4* | 207 ± 4 | |
| v | 146 ± 7 | 103 ± 7* | 78 ± 7* | 63 ± 5* | 55 ± 5* | 45 ± 4* | 38 ± 4* | 33 ± 4* | 146 ± 5 | |
| PCO2 (mmHg) | ||||||||||
| a | 41 ± 1 | 42 ± 1 | 42 ± 1 | 42 ± 0 | 42 ± 1 | 41 ± 1 | 39 ± 1 | 37 ± 1* | 33 ± 1* | |
| v | 50 ± 1 | 53 ± 1 | 56 ± 1 | 61 ± 1* | 70 ± 1* | 77 ± 2* | 83 ± 2* | 87 ± 3* | 43 ± 1* | |
| pH | ||||||||||
| a | 7.40 ± 0.01 | 7.40 ± 0.00 | 7.39 ± 0.00 | 7.38 ± 0.01 | 7.36 ± 0.01* | 7.32 ± 0.01* | 7.26 ± 0.01* | 7.20 ± 0.01* | 7.19 ± 0.01* | |
| v | 7.35 ± 0.01 | 7.35 ± 0.00 | 7.33 ± 0.01 | 7.30 ± 0.01* | 7.24 ± 0.01* | 7.17 ± 0.01* | 7.10 ± 0.01* | 7.03 ± 0.01* | 7.16 ± 0.01* | |
| Lactate (mmol l−1) | ||||||||||
| a | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.3 ± 0.1 | 2.0 ± 0.2 | 4.2 ± 0.3* | 7.7 ± 0.3* | 11.9 ± 0.4* | 16.4 ± 0.7* | 14.3 ± 0.6* | |
| v | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.7 ± 0.4 | 2.4 ± 0.4 | 4.3 ± 0.3* | 8.0 ± 0.4* | 12.1 ± 0.5* | 16.8 ± 0.8* | 13.7 ± 0.8* | |
| Glucose (mmol l−1) | ||||||||||
| a | 5.3 ± 0.3 | 5.3 ± 0.3 | 5.3 ± 0.3 | 5.4 ± 0.3 | 5.3 ± 0.3 | 5.2 ± 0.3 | 5.2 ± 0.3 | 5.0 ± 0.3 | 6.2 ± 0.2* | |
| v | 5.2 ± 0.3 | 5.2 ± 0.3 | 5.2 ± 0.3 | 5.2 ± 0.3 | 5.3 ± 0.3 | 5.2 ± 0.3 | 5.1 ± 0.3 | 4.9 ± 0.3 | 6.1 ± 0.2* | |
Values are means ± s.e.m. for 8 subjects. a, arterial. v, right atrium.
Different from rest, P < 0.05. PO2,PCO2 and pH values were corrected for changes in blood temperature.
Table 2.
Blood variables at rest, during constant load maximal exercise, and after 10 min of recovery
| Constant load exercise (min) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Rest | 0.8 | 1.5 | 3 | 4 | 5 | 6.9 ± 0.5 | 10 min recovery | ||
| Haemoglobin (g l−1) | |||||||||
| a | 151 ± 3 | 155 ± 3 | 159 ± 3* | 160 ± 4* | 165 ± 3* | 166 ± 3* | 169 ± 3* | 158 ± 4* | |
| v | 155 ± 3 | 158 ± 3 | 161 ± 4 | 163 ± 3 | 165 ± 3* | 166 ± 3* | 165 ± 3* | 157 ± 5 | |
| PO2 (mmHg) | |||||||||
| a | 118 ± 4 | 91 ± 4* | 91 ± 3* | 92 ± 4* | 94 ± 4* | 96 ± 4* | 101 ± 4* | 117 ± 3 | |
| v | 40 ± 1 | 22 ± 1* | 20 ± 1* | 20 ± 1* | 20 ± 1* | 20 ± 1* | 20 ± 1* | 49 ± 2* | |
| O2 saturation (%) | |||||||||
| a | 98.8 ± 0.2 | 97.1 ± 0.5 | 96.6 ± 0.3* | 96.1 ± 0.6* | 94.7 ± 0.6* | 94.3 ± 0.9* | 93.0 ± 1.0* | 96.9 ± 0.4* | |
| v | 70.8 ± 2.4 | 31.7 ± 2.8* | 22.9 ± 2.3* | 18.7 ± 1.7* | 16.6 ± 1.6* | 15.1 ± 1.8* | 12.5 ± 1.2* | 68.8 ± 1.3 | |
| O2 content (ml l−1) | |||||||||
| a | 204 ± 4 | 205 ± 4 | 209 ± 5 | 209 ± 6 | 213 ± 4* | 213 ± 4* | 213 ± 4* | 209 ± 5 | |
| v | 149 ± 7 | 68 ± 7* | 50 ± 6* | 42 ± 4* | 37 ± 4* | 34 ± 4* | 29 ± 3* | 146 ± 5 | |
| PCO2 (mmHg) | |||||||||
| a | 41 ± 1 | 41 ± 1 | 41 ± 1 | 38 ± 1 | 37 ± 1 | 36 ± 1* | 34 ± 1* | 33 ± 1* | |
| v | 51 ± 1 | 58 ± 2* | 71 ± 2* | 76 ± 2* | 78 ± 2* | 79 ± 2* | 82 ± 3* | 43 ± 1* | |
| pH | |||||||||
| a | 7.41 ± 0.01 | 7.39 ± 0.01 | 7.35 ± 0.01 | 7.27 ± 0.01* | 7.23 ± 0.01* | 7.20 ± 0.01* | 7.12 ± 0.02* | 7.17 ± 0.03* | |
| v | 7.35 ± 0.00 | 7.32 ± 0.01 | 7.22 ± 0.01* | 7.13 ± 0.01* | 7.09 ± 0.01* | 7.06 ± 0.01* | 7.00 ± 0.02* | 7.14 ± 0.03* | |
| Lactate (mmol l−1) | |||||||||
| a | 1.1 ± 0.1 | 1.9 ± 0.2 | 5.3 ± 0.4* | 10.8 ± 0.6* | 13.7 ± 0.8* | 16.0 ± 0.9* | 19.0 ± 1.1* | 13.8 ± 1.2* | |
| v | 1.0 ± 0.1 | 2.2 ± 0.2 | 5.4 ± 0.5* | 10.8 ± 0.6* | 13.4 ± 0.7* | 15.6 ± 0.9* | 18.6 ± 1.1* | 13.6 ± 1.2* | |
| Glucose (mmol l−1) | |||||||||
| a | 5.4 ± 0.2 | 5.3 ± 0.2 | 5.4 ± 0.2 | 5.3 ± 0.3 | 5.2 ± 0.3 | 5.2 ± 0.3 | 5.3 ± 0.2 | 7.3 ± 0.3* | |
| v | 5.3 ± 0.2 | 5.3 ± 0.2 | 5.3 ± 0.2 | 5.3 ± 0.2 | 5.2 ± 0.3 | 5.2 ± 0.2 | 5.2 ± 0.2 | 7.2 ± 0.3* | |
Values are means ± s.e.m. for 8 subjects. a, arterial. v, right atrium.
Different from rest, P < 0.05. PO2, PCO2 and pH values were corrected for changes in blood temperature.
Leg haemodynamics, O2 transport and O2 uptake
The rate of increase in LBF and O2 delivery during incremental exercise was attenuated at intensities above 50% of peak power, reaching a plateau at 73–88% (Fig. 5). The levelling off in LBF, associated with a plateau in leg vascular conductance, attenuated the increase in leg  (8.8 ± 0.5 versus 11.9 ± 0.7 ml W−1 min−1 at exhaustion compared to 50% peak power, respectively; P = 0.003), despite increasing O2 extraction. At the systemic level,
(8.8 ± 0.5 versus 11.9 ± 0.7 ml W−1 min−1 at exhaustion compared to 50% peak power, respectively; P = 0.003), despite increasing O2 extraction. At the systemic level,  reached a plateau at ∼80% peak power leading to the blunting of the rate of increase in O2 delivery per litre of
reached a plateau at ∼80% peak power leading to the blunting of the rate of increase in O2 delivery per litre of  (Fig. 6). In contrast to incremental cycling, LBF, O2 delivery and leg
(Fig. 6). In contrast to incremental cycling, LBF, O2 delivery and leg  increased linearly from rest to exhaustion during one-legged knee-extensor exercise (r2 = 0.994–0.999; P < 0.0002), thereby allowing the maintenance of constant LBF, leg O2 delivery and leg
increased linearly from rest to exhaustion during one-legged knee-extensor exercise (r2 = 0.994–0.999; P < 0.0002), thereby allowing the maintenance of constant LBF, leg O2 delivery and leg  per unit of work (slopes = 75.6, 16.3 and 13.4 ml W−1 min−1, respectively). As depicted in Fig. 7, LBF and leg
per unit of work (slopes = 75.6, 16.3 and 13.4 ml W−1 min−1, respectively). As depicted in Fig. 7, LBF and leg 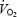 were higher during maximal cycling compared to knee-extensor exercise. However, leg
were higher during maximal cycling compared to knee-extensor exercise. However, leg 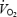 was lower during cycle compared to knee-extensor exercise when expressed per unit of work (8.8 ± 0.5 versus 12.2 ± 0.6 ml W−1 min−1, respectively; P < 0.01) or estimated active muscle mass (175 ± 9 versus 443 ± 34 ml kg−1 min−1, respectively; P < 0.01) due largely to the lower blood flow.
was lower during cycle compared to knee-extensor exercise when expressed per unit of work (8.8 ± 0.5 versus 12.2 ± 0.6 ml W−1 min−1, respectively; P < 0.01) or estimated active muscle mass (175 ± 9 versus 443 ± 34 ml kg−1 min−1, respectively; P < 0.01) due largely to the lower blood flow.
Figure 5. Leg haemodynamics during incremental exercise to exhaustion.
Leg blood flow, mean arterial pressure, leg vascular conductance, leg O2 delivery, leg a–v O2 difference and leg 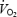 during incremental exercise to exhaustion plotted against the relative increase in power. Data are means ± s.e.m. for 10 subjects.
during incremental exercise to exhaustion plotted against the relative increase in power. Data are means ± s.e.m. for 10 subjects.
Figure 6. Relationship between blood flow, vascular conductance and O2 delivery and  .
.
Cardiac output and 2-legged blood flow, systemic and 2-legged vascular conductance, and systemic and 2-legged O2 delivery plotted against the increases in  during incremental exercise to exhaustion. Data are means ± s.e.m. for 6 subjects.
during incremental exercise to exhaustion. Data are means ± s.e.m. for 6 subjects.
Figure 7. Quadriceps muscle perfusion and  during maximal knee-extensor and cycling exercise.
during maximal knee-extensor and cycling exercise.
Maximal values for leg blood flow and  during maximal knee-extensor and cycling exercise expressed as absolute values (A), in relation to peak power (B; 103 ± 4 and 225 ± 10 W, respectively), and in relation to the estimated active muscle mass (C), assuming that all quadriceps muscles (2.9 kg) and all leg muscles (11.4 kg) were recruited during maximal knee-extensor and cycling exercise, respectively. Data are means ± s.e.m. for 10 subjects. * Different from knee-extensor exercise, P < 0.05.
during maximal knee-extensor and cycling exercise expressed as absolute values (A), in relation to peak power (B; 103 ± 4 and 225 ± 10 W, respectively), and in relation to the estimated active muscle mass (C), assuming that all quadriceps muscles (2.9 kg) and all leg muscles (11.4 kg) were recruited during maximal knee-extensor and cycling exercise, respectively. Data are means ± s.e.m. for 10 subjects. * Different from knee-extensor exercise, P < 0.05.
Discussion
This study employed two-legged cycling and one-legged knee-extension as exercise models for investigating whether O2 transport poses a limitation to aerobic power and capacity in humans. The key observations were: (1) during incremental cycling,  , LBF and O2 delivery reached a plateau at intensities below
, LBF and O2 delivery reached a plateau at intensities below  , accompanying a decline in SV and increases in central venous pressure and MAP, (2) with a blunted LBF, leg
, accompanying a decline in SV and increases in central venous pressure and MAP, (2) with a blunted LBF, leg  per unit of work declined despite the increasing O2 extraction, (3) when LBF increased linearly during knee-extensor exercise,
per unit of work declined despite the increasing O2 extraction, (3) when LBF increased linearly during knee-extensor exercise,  per unit of work was unaltered, (4) during constant load cycling, SV,
per unit of work was unaltered, (4) during constant load cycling, SV,  , and systemic O2 delivery and
, and systemic O2 delivery and  dropped before exhaustion, despite increasing or stable central venous pressure, MAP and O2 extraction, and (5) the impaired O2 supply during cycling was due to a fall in SV, as both the arterial O2 content and HR continued to increase. These results indicate that an inability of the circulatory system to sustain a linear increase in O2 delivery to the locomotor limb muscles markedly restrains aerobic power. Moreover, the impaired SV and O2 delivery during cycling together with the higher muscle
dropped before exhaustion, despite increasing or stable central venous pressure, MAP and O2 extraction, and (5) the impaired O2 supply during cycling was due to a fall in SV, as both the arterial O2 content and HR continued to increase. These results indicate that an inability of the circulatory system to sustain a linear increase in O2 delivery to the locomotor limb muscles markedly restrains aerobic power. Moreover, the impaired SV and O2 delivery during cycling together with the higher muscle  with unrestricted circulation during knee-extensor exercise support a preponderant central limitation to aerobic power and capacity in humans.
with unrestricted circulation during knee-extensor exercise support a preponderant central limitation to aerobic power and capacity in humans.
During incremental cycling exercise,  and systemic O2 delivery increased linearly to ∼80% of peak power but levelled off thereafter. Similarly, the initial linear increase in LBF and O2 delivery was attenuated at intensities above 50% of peak power, reaching a plateau at 73–88%. The tight 1: 1 relationship between systemic and locomotor limb O2 delivery versus
and systemic O2 delivery increased linearly to ∼80% of peak power but levelled off thereafter. Similarly, the initial linear increase in LBF and O2 delivery was attenuated at intensities above 50% of peak power, reaching a plateau at 73–88%. The tight 1: 1 relationship between systemic and locomotor limb O2 delivery versus  at low and moderate exercise intensities was therefore blunted at intensities above ∼80% of peak power. This novel finding refutes the theory that O2 delivery is linearly related to
at low and moderate exercise intensities was therefore blunted at intensities above ∼80% of peak power. This novel finding refutes the theory that O2 delivery is linearly related to  from rest to
from rest to  . This concept is based on linear regression analysis of: (1)
. This concept is based on linear regression analysis of: (1)  data that were not normalized (Asmussen & Nielsen, 1952; Chapman et al. 1960; Bevegård et al. 1963; Åstrand et al. 1964; Saltin, 1964; Grimsby et al. 1966), (2) haemodynamic data from longitudinal studies on human subjects undergoing changes in physical activity levels (Saltin et al. 1968), (3) cross-sectional data from subjects with different training status with the focus of the analysis on whether
data that were not normalized (Asmussen & Nielsen, 1952; Chapman et al. 1960; Bevegård et al. 1963; Åstrand et al. 1964; Saltin, 1964; Grimsby et al. 1966), (2) haemodynamic data from longitudinal studies on human subjects undergoing changes in physical activity levels (Saltin et al. 1968), (3) cross-sectional data from subjects with different training status with the focus of the analysis on whether  could explain the differences in
could explain the differences in  between well-trained and sedentary people (Ekblom & Hermansen, 1968; Ekblom, 1968), and (4) haemodynamic data in untrained humans who might have failed to attain maximal levels of exertion (Poliner et al. 1980; Higginbotham et al. 1986). Although these studies collectively documented a tight relationship between
between well-trained and sedentary people (Ekblom & Hermansen, 1968; Ekblom, 1968), and (4) haemodynamic data in untrained humans who might have failed to attain maximal levels of exertion (Poliner et al. 1980; Higginbotham et al. 1986). Although these studies collectively documented a tight relationship between  and
and  over a wide range of aerobic capacities, they provide neither insight into the relationship between systemic O2 delivery and
over a wide range of aerobic capacities, they provide neither insight into the relationship between systemic O2 delivery and  close to maximal exercise, nor specific information on the contribution of locomotor limb muscle O2 transport to
close to maximal exercise, nor specific information on the contribution of locomotor limb muscle O2 transport to  . The present findings in healthy trained subjects show that systemic and locomotor limb blood flow and O2 delivery are linearly related to
. The present findings in healthy trained subjects show that systemic and locomotor limb blood flow and O2 delivery are linearly related to  up to 50–90% of
up to 50–90% of  , levelling off before
, levelling off before  is reached. The critical consequence of the plateau in locomotor limb O2 delivery is the attenuation in the rate of rise in
is reached. The critical consequence of the plateau in locomotor limb O2 delivery is the attenuation in the rate of rise in  despite increasing O2 extraction.
despite increasing O2 extraction.
Restrictions in systemic and locomotor limb muscle O2 transport pose a more important limitation to 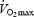 and maximal endurance capacity than suspected. In the exercising legs,
and maximal endurance capacity than suspected. In the exercising legs,  per unit of work declined from 11.9 ± 0.7 ml W−1 min−1 at 50% peak power to 8.8 ± 0.5 ml W−1 min−1 at exhaustion, suggesting that the concomitant exponential rise in leg lactate release accompanied a suppression in skeletal muscle aerobic ATP production. The estimate that leg O2 delivery and
per unit of work declined from 11.9 ± 0.7 ml W−1 min−1 at 50% peak power to 8.8 ± 0.5 ml W−1 min−1 at exhaustion, suggesting that the concomitant exponential rise in leg lactate release accompanied a suppression in skeletal muscle aerobic ATP production. The estimate that leg O2 delivery and  would have been ∼52% higher (i.e. both ∼1 l min−1) during cycling exercise if LBF increased linearly until exhaustion, illustrates the magnitude of the blunting of LBF and its effect on locomotor muscle
would have been ∼52% higher (i.e. both ∼1 l min−1) during cycling exercise if LBF increased linearly until exhaustion, illustrates the magnitude of the blunting of LBF and its effect on locomotor muscle  . Limitations in diffusive O2 transport from the muscle capillary to the mitochondrial cytochrome and/or oxidative capacity of mitochondria could also be restricting muscle
. Limitations in diffusive O2 transport from the muscle capillary to the mitochondrial cytochrome and/or oxidative capacity of mitochondria could also be restricting muscle  based on the observation that leg O2 extraction is not maximal at exhaustion (Roca et al. 1989). Yet, an increase in leg O2 extraction from the measured value of 87% to a hypothetical 100% would only increase leg
based on the observation that leg O2 extraction is not maximal at exhaustion (Roca et al. 1989). Yet, an increase in leg O2 extraction from the measured value of 87% to a hypothetical 100% would only increase leg  by 16% (i.e. ∼0.3 l min−1), although this is an overestimation given that ∼20% of the leg consists of non-muscle tissues with lower O2 extraction than contracting muscle.
by 16% (i.e. ∼0.3 l min−1), although this is an overestimation given that ∼20% of the leg consists of non-muscle tissues with lower O2 extraction than contracting muscle.
A critical question is whether the leg muscles can indeed increase  above the levels observed during maximal cycling exercise. An approach to answer this question is to determine whether quadriceps
above the levels observed during maximal cycling exercise. An approach to answer this question is to determine whether quadriceps  is elevated when systemic O2 transport is not limiting during maximal one-legged knee-extensor exercise (Andersen & Saltin, 1985). In contrast to cycling and in agreement with published reports (Andersen & Saltin, 1985; Richardson et al. 1993), leg
is elevated when systemic O2 transport is not limiting during maximal one-legged knee-extensor exercise (Andersen & Saltin, 1985). In contrast to cycling and in agreement with published reports (Andersen & Saltin, 1985; Richardson et al. 1993), leg  increased linearly during knee-extensor exercise to exhaustion in parallel with the rise in LBF. Leg
increased linearly during knee-extensor exercise to exhaustion in parallel with the rise in LBF. Leg  per unit of work at fatigue was therefore higher during knee-extensor than cycling exercise (Fig. 7). Moreover, assuming that the quadriceps femoris and all leg muscles are active during knee-extensor and cycling exercise, we estimated that
per unit of work at fatigue was therefore higher during knee-extensor than cycling exercise (Fig. 7). Moreover, assuming that the quadriceps femoris and all leg muscles are active during knee-extensor and cycling exercise, we estimated that  per kilogram of muscle was 3-fold higher during knee-extensor than cycling exercise (Richardson & Saltin 1998). Despite the uncertainty of the assumption, it is clear that the quadriceps muscles as the only muscles generating power during knee-extensor exercise, are consuming more O2 (i.e. 1.3 l min−1; 103 W; ∼2.9 kg) than they are as mere contributors to power generation during cycling (i.e. 2.0 l min−1; 225 W; ∼11.4 kg). Collectively, these observations reveal that during maximal whole body exercise: (1) locomotor muscle
per kilogram of muscle was 3-fold higher during knee-extensor than cycling exercise (Richardson & Saltin 1998). Despite the uncertainty of the assumption, it is clear that the quadriceps muscles as the only muscles generating power during knee-extensor exercise, are consuming more O2 (i.e. 1.3 l min−1; 103 W; ∼2.9 kg) than they are as mere contributors to power generation during cycling (i.e. 2.0 l min−1; 225 W; ∼11.4 kg). Collectively, these observations reveal that during maximal whole body exercise: (1) locomotor muscle 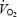 and exercise endurance could be improved if blood flow increased linearly, (2) the rates of mitochondrial oxidation and O2 transport from capillary to mitochondrial cytochrome are not maximal, and (3) convective O2 transport to contracting skeletal muscle fibres is severely restricted owing to the lower blood flow.
and exercise endurance could be improved if blood flow increased linearly, (2) the rates of mitochondrial oxidation and O2 transport from capillary to mitochondrial cytochrome are not maximal, and (3) convective O2 transport to contracting skeletal muscle fibres is severely restricted owing to the lower blood flow.
During incremental cycling,  and LBF plateaued at ∼80% peak power in parallel to a marked blunting of systemic and leg vascular conductance, indicating an enhanced sympathetic vasoconstrictor activity. This agrees with the plateau in LBF at high cycling intensities in humans (Knight et al. 1992; Rosenmeier et al. 2004) and the plateau in
and LBF plateaued at ∼80% peak power in parallel to a marked blunting of systemic and leg vascular conductance, indicating an enhanced sympathetic vasoconstrictor activity. This agrees with the plateau in LBF at high cycling intensities in humans (Knight et al. 1992; Rosenmeier et al. 2004) and the plateau in  and skeletal muscle blood flow before exhaustion in miniature swine running on a treadmill (Armstrong et al. 1987), but contrasts with the linear increase in LBF during one-legged knee-extensor exercise in humans when the systemic circulation is not compromised (Andersen & Saltin, 1985; Richardson et al. 1993). It therefore seems that during exercise with a large muscle mass, reflexes signalling the plateau in
and skeletal muscle blood flow before exhaustion in miniature swine running on a treadmill (Armstrong et al. 1987), but contrasts with the linear increase in LBF during one-legged knee-extensor exercise in humans when the systemic circulation is not compromised (Andersen & Saltin, 1985; Richardson et al. 1993). It therefore seems that during exercise with a large muscle mass, reflexes signalling the plateau in  override the local vasodilatory stimuli responsible for the partial or full linear increase in LBF with incremental cycling and knee-extensor exercise, respectively. In support of this, a human study showed that the blunting of LBF and vascular conductance at high cycling intensities is associated with an exponential rise in circulating noradrenaline outstripping the increase of the vasodilator ATP (Rosenmeier et al. 2004). It is likely that the upper body limb and postural muscles as well as the heart and respiratory muscles become more active, thereby contributing to the linear increase in systemic
override the local vasodilatory stimuli responsible for the partial or full linear increase in LBF with incremental cycling and knee-extensor exercise, respectively. In support of this, a human study showed that the blunting of LBF and vascular conductance at high cycling intensities is associated with an exponential rise in circulating noradrenaline outstripping the increase of the vasodilator ATP (Rosenmeier et al. 2004). It is likely that the upper body limb and postural muscles as well as the heart and respiratory muscles become more active, thereby contributing to the linear increase in systemic  above 80% peak power. This scenario implies that the upper body muscles and organs are competing with the exercising legs for the available
above 80% peak power. This scenario implies that the upper body muscles and organs are competing with the exercising legs for the available  (Harms et al. 1997, 1998). However, the redistribution of blood flow to upper body muscles and organs ought to be small as the plateau in
(Harms et al. 1997, 1998). However, the redistribution of blood flow to upper body muscles and organs ought to be small as the plateau in  accounts for the majority of the LBF response. On the other hand, perfusion pressure did not reduce LBF as MAP increased until exhaustion. Consequently, the suppression in blood flow to the exercising legs during cycling appears to be, for the most part, the result of the blunted
accounts for the majority of the LBF response. On the other hand, perfusion pressure did not reduce LBF as MAP increased until exhaustion. Consequently, the suppression in blood flow to the exercising legs during cycling appears to be, for the most part, the result of the blunted  and the overriding sympathetic vasoconstrictor activity to the muscle microvasculature (Pawelczyk et al. 1992).
and the overriding sympathetic vasoconstrictor activity to the muscle microvasculature (Pawelczyk et al. 1992).
The similar maximal haemodynamic responses and blunting of O2 transport before exhaustion during INC and CON provide further insight into the limits of cardiovascular regulation in exercising humans. Even though  was maintained over a longer period during constant load cycling, both types of maximal exercise were characterized by essentially the same peak
was maintained over a longer period during constant load cycling, both types of maximal exercise were characterized by essentially the same peak  (27 and 28 l min−1 for INC and CON, respectively), HR (190 and 192 beats min−1), MAP (129 and 136 mmHg), systemic O2 delivery (5.7 and 5.8 l min−1), systemic O2 extraction (84 and 87%) and
(27 and 28 l min−1 for INC and CON, respectively), HR (190 and 192 beats min−1), MAP (129 and 136 mmHg), systemic O2 delivery (5.7 and 5.8 l min−1), systemic O2 extraction (84 and 87%) and  (4.8 l min−1). This indicates that the limits of the cardiovascular system and a true
(4.8 l min−1). This indicates that the limits of the cardiovascular system and a true  were reached during both types of cycling. Five decades ago, Mitchell et al. (1958) provided data on the determinants of
were reached during both types of cycling. Five decades ago, Mitchell et al. (1958) provided data on the determinants of  . Arguing in favour of a limiting
. Arguing in favour of a limiting  , they found a lower
, they found a lower  during supramaximal compared to maximal exercise (18.2 versus 21.0 l min−1, respectively), but
during supramaximal compared to maximal exercise (18.2 versus 21.0 l min−1, respectively), but  was the same (2.81 versus 2.87 l min−1; n = 6) because of the widening of the systemic a–v O2 difference. Our study extends previous work by simultaneously looking at the dynamics of the central and exercising limb circulations, allowing an assessment of the contribution of the locomotor muscle to
was the same (2.81 versus 2.87 l min−1; n = 6) because of the widening of the systemic a–v O2 difference. Our study extends previous work by simultaneously looking at the dynamics of the central and exercising limb circulations, allowing an assessment of the contribution of the locomotor muscle to 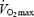 . Because LBF and
. Because LBF and  are impaired during maximal constant load cycling (González-Alonso & Calbet, 2003), we surmise that the locomotor skeletal muscles are the main tissue accounting for the restrictions in peripheral blood flow and O2 delivery during INC and CON.
are impaired during maximal constant load cycling (González-Alonso & Calbet, 2003), we surmise that the locomotor skeletal muscles are the main tissue accounting for the restrictions in peripheral blood flow and O2 delivery during INC and CON.
Within the central circulation, the impaired O2 supply was associated with a fall in SV (20–27 ml beat−1), as both arterial O2 content and HR continued to increase. This is congruent with reports during constant and incremental exercise showing a decline in SV (Keul et al. 1981; Higginbotham et al. 1986; Spina et al. 1992; Seals et al. 1994; Proctor et al. 1998; McCole et al. 1999; González-Alonso & Calbet, 2003; González-Alonso et al. 2004), but contrasts with the bulk of studies using incremental exercise showing either a plateau or an increase in SV with continuously increasing  from moderate to peak exercise (Åstrand et al. 1964; Poliner et al. 1980; Rubal et al. 1986; Spina et al. 1992; Gledhill et al. 1994; Seals et al. 1994; Fleg et al. 1994; Proctor et al. 1998). Differences in exercise protocols, levels of exertion, exercise mode, sex, age and training status might account for the discrepancy in the SV responses.
from moderate to peak exercise (Åstrand et al. 1964; Poliner et al. 1980; Rubal et al. 1986; Spina et al. 1992; Gledhill et al. 1994; Seals et al. 1994; Fleg et al. 1994; Proctor et al. 1998). Differences in exercise protocols, levels of exertion, exercise mode, sex, age and training status might account for the discrepancy in the SV responses.
The decline in SV described here could be attributed to alterations in cardiac preload, left ventricular afterload and/or left ventricular contractility (Rowell, 1974; Poliner et al. 1980; Higginbotham et al. 1986). However, a decline in preload does not seem to be a factor because in both trials central venous pressure continued to increase until exhaustion. Enhanced afterload might not be an important factor either, since SV increased early in exercise in parallel with increases in systolic blood pressure and MAP in both INC and CON and declined to a greater extent during CON when systolic blood pressure and MAP were maintained. Lastly, a depression in left ventricular contractility reducing SV is at odds with the finding that dP/dtmax at peak SV and at exhaustion were not different. The rate–pressure product of HR and MAP increased until exhaustion in both maximal tests, indicating that myocardial O2 demand was rising when SV declined. In this setting, an increase in myocardial  can only occur by an increase in O2 delivery provided by augmented coronary blood flow because the O2 extraction reserve is minimal. The impaired circulation and aerobic energy turnover in skeletal muscle raises the daunting possibility that alterations in cardiac metabolism contribute to the SV decline.
can only occur by an increase in O2 delivery provided by augmented coronary blood flow because the O2 extraction reserve is minimal. The impaired circulation and aerobic energy turnover in skeletal muscle raises the daunting possibility that alterations in cardiac metabolism contribute to the SV decline.
Alternatively, the observation that SV declined at a HR of 170–180 beats min−1 during both INC and CON raises the possibility that severe tachycardia reduces SV. Studies in humans and dogs manipulating HR by pacing the heart demonstrate that severe tachycardia leads to disproportional reductions in diastolic filling time and left ventricular end-diastolic volume which compromise SV and  (Templeton et al. 1972; Weisfeldt et al. 1978; Parke & Case, 1979; Sheriff et al. 1993). Consistent with the increase in core temperature to 39–40°C, human studies demonstrate that hyperthermia-induced tachycardia reduces SV during exercise (Fritzsche et al. 1999; González-Alonso et al. 1997, 1999, 2000a) and that blunting core hyperthermia and HR restores most of the fall in
(Templeton et al. 1972; Weisfeldt et al. 1978; Parke & Case, 1979; Sheriff et al. 1993). Consistent with the increase in core temperature to 39–40°C, human studies demonstrate that hyperthermia-induced tachycardia reduces SV during exercise (Fritzsche et al. 1999; González-Alonso et al. 1997, 1999, 2000a) and that blunting core hyperthermia and HR restores most of the fall in  evoked by heat stress (Nybo et al. 2001). Although studies independently altering HR and directly examining cardiac circulation, metabolism and function are required to determine the mechanism, it seems that the decline in SV during maximal exercise is related, at least in part, to the restriction in left ventricular filling time and left ventricular end-diastolic volume that accompanies severe tachycardia and hyperthermia.
evoked by heat stress (Nybo et al. 2001). Although studies independently altering HR and directly examining cardiac circulation, metabolism and function are required to determine the mechanism, it seems that the decline in SV during maximal exercise is related, at least in part, to the restriction in left ventricular filling time and left ventricular end-diastolic volume that accompanies severe tachycardia and hyperthermia.
In summary, the present findings in trained humans show that systemic and locomotor limb O2 delivery does not increase linearly from rest to  , but plateaus at intensities below
, but plateaus at intensities below  , resulting in the blunting of locomotor limb
, resulting in the blunting of locomotor limb  despite the increasing O2 extraction. Similarly, systemic O2 delivery and
despite the increasing O2 extraction. Similarly, systemic O2 delivery and  decline during constant load cycling despite the increasing O2 extraction. In both types of maximal exercise, the impaired systemic O2 delivery was associated with a decline in SV. The attenuation in LBF blunting leg O2 delivery and
decline during constant load cycling despite the increasing O2 extraction. In both types of maximal exercise, the impaired systemic O2 delivery was associated with a decline in SV. The attenuation in LBF blunting leg O2 delivery and  during incremental cycling appears to be largely related to the plateau in
during incremental cycling appears to be largely related to the plateau in  and an enhanced muscle sympathetic vasoconstrictor activity. In contrast to two-legged cycling, LBF and
and an enhanced muscle sympathetic vasoconstrictor activity. In contrast to two-legged cycling, LBF and  increased until volitional exhaustion during one-legged knee-extensor exercise when O2 transport was not limited. Collectively, these findings support the hypothesis that restrictions in O2 supply to locomotor limb muscles impose a limitation to aerobic power and capacity in humans.
increased until volitional exhaustion during one-legged knee-extensor exercise when O2 transport was not limited. Collectively, these findings support the hypothesis that restrictions in O2 supply to locomotor limb muscles impose a limitation to aerobic power and capacity in humans.
Acknowledgments
We give special thanks to the volunteer subjects for their enthusiasm. We also thank Peter Nissen, Troels Munch and Jacob Mørkeberg for the excellent technical assistance. This study was supported by the Gatorade Sports Science Institute and the Novo Nordisk Foundation. J.G.-A. and N.H.S. were supported by The Copenhagen Hospital System.
References
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RB, Delp MD, Goljan EF, Laughlin MH. Distribution of blood flow in muscles of miniature swine during exercise. J Appl Physiol. 1987;62:1285–1298. doi: 10.1152/jappl.1987.62.3.1285. [DOI] [PubMed] [Google Scholar]
- Asmussen E, Nielsen M. The cardiac output in rest and work determined simultaneously by the acetylene and the dye injection methods. Acta Physiol Scand. 1952;27:217–230. doi: 10.1111/j.1748-1716.1953.tb00937.x. [DOI] [PubMed] [Google Scholar]
- Åstrand PO. Experimental Studies of Physical Working Capacity in Relation to Sex and Age. Copenhagen: Munksgaard; 1952. [Google Scholar]
- Åstrand PO, Cuddy TE, Saltin B, Stenberg J. Cardiac output during submaximal and maximal work. J Appl Physiol. 1964;19:268–274. doi: 10.1152/jappl.1964.19.2.268. [DOI] [PubMed] [Google Scholar]
- Åstrand PO, Saltin B. Oxygen uptake during the first minutes of heavy muscular exercise. J Appl Physiol. 1961;16:971–976. doi: 10.1152/jappl.1961.16.6.971. [DOI] [PubMed] [Google Scholar]
- Barratt-Boyes BG, Wood EH. The oxygen saturation of blood in the venae cavae, right-heart chambers, and pulmonary vessels of healthy subjects. J Lab Clin Med. 1956;50:93–106. [PubMed] [Google Scholar]
- Bevegård S, Holmgren A, Johnsson B. Circulatory studies in well-trained athletes at rest and during heavy exercise, with special reference to stroke volume and the influence of body composition. Acta Physiol Scand. 1963;57:26–50. doi: 10.1111/j.1748-1716.1963.tb02572.x. [DOI] [PubMed] [Google Scholar]
- Chapman CB, Fisher JN, Sproule BJ. Behavior of stroke volume at rest and during exercise in human beings. J Clin Invest. 1960;39:1208–1213. doi: 10.1172/JCI104136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JR, Rossiter HB, Coats EM, Skasick A, Whipp BJ. The maximally attainable VO2 during exercise in humans: the peak vs. maximum issue. J Appl Physiol. 2003;95:1901–1907. doi: 10.1152/japplphysiol.00024.2003. [DOI] [PubMed] [Google Scholar]
- Ekblom B. Effect of physical training on oxygen transport system in man. Acta Physiol Scand Suppl. 1968;328:1–45. [PubMed] [Google Scholar]
- Ekblom B, Hermansen L. Cardiac output in athletes. J Appl Physiol. 1968;25:619–625. doi: 10.1152/jappl.1968.25.5.619. [DOI] [PubMed] [Google Scholar]
- Ekelund LG, Holmgren A. Central hemodynamics during exercise. Circ Res. 1967;21(Suppl. 1):I33–I43. [Google Scholar]
- Fleg JL, Schulman SP, O'Connor FC, Gerstenblith G, Becker LC, Fortney S, Goldberg AP, Lakatta EG. Cardiovascular responses to exhaustive upright cycle exercise in highly trained older men. J Appl Physiol. 1994;77:1500–1506. doi: 10.1152/jappl.1994.77.3.1500. [DOI] [PubMed] [Google Scholar]
- Fritzsche RG, Switzer TW, Hodgkinson BJ, Coyle EF. Stroke volume decline during prolonged exercise is influenced by the increase in heart rate. J Appl Physiol. 1999;86:799–805. doi: 10.1152/jappl.1999.86.3.799. [DOI] [PubMed] [Google Scholar]
- Froelicher VF, Brammell H, Davis G, Noguera I, Stewart A, Lancaster MC. A comparison of three maximal treadmill exercise protocols. J Appl Physiol. 1972;36:720–725. doi: 10.1152/jappl.1974.36.6.720. [DOI] [PubMed] [Google Scholar]
- Gledhill N, Cox D, Jamnik R. Endurance athletes' stroke volume does not plateau: major advantage is diastolic function. Med Sci Sports Exerc. 1994;26:1116–1121. [PubMed] [Google Scholar]
- González-Alonso J, Calbet J. Reductions in systemic and skeletal muscle blood flow and oxygen delivery limit maximal aerobic capacity in humans. Circulation. 2003;107:824–830. doi: 10.1161/01.cir.0000049746.29175.3f. [DOI] [PubMed] [Google Scholar]
- González-Alonso J, Dalsgaard MK, Osada T, Volianitis S, Dawson EA, Yoshiga CC, Secher NH. Brain and central haemodynamics and oxygenation during maximal exercise in humans. J Physiol. 2004;557:331–342. doi: 10.1113/jphysiol.2004.060574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Alonso J, Mora-Rodríguez R, Coyle EF. Dehydration markedly impairs cardiovascular function in hyperthermic endurance athletes during exercise. J Appl Physiol. 1997;82:1229–1236. doi: 10.1152/jappl.1997.82.4.1229. [DOI] [PubMed] [Google Scholar]
- González-Alonso J, Mora-Rodríguez R, Coyle EF. Stroke volume during exercise: interaction of environment and hydration. Am J Physiol. 2000a;278:H321–H330. doi: 10.1152/ajpheart.2000.278.2.H321. [DOI] [PubMed] [Google Scholar]
- González-Alonso J, Quistorff B, Krustrup P, Bangsbo J, Saltin B. Heat production in human skeletal muscle at onset of intense dynamic exercise. J Physiol. 2000b;524:603–615. doi: 10.1111/j.1469-7793.2000.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Alonso J, Teller C, Andersen SL, Jensen FB, Hyldig T, Nielsen B. Influence of body temperature on the development of fatigue during prolonged exercise in the heat. J Appl Physiol. 1999;86:1032–1039. doi: 10.1152/jappl.1999.86.3.1032. [DOI] [PubMed] [Google Scholar]
- Grimsby G, Nilsson NJ, Saltin B. Cardiac output during submaximal and maximal exercise in active middle-aged athletes. J Appl Physiol. 1966;21:1150–1156. doi: 10.1152/jappl.1966.21.4.1150. [DOI] [PubMed] [Google Scholar]
- Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Dempsey JA. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol. 1997;82:1573–1583. doi: 10.1152/jappl.1997.82.5.1573. [DOI] [PubMed] [Google Scholar]
- Harms CA, Wetter TJ, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Hanson P, Dempsey JA. Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J Appl Physiol. 1998;85:609–618. doi: 10.1152/jappl.1998.85.2.609. [DOI] [PubMed] [Google Scholar]
- Higginbotham MB, Morris KG, Williams RS, McHale PA, Coleman RE, Cobb FR. Regulation of stroke volume during submaximal and maximal upright exercise in normal man. Circ Res. 1986;58:281–291. doi: 10.1161/01.res.58.2.281. [DOI] [PubMed] [Google Scholar]
- Hill AV, Lupton H. Muscular exercise, lactic acid, and the supply and utilization of oxygen. Q J Med. 1923;16:135–171. [Google Scholar]
- Holmgren A. Circulatory changes during muscular work in man: with special reference to arterial and central venous pressures in the systemic circulation. Scand J Clin Lab Invest. 1956;8(Suppl. 24):1–97. [PubMed] [Google Scholar]
- Keul J, Dickhuth HH, Simon G, Lehmann M. Effect of static and dynamic exercise on heart volume, contractility, and left ventricular dimensions. Circ Res. 1981;48:I162–I170. [PubMed] [Google Scholar]
- Knight DR, Poole DC, Schaffartzik W, Gay HJ, Prediletto R, Hogan MC, Wagner P. Relationship between body and leg VO2 during maximal cycle ergometry. J Appl Physiol. 1992;73:1114–1121. doi: 10.1152/jappl.1992.73.3.1114. [DOI] [PubMed] [Google Scholar]
- McCole SD, Brown MD, Moore GE, Zmuda JM, Cwynar JD, Hagberg JM. Cardiovascular hemodynamics with increasing exercise intensities in postmenopausal women. J Appl Physiol. 1999;87:2334–2340. doi: 10.1152/jappl.1999.87.6.2334. [DOI] [PubMed] [Google Scholar]
- Meyers J, Walsh D, Sullivan M, Froelicher V. Effect of sampling on variability and plateau in oxygen uptake. J Appl Physiol. 1990;68:404–410. doi: 10.1152/jappl.1990.68.1.404. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Sproule BJ, Chapman CB. The physiological meaning of the maximal oxygen intake test. J Clin Invest. 1958;37:538–547. doi: 10.1172/JCI103636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nybo L, Jensen T, Nielsen B, González-Alonso J. Effects of hyperthermia with and without dehydration on VO2 kinetics during intense exercise. J Appl Physiol. 2001;90:1057–1064. doi: 10.1152/jappl.2001.90.3.1057. [DOI] [PubMed] [Google Scholar]
- Parke JO, Case RB. Normal left ventricular function. Circulation. 1979;60:4–12. doi: 10.1161/01.cir.60.1.4. [DOI] [PubMed] [Google Scholar]
- Pawelczyk JA, Hanel B, Pawelczyk RA, Warberg J, Secher NH. Leg vasoconstriction during dynamic exercise with reduced cardiac output. J Appl Physiol. 1992;73:1838–1846. doi: 10.1152/jappl.1992.73.5.1838. [DOI] [PubMed] [Google Scholar]
- Poliner LR, Dehmer GJ, Lewis SE, Parkey RW, Blomqvist CG, Willerson JT. Left ventricular performance in normal subjects: a comparison of the responses to exercise in the upright and supine positions. Circulation. 1980;62:528–534. doi: 10.1161/01.cir.62.3.528. [DOI] [PubMed] [Google Scholar]
- Pollock ML, Bohannon RL, Cooper KH, Ayres JJ, Ward A, White SR, Linnerud AC. A comparative analysis of four protocols for maximal treadmill stress testing. Am Heart J. 1976;92:39–46. doi: 10.1016/s0002-8703(76)80401-2. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Beck KC, Shen PH, Eickhoff TJ, Halliwill JR, Joyner MJ. Influence of age and gender on cardiac output-VO2 relationships during submaximal cycle ergometry. J Appl Physiol. 1998;84:599–605. doi: 10.1152/jappl.1998.84.2.599. [DOI] [PubMed] [Google Scholar]
- Reeves JT, Groves BM, Cymerman A, Sutton JR, Wagner PD, Turkevich D, Houston CS. Operation Everest II: cardiac filling pressures during cycle exercise at sea level. Resp Physiol. 1990;80:147–154. doi: 10.1016/0034-5687(90)90078-d. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Poole DC, Knight DR, Kurdak SS, Hogan MC, Grassi B, Johnson EC, Kendrick KF, Erickson BK, Wagner PD. High muscle blood flow in man: is maximal O2 extraction compromised? J Appl Physiol. 1993;75:1911–1926. doi: 10.1152/jappl.1993.75.4.1911. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Saltin B. Human muscle blood flow and metabolism studied in the isolated quadricepes muscles. Med Sci Sports Exerc. 1998;30:28–33. doi: 10.1097/00005768-199801000-00005. [DOI] [PubMed] [Google Scholar]
- Roca J, Hogan MC, Story D, Bebout DE, Haab P, Gonzalez R, Ueno O, Wagner PD. Evidence for tissue diffusion limitation of VO2max in normal humans. J Appl Physiol. 1989;67:291–299. doi: 10.1152/jappl.1989.67.1.291. [DOI] [PubMed] [Google Scholar]
- Rosenmeier JB, Hansen J, González-Alonso J. Circulating ATP-induced vasodilatation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J Physiol. 2004;558:351–365. doi: 10.1113/jphysiol.2004.063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev. 1974;54:75–159. doi: 10.1152/physrev.1974.54.1.75. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Human Cardiovascular Control. New York: Oxford University Press; 1993. [Google Scholar]
- Rowell LB, O'Leary DS, Kellogg DL., Jr . Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems, chap. 17. American Physiological Society, Bethesda, USA: 1996. Integration of cardiovascular control systems in dynamic exercise; pp. 770–838. [Google Scholar]
- Rowell LB, Taylor HL, Wang Y. Limitations to prediction of maximal oxygen intake. J Appl Physiol. 1964;19:919–927. doi: 10.1152/jappl.1964.19.5.919. [DOI] [PubMed] [Google Scholar]
- Rubal BJ, Moody JM, Damore S, Bunker SR, Diaz NM. Left ventricular performance of the athletic heart during upright exercise: a heart rate-controlled study. Med Sci Sports Exerc. 1986;18:134–140. [PubMed] [Google Scholar]
- Saltin B. Circulatory response to submaximal and maximal exercise after thermal dehydration. J Appl Physiol. 1964;19:1125–1132. doi: 10.1152/jappl.1964.19.6.1125. [DOI] [PubMed] [Google Scholar]
- Saltin B, Blomqvist G, Mitchell JH, Johnson RL, Jr, Wildenthal K, Chapman CB. Response to exercise after bed rest and after training. Circulation. 1968;38(5 Suppl.):VII1–78. [PubMed] [Google Scholar]
- Seals DR, Hagberg JM, Spina RJ, Rogers MA, Schechtman KB, Ehsani AA. Enhanced left ventricular performance in endurance trained older men. Circulation. 1994;89:198–205. doi: 10.1161/01.cir.89.1.198. [DOI] [PubMed] [Google Scholar]
- Sheriff DD, Zhou XP, Scher AM, Rowell LB. Dependence of cardiac filling pressure on cardiac output during rest and dynamic exercise in dogs. Am J Physiol. 1993;265:H316–H322. doi: 10.1152/ajpheart.1993.265.1.H316. [DOI] [PubMed] [Google Scholar]
- Spina RJ, Ogawa T, Martin WH, 3rd, Coggan AR, Holloszy JO, Ehsani AA. Exercise training prevents decline in stroke volume during exercise in young healthy subjects. J Appl Physiol. 1992;72:2458–2462. doi: 10.1152/jappl.1992.72.6.2458. [DOI] [PubMed] [Google Scholar]
- Taylor HL, Buskirk E, Henschel A. Maximal oxygen intake as an objective measure of cardio-respiratory performance. J Appl Physiol. 1955;8:73–80. doi: 10.1152/jappl.1955.8.1.73. [DOI] [PubMed] [Google Scholar]
- Templeton GH, Ecker RR, Mitchel JH. Left ventricular stiffness during diastole and systole: the influence of changes in volume and inotropic state. Cardiovasc Res. 1972;6:95–100. doi: 10.1093/cvr/6.1.95. [DOI] [PubMed] [Google Scholar]
- Weisfeldt ML, Frederiksen JW, Yin FCP, Weiss JL. Evidence of incomplete left ventricular relaxation in the dog. J Clin Invest. 1978;62:1296–1302. doi: 10.1172/JCI109250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whipp BJ, Wasserman K. Oxygen kinetics for various intensities of constant-load exercise. J Appl Physiol. 1972;33:351–356. doi: 10.1152/jappl.1972.33.3.351. [DOI] [PubMed] [Google Scholar]









