Abstract
The effects of changes in shivering intensity on the relative contributions of plasma glucose, muscle glycogen, lipids and proteins to total heat production are unclear in humans. The goals of this study were: (1) to determine whether plasma glucose starts playing a more prominent role as shivering intensifies, (2) to quantify overall changes in fuel use in relation to the severity of cold exposure, and (3) to establish whether the fuel selection pattern of shivering is different from the classic fuel selection pattern of exercise. Using a combination of indirect calorimetry and stable isotope methodology, fuel metabolism was monitored in non-acclimatized adult men exposed for 90 mins to 10°C (low-intensity shivering (L)) or 5°C (moderate-intensity shivering (M)). Results show that plasma glucose oxidation is strongly stimulated by moderate shivering (+122% from L to M), but the relative contribution of this pathway to total heat generation always remains minor (< 15% of total heat production). Instead, muscle glycogen is responsible for most of the increase in heat production between L and M. By itself, the increase in CHO oxidation is responsible for the 100 W increase in metabolic rate observed between L and M, because rates of lipid and protein oxidation remain constant. This high reliance on CHO is not compatible with the well known fuel selection pattern of exercise, when considering the relatively low metabolic rates elicited by shivering (∼30% 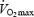 for M). We conclude that shivering and exercise of similar energy requirements appear to be supported by different fuel mixtures. Investigating the physiological mechanisms underlying why a muscle producing only heat (shivering), or significant movement (exercise), shows a different pattern of fuel selection at the same power output strikes us as a fascinating area for future research.
for M). We conclude that shivering and exercise of similar energy requirements appear to be supported by different fuel mixtures. Investigating the physiological mechanisms underlying why a muscle producing only heat (shivering), or significant movement (exercise), shows a different pattern of fuel selection at the same power output strikes us as a fascinating area for future research.
In humans exposed to cold, carbohydrates (CHO) can provide up to 60% of all the heat generated (Haman et al. 2002), and two separate sources of CHO are available for thermogenesis: muscle glycogen and plasma glucose. During low-intensity shivering, we have recently shown that muscle glycogen is the dominant source of CHO, providing at least 3 times more glucosyl units than plasma glucose (Haman et al. 2002). The importance of plasma glucose also remains minor when the size of glycogen stores is experimentally increased or decreased (Haman et al. 2004c). However, indirect evidence suggests that circulating glucose may start playing a more important role at the higher metabolic rates necessary to survive during more extreme cold exposure. Using a continuous infusion of labelled glucose, Tipton et al. (1997) showed that the rate of disappearance (Rd,Glu) increases by as much as 2-fold between low- and moderate-intensity shivering. However, the exact fate of glucose leaving the circulation is still unclear because Rd,Glu measurements cannot discriminate between oxidative and non-oxidative disposal. A large increase in plasma glucose utilization would not be surprising because much higher rates of oxidation can be elicited by exercise (6–8 mg kg−1 min−1) than observed during low-intensity shivering (1.2 mg kg−1 min−1) (Coggan et al. 1990; Bosch, 1993; Bosch et al. 1994, 1996; Friedlander et al. 1997; Péronnet et al. 1998; Jeukendrup et al. 1999; Van Loon et al. 2001; Haman et al. 2002). Direct measurements of plasma glucose oxidation are needed to establish whether this fuel plays a more important thermogenic role when shivering intensifies. Therefore, the main goal of this study is to quantify the contribution of plasma glucose to total heat production during moderate-intensity shivering (3.5 times resting metabolic rate (RMR) or 60% of maximal shivering 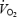 (Shivpeak)) and to compare our results with values previously obtained during low-intensity shivering (2.5 times RMR or 40% Shivpeak) (Haman et al. 2002). In view of the changes in Rd,Glu previously reported by Tipton et al. (1997), we hypothesize that the absolute rate of plasma glucose oxidation as well as the relative contribution of this fuel to total heat production will increase between low- (L) and moderate-intensity shivering (M).
(Shivpeak)) and to compare our results with values previously obtained during low-intensity shivering (2.5 times RMR or 40% Shivpeak) (Haman et al. 2002). In view of the changes in Rd,Glu previously reported by Tipton et al. (1997), we hypothesize that the absolute rate of plasma glucose oxidation as well as the relative contribution of this fuel to total heat production will increase between low- (L) and moderate-intensity shivering (M).
This paper also addresses another important issue: how does overall fuel selection vary in relation to the severity of cold exposure? The effect of shivering intensity on the relative use of CHO, lipids and proteins has never been determined (Weller et al. 1998; Haman et al. 2002). Over the last two decades, a number of studies have reported rates of CHO and lipid oxidation for low-intensity shivering (Vallerand & Jacobs, 1989, 1990; Vallerand et al. 1989, 1993, 1995, 1999; MacNaughton et al. 1990; Glickman-Weiss et al. 1993, 1994; Weller et al. 1998; Haman et al. 2002) or moderate-intensity shivering (Martineau & Jacobs, 1988, 1989a; Tikuisis et al. 2000, 2002), but only a handful have accounted for proteins (Vallerand et al. 1995; Haman et al. 2002). Even though the contribution of proteins is generally thought to be minor (< 10%), the reported dominance of either CHO or lipids remains ambiguous (Weller et al. 1998; Haman et al. 2002). This study is the first to compare rates of CHO, lipid and protein oxidation at low and moderate shivering intensities (40 and 60% Shivpeak). If the fuel selection patterns of shivering and exercise are identical, we would anticipate that lipids should dominate at all shivering intensities because extreme cold exposure can only elicit low metabolic rates compared to exercise (< 5 RMR or ∼40% 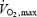 ).
).
Methods
Subjects
Eight healthy men volunteered for this study. It was approved by the Health Sciences Ethical Committee of the University of Ottawa, and written consent was obtained from the participants. Physical characteristics of the subjects are presented in Table 1. Morphological measurements (height, weight, percentage body fat) and maximal oxygen consumption (ramp treadmill protocol) were obtained 5–7 days before the experiments.
Table 1.
Physical characteristics of subjects for L and M
| L | M | |
|---|---|---|
| Age (years) | 24.7 ± 1.5 | 23.3 ± 0.4 |
| Body mass (kg) | 78.1 ± 4.8 | 71.7 ± 3.0 |
| Height (cm) | 178.2 ± 2.5 | 174.0 ± 1.0 |
| Body surface area (m2) | 2.0 ± 0.1 | 1.9 ± 0.1 |
| Percent body fat* (%) | 13.3 ± 1.9 | 12.7 ± 0.8 |
| Estimated Shivpeak*† (ml kg−1 min−1) | 22.1 ± 1.9 | 22.2 ± 1.2 |
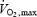 ‡ (ml kg−1 min−1) ‡ (ml kg−1 min−1) |
56.4 ± 2.9 | 53.1 ± 2.5 |
Underwater weighing; Brosek et al. (1963).
From Eyolfson et al. (2001); Shivpeak = 30.5 + (0.348 × 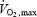 ) − (0.909 × BMI) − (0.233 × age), where
) − (0.909 × BMI) − (0.233 × age), where 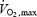 is in ml kg−1 min−1, BMI is the body mass index.
is in ml kg−1 min−1, BMI is the body mass index.
Progressive treadmill test.
Experimental protocol
Experiments were conducted between 08.00 h and 12.00 h, following 36-h without heavy physical activity. The last evening meal was standardized (∼4150 kJ (∼990 kcal), ∼52% CHO, ∼18% lipids, ∼30% proteins) and subjects were asked to report to the laboratory at 08.00 h the next morning after a 12–14 h fast. Ingestion of carbohydrates from plants naturally rich in 13C (C4 photosynthetic cycle) was avoided to maintain low 13C background enrichment in plasma glucose and expired CO2. Upon their arrival in the laboratory, subjects wearing only shorts were instrumented with thermal probes and an indwelling catheter (18G, 32 mm, Medical Inc., Arlington, TX, USA) was placed in an antecubital vein for blood sampling (left arm). After emptying their bladder, subjects were fitted with a ‘liquid-conditioned suit’ (LCS: One-piece CORETEC, Delta Temax, Inc., Pembroke, ON, Canada) and remained seated for 120 min at ambient conditions (25.5 ± 0.2°C, 759 ± 2 mmHg, 45 ± 4% relative humidity). Following this habituation period, subjects were transferred to an environmental chamber (5.7 ± 0.1°C, 759 ± 2 mmHg, 69 ± 2% relative humidity) and water at 5°C was perfused through the LCS using a temperature-controlled circulation bath (Endocal, NESLAB, Thermo Electron Corporation; and Model 200-00, Micropump Inc., Vancouver, WA, USA). Thermal response, metabolic rate and fuel utilization rates were measured at 26°C and during the next 90 min at 5°C.
Thermal response
Changes in heat production 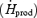 , and oesophageal (Tes), and mean skin temperatures
, and oesophageal (Tes), and mean skin temperatures 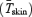 were determined as previously described (Haman et al. 2002).
were determined as previously described (Haman et al. 2002). 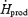 was calculated by indirect respiratory calorimetry corrected for protein oxidation (see below). Relative shivering intensity (%Shivpeak) was determined by dividing
was calculated by indirect respiratory calorimetry corrected for protein oxidation (see below). Relative shivering intensity (%Shivpeak) was determined by dividing 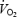 (ml kg−1 min−1) in the cold by the estimated shivering peak value, as described by Eyolfson et al. (2001). Tes and
(ml kg−1 min−1) in the cold by the estimated shivering peak value, as described by Eyolfson et al. (2001). Tes and 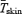 were monitored continuously prior to and during cold exposure using a paediatric oesophageal probe (Mon-a-therm general purpose, Mallinckrodt Medical Inc, St Louis, MO, USA) and heat flux transducers (area-weighed equation from 12 sites: forehead, chest, biceps, forearm, abdomen, lower and upper back, front and back calf, quadriceps, hamstrings and finger tip (Dubois & Dubois, 1916)).
were monitored continuously prior to and during cold exposure using a paediatric oesophageal probe (Mon-a-therm general purpose, Mallinckrodt Medical Inc, St Louis, MO, USA) and heat flux transducers (area-weighed equation from 12 sites: forehead, chest, biceps, forearm, abdomen, lower and upper back, front and back calf, quadriceps, hamstrings and finger tip (Dubois & Dubois, 1916)).
Metabolic rate and fuel utilization
Ventilation 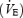 , oxygen consumption
, oxygen consumption 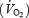 and carbon dioxide production
and carbon dioxide production 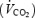 were measured by open-circuit spirometry (250 l, chain-compensated gasometer, Warren Collins Inc., Braintree, MA, USA) as described by Haman et al. (2002). Oxygen and carbon dioxide concentrations in dry expired gases were determined using calibrated electrochemical gas analysers (AMETEK Model S-3 A/1 and CD 3 A, Applied Electrochemistry, Pittsburg, PA, USA). Total protein (RPox), carbohydrate (RGox) and lipid (RFox) oxidation rates (in g min−1) were calculated as previously described (Haman et al. 2002, 2004c):
were measured by open-circuit spirometry (250 l, chain-compensated gasometer, Warren Collins Inc., Braintree, MA, USA) as described by Haman et al. (2002). Oxygen and carbon dioxide concentrations in dry expired gases were determined using calibrated electrochemical gas analysers (AMETEK Model S-3 A/1 and CD 3 A, Applied Electrochemistry, Pittsburg, PA, USA). Total protein (RPox), carbohydrate (RGox) and lipid (RFox) oxidation rates (in g min−1) were calculated as previously described (Haman et al. 2002, 2004c):
where 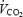 (l min−1) and
(l min−1) and 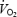 (l min−1) were corrected for the volumes of O2 and CO2 corresponding to protein oxidation (1.010 and 0.843 l g−1, respectively).
(l min−1) were corrected for the volumes of O2 and CO2 corresponding to protein oxidation (1.010 and 0.843 l g−1, respectively).
RPox was estimated from urinary urea excretion (UREAurine) in urine samples collected for 120 min during the habituation period, and 90 min in the cold. Urinary concentrations were determined on a Synchron Clinical System (CX7, Beckman, Anaheim, CA, USA). Energy potentials of 16.3 kJ g−1 (carbohydrates), 40.8 kJ g−1 (lipids), and 19.7 kJ g−1 (proteins) were used to calculate the relative contributions of each fuel to total heat production (Elia, 1991; Péronnet & Massicotte, 1991).
Plasma glucose oxidation
The rate of plasma glucose oxidation was estimated by repeated [13C]glucose ingestions (Haman et al. 2002, 2004c). On the evening before the experiment, 6.4 ± 0.5 g of glucose (natural enrichment 13C/C = 0.01098) was diluted in 700 ml water, and further enriched with [U-13C]glucose (13C/C > 99%, Isotec, Miamisburg, OH, USA) to obtain a final 13C/C ratio of 3.17 ± 0.42 (Rexo). The solution was divided into seven equal doses. Following the collection of gases and blood samples for the preingestion measurement of natural 13C/C in plasma and expired CO2 (t =−120 min), subjects ingested the first dose of exogenous [13C]glucose. Subsequent doses were taken every 30 min until the end of the experiment. Blood samples (7 ml each) and expired gas samples (10 ml) were taken just before ingestion of each [13C] glucose dose. Upon collection, blood samples were placed on ice and spun in a refrigerated centrifuge. Plasma was separated and stored at −20°C until analysed.
Plasma glucose was isolated by double-bed ion exchange chromatography with superimposed columns (resins: AG 50 W-X8 H+, 200–400 mesh, and AG 1-X8 chloride, 200–400 mesh). Following evaporation, glucose was combusted (60 min at 400°C) in the presence of CuO, and CO2 was recovered. Measurements of 13C/12C in expired CO2 (Rexp), and in CO2 obtained from glucose combustion (Rglu) were determined in a Prism mass spectrometer (Micromass, Manchester, UK). Isotopic composition was expressed as percentage 13C/C.
The rate of plasma glucose oxidation (RGox-plasma) was calculated from 13CO2 production at the mouth and plasma glucose isotopic composition (Fig. 1) using the following equation (Wolfe, 1992; Derman et al. 1996):
where 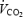 is in l min−1 (STPD), Rref-exp is the isotopic composition of expired CO2 prior to the ingestion of the first [13C] glucose dose, Rref-glu is the isotopic composition of plasma glucose before ingestion of the first [13C]glucose dose, k1 (0.7426 l g−1) is the volume of CO2 produced from the complete oxidation of glucose, and k2 is the fractional recovery (at the mouth) of CO2 produced in tissues (Leijssen & Elia, 1996). Fractional 13CO2 recovery values (k2) of 0.8 and 1 were used before and during cold exposure, respectively (Wolfe, 1992). RGox-plasma was corrected to account for the fraction of plasma glucose oxidized from exogenous sources (Haman et al. 2002). Oxidation of glucose derived from muscle glycogen stores (RGox-muscle in g min−1), directly or through the lactate shuttle (Brooks et al. 1999), was calculated by subtracting RGox-plasma from RGox.
is in l min−1 (STPD), Rref-exp is the isotopic composition of expired CO2 prior to the ingestion of the first [13C] glucose dose, Rref-glu is the isotopic composition of plasma glucose before ingestion of the first [13C]glucose dose, k1 (0.7426 l g−1) is the volume of CO2 produced from the complete oxidation of glucose, and k2 is the fractional recovery (at the mouth) of CO2 produced in tissues (Leijssen & Elia, 1996). Fractional 13CO2 recovery values (k2) of 0.8 and 1 were used before and during cold exposure, respectively (Wolfe, 1992). RGox-plasma was corrected to account for the fraction of plasma glucose oxidized from exogenous sources (Haman et al. 2002). Oxidation of glucose derived from muscle glycogen stores (RGox-muscle in g min−1), directly or through the lactate shuttle (Brooks et al. 1999), was calculated by subtracting RGox-plasma from RGox.
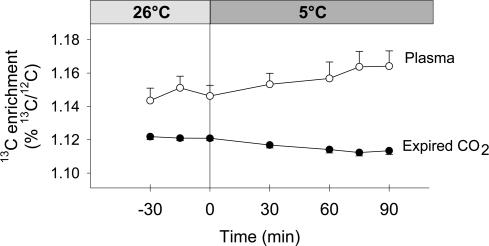
Figure 1. Isotopic enrichment (%13C/12C) of expired CO2 (•) and plasma glucose (○) at 26°C and during moderate-intensity shivering (at 5°C, M).
Values for low-intensity shivering (L) are presented in Haman et al. (2002).
Blood analysis
Plasma glucose and lactate concentrations were measured spectrophotometrically at 340 nm on a Beckman DU 640 (Bergmeyer, 1985). Total plasma non-esterified fatty acid (NEFA) concentration was determined using a kit (NEFA C, Wako Chemicals, Osaka, Japan) and insulin concentration was measured using a radioimmunoassay (no. KTSP-11001, Medicorp Inc, Montréal, Quebéc, Canada).
Statistical analyses
Thermal and metabolic parameters measured in adult male subjects exposed to 5°C (M; n = 8; this study) and to 10°C (L; n = 6; from a previous study (Haman et al. 2002)) were compared. Shivering values previously published for 10°C in Haman et al. (2002) were reported for 90–120 min of cold exposure. Here, we have used shivering values for 60–90 min at 10°C for our comparison with shivering values at 5°C to standardize cold exposure duration between intensities. Although different subjects were used in L and M, care was taken to normalize for subjects' age, morphology and body composition (Table 1). In addition, all experiments were conducted at the same time of day (08.00–12.00 h) in 12–14 h postabsorptive men having eaten a standardized evening meal.
Changes in 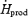 , Tes,
, Tes, 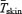 , fuel selection and blood metabolite concentrations were assessed by two-way analysis of variance for repeated measures. For each sampling time, a Bonferroni t test was used to detect differences with control values (before cold exposure). Differences in
, fuel selection and blood metabolite concentrations were assessed by two-way analysis of variance for repeated measures. For each sampling time, a Bonferroni t test was used to detect differences with control values (before cold exposure). Differences in 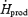 , oxidation rates of CHO (RGox-plasma, RGox-muscle), lipids (RFox) and proteins (RPox), and plasma metabolite concentrations over the last 30 min of cold exposure were determined using a one-way analysis of variance to verify the main effect of shivering intensity (L versus M). Statistical differences were considered significant when P≤ 0.05. All values given are means ± standard error of the mean (s.e.m.).
, oxidation rates of CHO (RGox-plasma, RGox-muscle), lipids (RFox) and proteins (RPox), and plasma metabolite concentrations over the last 30 min of cold exposure were determined using a one-way analysis of variance to verify the main effect of shivering intensity (L versus M). Statistical differences were considered significant when P≤ 0.05. All values given are means ± standard error of the mean (s.e.m.).
Results
Thermal response
Changes in 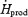 , Tes and
, Tes and 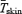 are presented in Fig. 2. By the end of cold exposure,
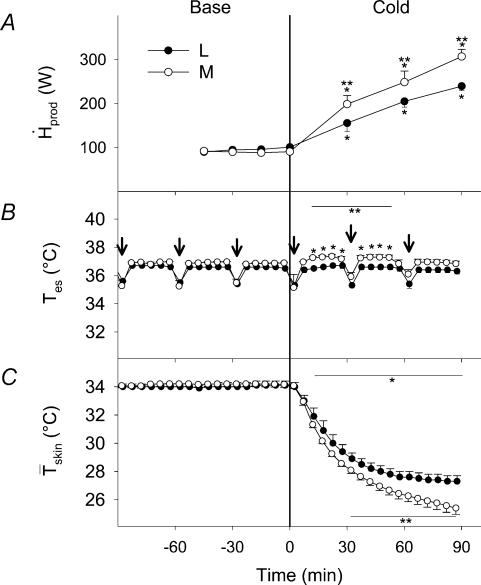
are presented in Fig. 2. By the end of cold exposure, 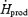 increased 2.6-fold for L (from 95.3 ± 2.2 to 243.8 ± 4.2 W) and 3.5-fold for M (from 89.5 ± 3.5 to 292.1 ± 18.2 W) (Fig. 2A). Shivering intensity was equivalent to 42.0 ± 3.4% Shivpeak (L) and 57.4 ± 3.1% Shivpeak (M). Tes remained constant at 36.4 ± 0.1°C for L throughout cold exposure, whereas a slight transient increase was observed for M in the first 60 min (from 36.8 ± 0.2°C at baseline to 37.0 ± 0.1°C after 60 min in the cold) (Fig. 2B).
increased 2.6-fold for L (from 95.3 ± 2.2 to 243.8 ± 4.2 W) and 3.5-fold for M (from 89.5 ± 3.5 to 292.1 ± 18.2 W) (Fig. 2A). Shivering intensity was equivalent to 42.0 ± 3.4% Shivpeak (L) and 57.4 ± 3.1% Shivpeak (M). Tes remained constant at 36.4 ± 0.1°C for L throughout cold exposure, whereas a slight transient increase was observed for M in the first 60 min (from 36.8 ± 0.2°C at baseline to 37.0 ± 0.1°C after 60 min in the cold) (Fig. 2B). 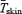 decreased by 20% for L (from 34.0 ± 0.02 to 27.2 ± 0.02°C) and by 25% for M (from 34.2 ± 0.2°C to 25.8 ± 0.4°C) during cold exposure (Fig. 2C).
decreased by 20% for L (from 34.0 ± 0.02 to 27.2 ± 0.02°C) and by 25% for M (from 34.2 ± 0.2°C to 25.8 ± 0.4°C) during cold exposure (Fig. 2C).
Figure 2. Heat production (A,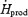 ), and oesophageal (B, Tes) and mean skin (C,
), and oesophageal (B, Tes) and mean skin (C,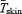 ) temperature before (Base) and during cold exposure for L (•) and M (○).
) temperature before (Base) and during cold exposure for L (•) and M (○).
Arrows indicate the times at which [13C]glucose solutions were ingested. *Significantly different from control values before cold exposure **Significantly different from L.
Metabolic response and fuel utilization
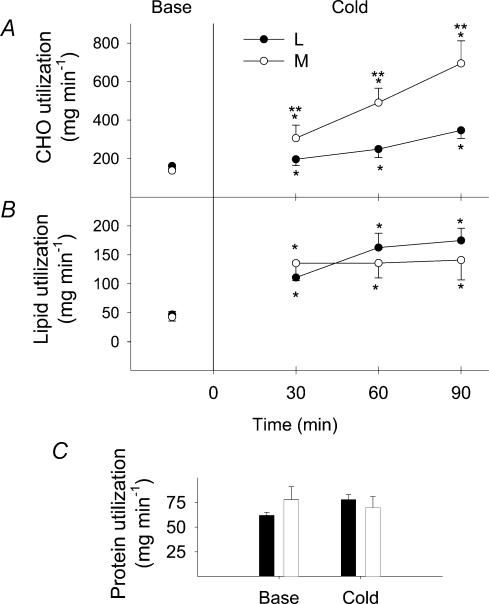
Changes in absolute rates of oxidation for CHO (RGox), lipids (RFox) and proteins (RPox) are shown in Fig. 3 for both shivering intensities. In response to cold exposure, RGox increased by 2.2-fold for L (from 165 ± 9 to 358 ± 41 mg min−1) and by 5-fold for M (from 138 ± 12 to 694 ± 118 mg min−1) (Fig. 3A). RFox increased by 3.8-fold for L (from 39 ± 2 to 175 ± 21 mg min−1) and by 3.3-fold for M (from 43 ± 5 to 141 ± 34 mg min−1) (Fig. 3B). In contrast, RPox remained unaffected by cold exposure at both shivering intensities, averaging 62 ± 3 and 76 ± 13 mg min−1 at baseline and 78 ± 5 and 70 ± 11 mg min−1 during cold exposure for L and M, respectively (Fig. 3C). In the last 30 min of cold exposure, no significant difference between shivering intensities was found for RFox and RPox, but RGox was 1.9-fold higher in M compared to L.
Figure 3. Absolute carbohydrate (A, CHO), lipid (B) and protein (C) utilization rates before (Base) and during cold exposure for L (•) and M (○).
*Significantly different from control values before cold exposure **Significantly different from L.
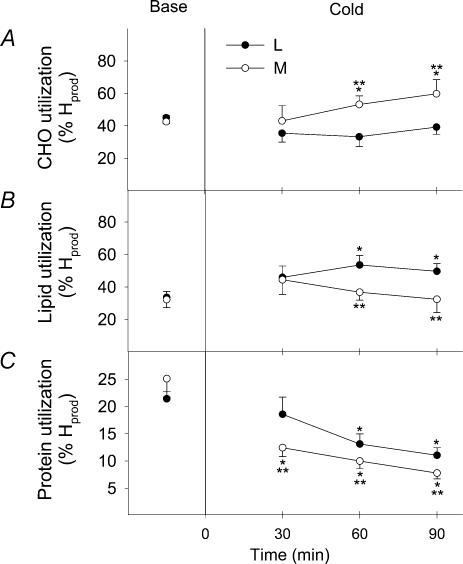
Changes in the relative contributions of CHO (%RGox), lipids (%RFox) and proteins (%RPox) to total heat production are shown in Fig. 4 for both shivering intensities. Before cold exposure, the fuel selection patterns of L and M were the same. %RGox was not affected by cold exposure in L, but showed a 1.4-fold increase in M, to reach a maximum of 59.8 ± 8.6% 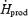 (Fig. 4A). %RFox increased by 1.5-fold in L (from 33.6 ± 3.7%
(Fig. 4A). %RFox increased by 1.5-fold in L (from 33.6 ± 3.7% 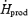 to 49.7 ± 4.8%
to 49.7 ± 4.8% 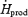 ), but remained constant in M (32.3 ± 5.0%
), but remained constant in M (32.3 ± 5.0% 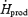 versus 32.4 ± 8.1%
versus 32.4 ± 8.1% 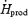 ) (Fig. 4B). %RPox decreased from 21.4 ± 11.0 to 11.0 ± 1.4%
) (Fig. 4B). %RPox decreased from 21.4 ± 11.0 to 11.0 ± 1.4% 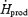 in L and from 25.1 ± 3.4 to 7.8 ± 1.1%
in L and from 25.1 ± 3.4 to 7.8 ± 1.1% 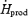 in M during cold exposure (Fig. 4C). Comparisons between shivering intensities during the last 30 min of cold exposure show that RGox was 1.4-fold higher for M than for L (Fig. 4A), and that percentage RFox (Fig. 4B) and percentage RPox (Fig. 4C) were 1.5-fold higher for L than for M.
in M during cold exposure (Fig. 4C). Comparisons between shivering intensities during the last 30 min of cold exposure show that RGox was 1.4-fold higher for M than for L (Fig. 4A), and that percentage RFox (Fig. 4B) and percentage RPox (Fig. 4C) were 1.5-fold higher for L than for M.
Figure 4. Relative contributions of carbohydrates (A, CHO), lipids (B) and proteins (C) to total heat production before (Base) and during cold exposure for L (•) and M (○).
*Significantly different from control values before cold exposure **Significantly different from L.
Plasma concentrations
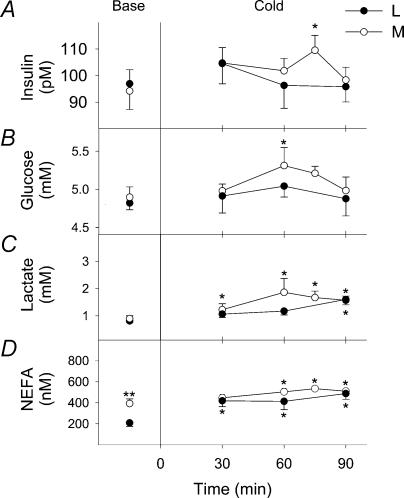
Changes in plasma concentrations of insulin, glucose, lactate and NEFA are presented in Fig. 5. Transient increases in insulin (from 96.9 ± 5.2 to 109.5 ± 5.6 pm at 75 min, Fig. 5A) and glucose levels (from 4.8 ± 0.1 to 5.3 ± 0.2 mm at 60 min) were observed in M. However, no such changes in insulin and glucose concentrations took place in L. Cold exposure caused significant increases in lactate levels after 30 min for M (from 0.8 ± 0.1 to 1.6 ± 0.5 mm) and 90 min for L (from 0.9 ± 0.11 to 1.61 ± 0.15 mm) (Fig. 5C). Significant increases in NEFA concentrations were also found for L (from 0.21 ± 0.03 to 0.52 ± 0.01 mm at 90 min) and for M (0.39 ± 0.04 to 0.51 ± 0.02 mm at 90 min) (Fig. 5D).
Figure 5. Plasma insulin (A), glucose (B), lactate (C) and non-esterified fatty acid (D, NEFA) concentrations before (Base) and during cold exposure for L (•) and M (○).
*Significantly different from control values before cold exposure **Significantly different from L.
CHO oxidation: plasma glucose versus muscle glycogen
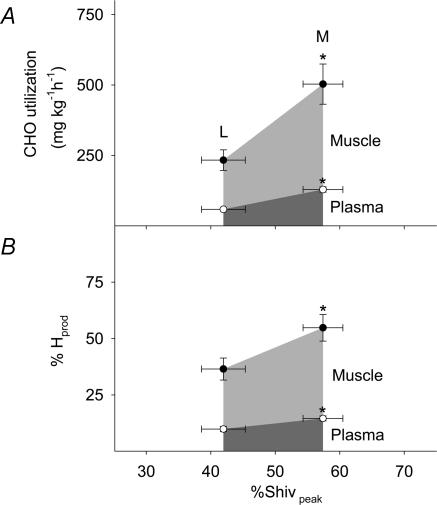
Absolute oxidation rates and relative contributions of plasma glucose and muscle glycogen are shown in Fig. 6 as a function of relative shivering intensity (%Shivpeak). RGox-plasma and RGox-muscle were significantly lower for L than for M (58.4 ± 2.7 versus 129.0 ± 9.2 mg kg−1 h−1 for RGox-plasma and 175 ± 32 versus 374 ± 67 mg kg−1 h−1 for RGox-muscle) (Fig. 6A). Table 2 summarizes average values for all variables of fuel utilization estimated between 60 and 90 min of cold exposure. 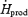 was 1.4-fold higher for M than for L. RGox, RGox-plasma and RGox-muscle were ∼2-fold higher for M than for L, whereas the relative contribution of these fuels to total
was 1.4-fold higher for M than for L. RGox, RGox-plasma and RGox-muscle were ∼2-fold higher for M than for L, whereas the relative contribution of these fuels to total 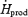 was 1.5-fold higher for M than for L. Even though the relative contribution of lipids was 30% lower for M than for L, no differences in RFox and RPox were observed between shivering intensities.
was 1.5-fold higher for M than for L. Even though the relative contribution of lipids was 30% lower for M than for L, no differences in RFox and RPox were observed between shivering intensities.
Figure 6. Absolute oxidation rates (A) and relative contributions of plasma glucose (○) and muscle glycogen (•) (B) to total heat production as a function of relative shivering intensity (%Shivpeak) for L and M.
*Significantly different from L.
Table 2.
Absolute oxidation (mg kg−1 min−1) and relative (% 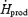 ) contributions of glucose (Total: RGox; plasma glucose:RGox-plasma; and muscle glycogen:RGox-muscle), lipid (RFox) and protein oxidation (RPox) to total heat production (
) contributions of glucose (Total: RGox; plasma glucose:RGox-plasma; and muscle glycogen:RGox-muscle), lipid (RFox) and protein oxidation (RPox) to total heat production (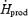 ) during low-intensity (L, 2.3 times RMR) and moderate-intensity shivering (M, 3.5 times RMR)
) during low-intensity (L, 2.3 times RMR) and moderate-intensity shivering (M, 3.5 times RMR)
| L | M | |
|---|---|---|
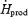 (kJ kg−1 h−1) (kJ kg−1 h−1) |
10.5 ± 0.9 | 14.7 ± 0.9* |
| Total glucose (RGox) | ||
| mg kg−1 h−1 | 233 ± 32 | 503 ± 71* |
%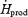
|
36.5 ± 4.9 | 54.8 ± 5.9* |
| Plasma glucose (RGox-plasma) | ||
| mg kg−1 h−1 | 58 ± 3 | 129 ± 9* |
%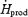
|
9.9 ± 1.1 | 14.6 ± 1.1* |
| Muscle glycogen (RGox-muscle) | ||
| mg kg−1 h−1 | 175 ± 32 | 374 ± 67* |
%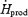
|
26.6 ± 4.6 | 40.2 ± 5.7* |
| Lipids (RFox) | ||
| mg kg−1 h−1 | 133 ± 22.4 | 138 ± 24.6 |
%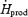
|
51.5 ± 5.1 | 36.8 ± 5.4* |
| Proteins (RPox) | ||
| mg kg−1 h−1 | 60.0 ± 5.6 | 59.4 ± 6.9 |
%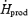
|
12.0 ± 1.6 | 8.4 ± 1.0 |
Significantly different from L.
Discussion
Plasma glucose oxidation is much more strongly stimulated by moderate than by low-intensity shivering (+122% from L to M), but the relative contribution of this pathway to total heat generation always remains minor (< 15% 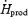 ) (Table 2, Fig. 6). In contrast, muscle glycogen becomes the dominant fuel during moderate shivering, providing 40% of total heat produced or 75% of total CHO oxidized. By itself, the increase in CHO oxidation is responsible for the 100 W increase in metabolic rate observed between L and M, because rates of lipid and protein oxidation remain constant (Table 2, Figs 3, 4 and 7). This high reliance on CHO is not compatible with the well known fuel selection pattern of exercise, when considering the relatively low metabolic rates elicited by moderate shivering (∼30%
) (Table 2, Fig. 6). In contrast, muscle glycogen becomes the dominant fuel during moderate shivering, providing 40% of total heat produced or 75% of total CHO oxidized. By itself, the increase in CHO oxidation is responsible for the 100 W increase in metabolic rate observed between L and M, because rates of lipid and protein oxidation remain constant (Table 2, Figs 3, 4 and 7). This high reliance on CHO is not compatible with the well known fuel selection pattern of exercise, when considering the relatively low metabolic rates elicited by moderate shivering (∼30% 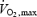 ). We show that shivering and exercise of similar energy requirements appear to be supported by different fuel mixtures. However, the fuel selection patterns for the two modes of muscle contraction can be reconciled, if relative power output is expressed as percentage
). We show that shivering and exercise of similar energy requirements appear to be supported by different fuel mixtures. However, the fuel selection patterns for the two modes of muscle contraction can be reconciled, if relative power output is expressed as percentage 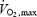 for exercise, but percentage Shivpeak for shivering (see Fig. 7).
for exercise, but percentage Shivpeak for shivering (see Fig. 7).
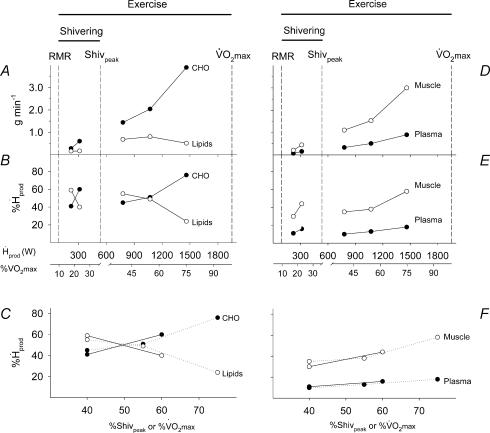
Figure 7. Comparison of absolute oxidation rates and relative contributions of carbohydrates (CHO; A, B and C, •), lipids (A, B and C, ○), plasma glucose (D, E and F, •) and muscle glycogen (D, E and F, ○) to total heat production (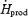 in watts) and relatively to maximal exercise output (%
in watts) and relatively to maximal exercise output (%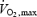 ) between shivering (present study) and exercise (3 intensities, Van Loon et al. 2001).
) between shivering (present study) and exercise (3 intensities, Van Loon et al. 2001).
Shivering and exercise values for A, B, D and E are expressed as a function of absolute heat production while those for C and F are presented relatively to maximal power output for shivering (%Shivpeak, estimated as described by Eyolfson et al. 2001) or for exercise (% 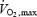 ). In C and F, continuous lines join shivering values and dotted lines exercise values.
). In C and F, continuous lines join shivering values and dotted lines exercise values.
Blood glucose
We examined whether RGox-plasma begins playing a more prominent role when shivering intensifies. Plasma glucose fluxes were investigated previously by Tipton et al. (1997) during moderate-intensity shivering, but these authors measured glucose disappearance rate, or the sum of glucose oxidation and non-oxidative disposal. Because rates of oxidation and non-oxidative disposal are often affected differently, direct measurements of glucose oxidation are essential to establish the role of this substrate in heat production. For example, Vallerand et al. (1995) found that low-intensity shivering causes a small, 25% increase in glucose disappearance rate, but subsequent measurements showed that RGox-plasma actually increases by 150% (a change proportional to the change in metabolic rate) (Haman et al. 2002). The present study provides the first direct measurements of RGox-plasma during moderate-intensity shivering. It shows that RGox-plasma doubles between low- and moderate-intensity shivering (from 58 ± 3 to 129 ± 9 mg kg−1 h−1) (Table 2), a finding consistent with the results of Tipton et al. (1997). The observed increase in glucose oxidation caused a small increment in the relative contribution of plasma glucose to total heat production, but this fuel remained responsible for < 15% 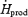 (Table 2 and Fig. 6).
(Table 2 and Fig. 6).
Maintaining a low thermogenic contribution for plasma glucose may be necessary to prevent hypoglycaemia (Passias et al. 1996). During cold exposure, animal (Cassidy et al. 1925, 1926; Dworkin & Finney, 1927; Silva & Boulant, 1984) and human studies (Haight & Keating, 1973; Gale et al. 1981; Passias et al. 1996) have reported that hypoglycaemia inhibits shivering thermogenesis. For example, Passias et al. (1996) showed that 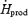 was decreased by 20% during a hyperinsulinaemic, hypoglycaemic clamp (2.8 mm plasma glucose), and a complete inhibition of shivering was observed below 2.5 mm plasma glucose (Gale et al. 1981). This inhibitory effect seems to occur centrally (inhibition of cold-sensitive neurones within the preoptic anterior hypothalamus), rather than peripherally (lack of substrate to support shivering) (Gale et al. 1981). To date, however, no evidence that cold exposure alone can elicit hypoglycaemia in humans is available. Even in the study monitoring the most prolonged and intense cold stress (3–4 h at 60–70% Shivpeak), Tikuisis et al. (2002) found a slight increase in glycaemia (+13%). Here, moderate-intensity shivering caused a transient increase in glucose levels, and no change was observed during low-intensity shivering (Fig. 5B). It is unknown whether extreme shivering (maximal intensity for > 5 h) would cause hypoglycaemia. The fact that plasma glucose remains a minor contributor to total heat production during moderate shivering suggests that euglycaemia is also maintained during maximal cold stress.
was decreased by 20% during a hyperinsulinaemic, hypoglycaemic clamp (2.8 mm plasma glucose), and a complete inhibition of shivering was observed below 2.5 mm plasma glucose (Gale et al. 1981). This inhibitory effect seems to occur centrally (inhibition of cold-sensitive neurones within the preoptic anterior hypothalamus), rather than peripherally (lack of substrate to support shivering) (Gale et al. 1981). To date, however, no evidence that cold exposure alone can elicit hypoglycaemia in humans is available. Even in the study monitoring the most prolonged and intense cold stress (3–4 h at 60–70% Shivpeak), Tikuisis et al. (2002) found a slight increase in glycaemia (+13%). Here, moderate-intensity shivering caused a transient increase in glucose levels, and no change was observed during low-intensity shivering (Fig. 5B). It is unknown whether extreme shivering (maximal intensity for > 5 h) would cause hypoglycaemia. The fact that plasma glucose remains a minor contributor to total heat production during moderate shivering suggests that euglycaemia is also maintained during maximal cold stress.
Muscle glycogen
The oxidation rate of muscle glycogen (RGox-muscle) doubles between low- and moderate-intensity shivering (Table 2 and Fig. 6), bringing the relative importance of this fuel above that of all others (40% 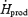 versus 37% for lipids, 15% for plasma glucose, and 8% for proteins). At all shivering intensities, muscle glycogen remains the main source of CHO, providing 75–80% of total glucosyl units oxidized. Such high reliance on muscle glycogen is consistent with previous results by Martineau & Jacobs (1988) reporting a 20% decrease in glycogen content in the vastus lateralis after 90 min of moderate-intensity shivering. The effects of changes in CHO stores on the partitioning between blood glucose and muscle glycogen have never been quantified during moderate-intensity shivering. During low-intensity shivering, however, large increases in muscle glycogen oxidation have been demonstrated in glycogen-depleted and glycogen-loaded individuals (Haman et al. 2004c). Even though RGox-muscle is more than 2 times lower in glycogen-depleted (∼170 mg min−1) than in glycogen-loaded individuals (∼380 mg min−1), muscle glycogen remains responsible for a significant fraction of total heat production (> 20%
versus 37% for lipids, 15% for plasma glucose, and 8% for proteins). At all shivering intensities, muscle glycogen remains the main source of CHO, providing 75–80% of total glucosyl units oxidized. Such high reliance on muscle glycogen is consistent with previous results by Martineau & Jacobs (1988) reporting a 20% decrease in glycogen content in the vastus lateralis after 90 min of moderate-intensity shivering. The effects of changes in CHO stores on the partitioning between blood glucose and muscle glycogen have never been quantified during moderate-intensity shivering. During low-intensity shivering, however, large increases in muscle glycogen oxidation have been demonstrated in glycogen-depleted and glycogen-loaded individuals (Haman et al. 2004c). Even though RGox-muscle is more than 2 times lower in glycogen-depleted (∼170 mg min−1) than in glycogen-loaded individuals (∼380 mg min−1), muscle glycogen remains responsible for a significant fraction of total heat production (> 20% 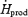 ), and it can still supply > 75% of all CHO oxidized in glycogen-depleted individuals (Haman et al. 2004c). To date, the fuel selection studies available for shivering humans leave us with an intriguing paradox: small muscle glycogen reserves become a dominant heat source as shivering intensifies, but a further reduction of these CHO stores do not seem to compromise heat production. Resolving this apparent contradiction will require investigating shivering endurance ( > 5 h) at high thermogenic rates ( > 75% Shivpeak).
), and it can still supply > 75% of all CHO oxidized in glycogen-depleted individuals (Haman et al. 2004c). To date, the fuel selection studies available for shivering humans leave us with an intriguing paradox: small muscle glycogen reserves become a dominant heat source as shivering intensifies, but a further reduction of these CHO stores do not seem to compromise heat production. Resolving this apparent contradiction will require investigating shivering endurance ( > 5 h) at high thermogenic rates ( > 75% Shivpeak).
Lipids
Our study shows that the relative importance of lipids decreases between low- and moderate-intensity shivering (52% 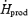 for L versus 37% for M; Table 2), because the absolute rate of lipid oxidation remains constant (133 ± 22 mg lipids kg−1 h−1 for L versus 138 ± 25 for M; Table 2). The 50% increase in
for L versus 37% for M; Table 2), because the absolute rate of lipid oxidation remains constant (133 ± 22 mg lipids kg−1 h−1 for L versus 138 ± 25 for M; Table 2). The 50% increase in 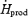 between L and M had no effect on RFox, raising the possibility that maximal shivering RFox was already reached at L. Surprisingly, the highest RFox values measured during shivering are more than 3 times lower than reported for sustained exercise (Achten et al. 2002). This suggests that maximal rates of lipid oxidation may be different for shivering and exercise. Such a difference could be explained, at least in part, by lower plasma catecholamine levels during exercise, but further work is needed to investigate this possibility.
between L and M had no effect on RFox, raising the possibility that maximal shivering RFox was already reached at L. Surprisingly, the highest RFox values measured during shivering are more than 3 times lower than reported for sustained exercise (Achten et al. 2002). This suggests that maximal rates of lipid oxidation may be different for shivering and exercise. Such a difference could be explained, at least in part, by lower plasma catecholamine levels during exercise, but further work is needed to investigate this possibility.
Previous studies during moderate-intensity shivering in cold water ( > 3.5 times RMR) have all reported that lipids accounted for ∼60% 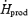 (Martineau & Jacobs, 1988, 1989a,b; Jacobs, 1997; Tikuisis et al. 2000, 2002), and therefore that this fuel played a more important role than measured here (37%
(Martineau & Jacobs, 1988, 1989a,b; Jacobs, 1997; Tikuisis et al. 2000, 2002), and therefore that this fuel played a more important role than measured here (37% 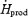 in M). The exact reasons for this discrepancy are unknown, but could be related to: (1) differences in nutritional state (e.g. size of CHO reserves) (Martineau & Jacobs, 1989b; Young et al. 1989; Haman et al. 2004c), (2) differences in individual shivering pattern (relative importance of burst versus continuous shivering) (Haman et al. 2004a), (3) differences in experimental cooling method (liquid-conditioned suit in this study versus water immersion in all others), or (4) to ignoring or including protein oxidation in the calculations.
in M). The exact reasons for this discrepancy are unknown, but could be related to: (1) differences in nutritional state (e.g. size of CHO reserves) (Martineau & Jacobs, 1989b; Young et al. 1989; Haman et al. 2004c), (2) differences in individual shivering pattern (relative importance of burst versus continuous shivering) (Haman et al. 2004a), (3) differences in experimental cooling method (liquid-conditioned suit in this study versus water immersion in all others), or (4) to ignoring or including protein oxidation in the calculations.
Proteins
At both shivering intensities, absolute rates of protein oxidation remained unaffected by cold exposure. Therefore, the observed 2.3-fold (L) and 3.5-fold increase (M) in metabolic rate were accompanied by a decrease in the relative contribution of proteins (21–25% 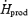 before cold exposure versus 12% in L and 8% in M). However, proteins do not always play such a minor role in heat generation. For example, protein oxidation can partly compensate for the lowered contribution of CHO in glycogen-depleted individuals, where they can provide up to 20%
before cold exposure versus 12% in L and 8% in M). However, proteins do not always play such a minor role in heat generation. For example, protein oxidation can partly compensate for the lowered contribution of CHO in glycogen-depleted individuals, where they can provide up to 20% 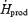 (Haman et al. 2004c). Under such conditions, it is clear that failing to account for proteins in the overall fuel budget leads to a significant overestimation of the rates of CHO and lipid oxidation.
(Haman et al. 2004c). Under such conditions, it is clear that failing to account for proteins in the overall fuel budget leads to a significant overestimation of the rates of CHO and lipid oxidation.
Patterns of fuel selection: shivering versus exercise
Several years ago, Tipton et al. (1997) noticed that Rd,Glu was significantly lower during shivering than exercise of similar metabolic rate, and they suggested that the two processes were not metabolically analogous. To extend this observation, we have compared the overall fuel selection patterns of shivering (this study) and exercise (using recent data from Van Loon et al. (2001)). This comparison is summarized in Fig. 7 where differences in fuel metabolism are highlighted as follows: (i) the left panels deal with total CHO and total lipid oxidation (Fig. 7A, B and C), whereas the right panels focus strictly on CHO, and present the partitioning between muscle glycogen and plasma glucose (Fig. 7D, E and F); (ii) Absolute rates of oxidation are shown in the upper panels (Fig. 7A and D); (iii) The relative contributions of the different fuels to total heat production are shown as a function of absolute metabolic rate 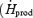 in watts) and relatively to maximal exercise output (%
in watts) and relatively to maximal exercise output (% 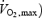 ) in the middle panels (Fig. 7B and E), and as a function of relative metabolic rate expressed as percentage Shivpeak for shivering and percentage
) in the middle panels (Fig. 7B and E), and as a function of relative metabolic rate expressed as percentage Shivpeak for shivering and percentage 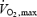 for exercise (Figs 7C and F). The first obvious distinction between the two activities is that oxidation rates of all the fuels are 2–7 times lower for shivering than for exercise, reflecting large differences in total metabolism (100–500 W for shivering versus 100–2000 W for exercise) (Fig. 7A and D). Assuming that lipids remain dominant at exercise intensities below 700 W, our analysis reveals that shivering and exercise are probably supported by different fuel mixtures (Fig. 7B and E). However, it is somewhat premature to be certain of this difference because the ranges of metabolic rates for which data are available for shivering and exercise do not overlap. Additional measurements of fuel oxidation will be necessary for maximal shivering (approaching 40%
for exercise (Figs 7C and F). The first obvious distinction between the two activities is that oxidation rates of all the fuels are 2–7 times lower for shivering than for exercise, reflecting large differences in total metabolism (100–500 W for shivering versus 100–2000 W for exercise) (Fig. 7A and D). Assuming that lipids remain dominant at exercise intensities below 700 W, our analysis reveals that shivering and exercise are probably supported by different fuel mixtures (Fig. 7B and E). However, it is somewhat premature to be certain of this difference because the ranges of metabolic rates for which data are available for shivering and exercise do not overlap. Additional measurements of fuel oxidation will be necessary for maximal shivering (approaching 40% 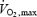 ) and for very low intensity exercise (below 30%
) and for very low intensity exercise (below 30% 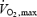 ) to confirm this conclusion.
) to confirm this conclusion.
The fuel selection pattern of mammalian exercise has been well characterized. Exercise intensity, expressed relatively to the aerobic maximum (% 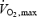 ), determines the balance between lipid and carbohydrate utilization (Weber & Haman, 2004). The contribution of carbohydrates increases progressively, and that of lipids decreases progressively as exercise intensifies. Therefore, at some intermediate work intensity called the ‘crossover point’ (Brooks & Mercier, 1994), the oxidation of each one of these two fuels is responsible for half the metabolic rate of the whole organism. In our present analysis (Fig. 7B), we can see that the crossover point is more than 3 times lower for shivering (∼300 W or ∼20%
), determines the balance between lipid and carbohydrate utilization (Weber & Haman, 2004). The contribution of carbohydrates increases progressively, and that of lipids decreases progressively as exercise intensifies. Therefore, at some intermediate work intensity called the ‘crossover point’ (Brooks & Mercier, 1994), the oxidation of each one of these two fuels is responsible for half the metabolic rate of the whole organism. In our present analysis (Fig. 7B), we can see that the crossover point is more than 3 times lower for shivering (∼300 W or ∼20% 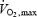 ) than for exercise (∼1100 W or ∼60%
) than for exercise (∼1100 W or ∼60% 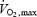 ). This large discrepancy is probably linked to differences in fibre recruitment, because CHO use was recently shown to covary with the specific activation of type II muscle fibres (Haman et al. 2004b). Surprisingly, however, the two patterns of fuel selection are completely reconciled when the relative contributions of CHO and lipid oxidation (%
). This large discrepancy is probably linked to differences in fibre recruitment, because CHO use was recently shown to covary with the specific activation of type II muscle fibres (Haman et al. 2004b). Surprisingly, however, the two patterns of fuel selection are completely reconciled when the relative contributions of CHO and lipid oxidation (% 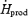 ) are expressed in relation to Shivpeak for shivering, and to
) are expressed in relation to Shivpeak for shivering, and to 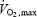 for exercise (see Fig. 7C). At the present time, we have no satisfying mechanistic explanation for this intriguing convergence.
for exercise (see Fig. 7C). At the present time, we have no satisfying mechanistic explanation for this intriguing convergence.
Conclusion
This study shows that plasma glucose oxidation always remains a minor pathway for heat generation (< 15% 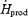 ), even when shivering intensifies and carbohydrates become the dominant thermogenic fuel. Instead, muscle glycogen is responsible for most of the increase in heat production between low- and moderate-intensity shivering. Oxidative fuel selection patterns during cold exposure and exercise at the same metabolic rate are probably very different, because CHO appears to play a much more important role during shivering. Investigating the physiological mechanisms underlying why a muscle producing only heat (shivering), or significant movement (exercise), shows a different pattern of fuel selection at the same power output strikes us as a fascinating area for future research.
), even when shivering intensifies and carbohydrates become the dominant thermogenic fuel. Instead, muscle glycogen is responsible for most of the increase in heat production between low- and moderate-intensity shivering. Oxidative fuel selection patterns during cold exposure and exercise at the same metabolic rate are probably very different, because CHO appears to play a much more important role during shivering. Investigating the physiological mechanisms underlying why a muscle producing only heat (shivering), or significant movement (exercise), shows a different pattern of fuel selection at the same power output strikes us as a fascinating area for future research.
References
- Achten J, Gleeson M, Jeukendrup AE. Determination of the exercise intensity that elicits maximal fat oxidation. Med Sci Sports Exerc. 2002;34:92–97. doi: 10.1097/00005768-200201000-00015. [DOI] [PubMed] [Google Scholar]
- Bergmeyer HU. Methods of Enzymatic Analysis. VIII. Weinheim: VCH; 1985. [Google Scholar]
- Bosch AN. Influence of carbohydrate loading on fuel substrate turnover and oxidation during prolonged exercise. J Appl Physiol. 1993;74:1921–1927. doi: 10.1152/jappl.1993.74.4.1921. [DOI] [PubMed] [Google Scholar]
- Bosch AN, Dennis SC, Noakes TD. Influence of carbohydrate ingestion on fuel substrate turnover and oxidation during prolonged exercise. J Appl Physiol. 1994;76:2364–2372. doi: 10.1152/jappl.1994.76.6.2364. [DOI] [PubMed] [Google Scholar]
- Bosch AN, Weltan SM, Dennis SC, Noakes TD. Fuel substrate turnover and oxidation and glycogen sparing with carbohydrate ingestion in non-carbohydrate-loaded cyclists. Eur J Physiol. 1996;432:1003–1010. doi: 10.1007/s004240050228. [DOI] [PubMed] [Google Scholar]
- Brooks GA, Fahey TD, White TP, Baldwin KM. Exercise Physiology – Human Bioenergetics and its Applications. Toronto: Mayfield Publishing Co; 1999. [Google Scholar]
- Brooks GA, Mercier J. Balance of carbohydrate and lipid utilization during exercise: the ‘crossover’ concept. J Appl Physiol. 1994;76:2253–2261. doi: 10.1152/jappl.1994.76.6.2253. [DOI] [PubMed] [Google Scholar]
- Brosek JF, Grande JT, Andersen JT, Keys A. Densiometric analysis of body composition: review of some quantitative assumptions. Ann N Y Acad Sci. 1963;110:113–140. doi: 10.1111/j.1749-6632.1963.tb17079.x. [DOI] [PubMed] [Google Scholar]
- Cassidy JG, Dworkin S, Finney HW. Insulin and the mechanism of hibernation. Am J Physiol. 1925;73:417–428. [Google Scholar]
- Cassidy JG, Dworkin S, Finney HW. The action of insulin on the domestic fowl. Am J Physiol. 1926;75:609–615. [Google Scholar]
- Coggan AR, Kohrt WM, Spina RJ, Bier DM, Holloszy JO. Endurance training decreases plasma glucose turnover and oxidation during moderate-intensity exercise in men. J Appl Physiol. 1990;68:990–996. doi: 10.1152/jappl.1990.68.3.990. [DOI] [PubMed] [Google Scholar]
- Derman KD, Hawley JA, Noakes TD, Dennis SC. Fuel kinetics during intense running and cycling when fed carbohydrate. Eur J Appl Physiol. 1996;74:36–43. doi: 10.1007/BF00376492. [DOI] [PubMed] [Google Scholar]
- Dubois D, Dubois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Inter Med. 1916;17:863–871. [Google Scholar]
- Dworkin S, Finney HW. Artificial hibernation in the woodchuck (Arctomys monax) Am J Physiol. 1927;80:75–81. [Google Scholar]
- Elia M. Energy equivalents of CO2 and their importance in assessing energy expenditure when using tracer techniques. Am J Physiol Endocrinol Metab. 1991;23:E75–E88. doi: 10.1152/ajpendo.1991.260.1.E75. [DOI] [PubMed] [Google Scholar]
- Eyolfson DA, Tikuisis P, Xu X, Weseen G, Giesbrecht GG. Measurement and prediction of peak shivering intensity in humans. Eur J Appl Physiol. 2001;84:100–106. doi: 10.1007/s004210000329. [DOI] [PubMed] [Google Scholar]
- Friedlander AL, Casazza GA, Huie MJ, Horning MA, Brooks GA. Endurance training alters glucose kinetics in response to the same absolute, but not the same relative workload. J Appl Physiol. 1997;82:1360–1369. doi: 10.1152/jappl.1997.82.4.1360. [DOI] [PubMed] [Google Scholar]
- Gale EA, Bennett T, Green JH, MacDonald IA. Hypoglycaemia, hypothermia and shivering in man. Clin Sci (Lond) 1981;61:463–469. doi: 10.1042/cs0610463. [DOI] [PubMed] [Google Scholar]
- Glickman-Weiss EL, Nelson AG, Hearon CM, Vasanthakumar SR, Stringer BT. Does feeding regime affect physiologic and thermal responses during exposure to 8, 20, and 27°C? Eur J Appl Physiol. 1993;67:30–34. doi: 10.1007/BF00377700. [DOI] [PubMed] [Google Scholar]
- Glickman-Weiss EL, Nelson AG, Hearon CM, Windhauser M, Heltz D. The thermogenic effect of carbohydrate feeding during exposure to 8, 12 and 27°C. Eur J Appl Physiol. 1994;68:291–297. doi: 10.1007/BF00571445. [DOI] [PubMed] [Google Scholar]
- Haight JSJ, Keating RW. Failure of thermoregulation in the cold during hypoglycaemia induced by exercise and ethanol. J Physiol. 1973;229:87–97. doi: 10.1113/jphysiol.1973.sp010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haman F, Legault SR, Rakobowchuk M, Ducharme MB, Weber JM. Effects of carbohydrate availability on sustained shivering II. Relating muscle recruitment to fuel selection. J Appl Physiol. 2004a;96:41–49. doi: 10.1152/japplphysiol.00428.2003. [DOI] [PubMed] [Google Scholar]
- Haman F, Legault SR, Weber JM. Fuel selection during intense shivering in humans: EMG pattern reflects carbohydrate oxidation. J Physiol. 2004b;556:305–313. doi: 10.1113/jphysiol.2003.055152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haman F, Péronnet F, Kenny GP, Doucet E, Massicotte D, Lavoie C, Weber JM. Effects of carbohydrate availability on sustained shivering I. Oxidation of plasma glucose, muscle glycogen, and proteins. J Appl Physiol. 2004c;96:32–40. doi: 10.1152/japplphysiol.00427.2003. [DOI] [PubMed] [Google Scholar]
- Haman F, Péronnet F, Kenny GP, Massicotte D, Lavoie C, Scott C, Weber JM. Effect of cold exposure on fuel utilization in humans: plasma glucose, muscle glycogen, and lipids. J Appl Physiol. 2002;93:77–84. doi: 10.1152/japplphysiol.00773.2001. [DOI] [PubMed] [Google Scholar]
- Jacobs I. Energy Metabolism in Cold-Stressed Females: Implications for Predictive Modeling. North York, Ontario: Defence and Civil Institute of Environmental Medicine; 1997. [Google Scholar]
- Jeukendrup AE, Raben A, Gijsen A, Stegen JHCH, Brouns F, Saris WHM, Wagenmakers JM. Glucose kinetics during prolonged exercise in highly trained human subjects: effect of glucose ingestion. J Physiol. 1999;515:579–589. doi: 10.1111/j.1469-7793.1999.579ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijssen DP, Elia M. Recovery of 13CO2 and 14CO2 in human bicarbonate studies: a critical review with original data. Clin Sci (Lond) 1996;91:665–677. doi: 10.1042/cs0910665. [DOI] [PubMed] [Google Scholar]
- MacNaughton K, Sathasivam P, Vallerand A, Graham T. Influence of caffeine on metabolic responses of men at rest in 28 and 5°C. J Appl Physiol. 1990;68:1889–1895. doi: 10.1152/jappl.1990.68.5.1889. [DOI] [PubMed] [Google Scholar]
- Martineau L, Jacobs I. Muscle glycogen utilization during shivering thermogenesis in humans. J Appl Physiol. 1988;65:2046–2050. doi: 10.1152/jappl.1988.65.5.2046. [DOI] [PubMed] [Google Scholar]
- Martineau L, Jacobs I. Free fatty acid availability and temperature regulation in cold water. J Appl Physiol. 1989a;67:2466–2472. doi: 10.1152/jappl.1989.67.6.2466. [DOI] [PubMed] [Google Scholar]
- Martineau L, Jacobs I. Muscle glycogen availability and temperature regulation in humans. J Appl Physiol. 1989b;66:72–78. doi: 10.1152/jappl.1989.66.1.72. [DOI] [PubMed] [Google Scholar]
- Passias TC, Meneilly GS, Mekjavic IB. Effect of hypoglycemia on thermoregulatory responses. J Appl Physiol. 1996;80:1021–1032. doi: 10.1152/jappl.1996.80.3.1021. [DOI] [PubMed] [Google Scholar]
- Péronnet F, Massicotte D. Table of nonprotein respiratory quotient: an update. Can J Spt Sci. 1991;16:23–29. [PubMed] [Google Scholar]
- Péronnet F, Rhéaume N, Lavoie C, Hillaire-Marcel C, Massicotte D. Oral [13C]glucose oxidation during prolonged exercise after high- and low-carbohydrate diets. J Appl Physiol. 1998;85:723–730. doi: 10.1152/jappl.1998.85.2.723. [DOI] [PubMed] [Google Scholar]
- Silva LN, Boulant JA. Effects of osmotic pressure, glucose, and temperature on neurons in preoptic tissue slices. Am J Physiol Reg Integr Comp Physiol. 1984;16:R335–R345. doi: 10.1152/ajpregu.1984.247.2.R335. [DOI] [PubMed] [Google Scholar]
- Tikuisis P, Eyolfson DA, Xu X, Giesbrecht GG. Shivering endurance and fatigue during cold water immersion in humans. Eur J Appl Physiol. 2002;87:50–58. doi: 10.1007/s00421-002-0589-1. [DOI] [PubMed] [Google Scholar]
- Tikuisis P, Jacobs I, Moroz D, Vallerand AL, Martineau L. Comparison of thermoregulatory responses between men and women immersed in cold water. J Appl Physiol. 2000;89:1403–1411. doi: 10.1152/jappl.2000.89.4.1403. [DOI] [PubMed] [Google Scholar]
- Tipton MJ, Franks GM, Meneilly GS, Mekjavic IB. Substrate utilisation during exercise and shivering. Eur J Appl Physiol. 1997;76:103–108. doi: 10.1007/s004210050220. [DOI] [PubMed] [Google Scholar]
- Vallerand AL, Jacobs I. Rates of energy substrate utilization during human cold exposure. Eur J Appl Physiol. 1989;58:873–878. doi: 10.1007/BF02332221. [DOI] [PubMed] [Google Scholar]
- Vallerand AL, Jacobs I. Influence of cold exposure on plasma triglyceride clearance in humans. Metabolism. 1990;39:1211–1218. doi: 10.1016/0026-0495(90)90097-v. [DOI] [PubMed] [Google Scholar]
- Vallerand AL, Jacobs I, Kavanagh MF. Mechanism of enhanced cold tolerance by an ephedrine-caffeine mixture in humans. J Appl Physiol. 1989;67:438–444. doi: 10.1152/jappl.1989.67.1.438. [DOI] [PubMed] [Google Scholar]
- Vallerand AL, Tikuisis P, Ducharme MB, Jacobs I. Is energy substrate mobilization a limiting factor for cold thermogenesis? Eur J Appl Physiol. 1993;67:239–244. doi: 10.1007/BF00864222. [DOI] [PubMed] [Google Scholar]
- Vallerand AL, Zamecnik J, Jones PJ, Jacobs I. Cold stress increases lipolysis, FFA Ra and TG/FFA cycling in humans. Aviat Space Environ Med. 1999;70:42–50. [PubMed] [Google Scholar]
- Vallerand A, Zamenik J, Jacobs I. Plasma glucose turnover during cold stress in humans. J Appl Physiol. 1995;78:1296–1302. doi: 10.1152/jappl.1995.78.4.1296. [DOI] [PubMed] [Google Scholar]
- Van Loon LJ, Greenhaff PL, Constantin-Teodosiu D, Saris WH, Wagenmakers AJ. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J Physiol. 2001;536:295–304. doi: 10.1111/j.1469-7793.2001.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J-M, Haman F. Oxidative fuel selection: Adjusting mix and flux to stay alive. 3rd International Conference, Comparative Physiology and Biochemistry; Elsevier, Ithala, South Africa. 2004. pp. 22–31. [Google Scholar]
- Weller AS, Greenhaff PL, Macdonald IA. Physiological responses to moderate cold stress in man and the influence of prior prolonged exhaustive exercise. Exp Physiol. 1998;83:679–695. doi: 10.1113/expphysiol.1998.sp004149. [DOI] [PubMed] [Google Scholar]
- Wolfe RR. Radioactive and Stable Isotope Tracers in Biomedicine – Principles and Practice of Kinetic Analysis. New York: Wiley-Liss; 1992. [Google Scholar]
- Young AJ, Sawka MN, Neufer PD, Muza SR, Askew EW, Pandolf KB. Thermoregulation during cold water immersion is impaired by low glycogen levels. J Appl Physiol. 1989;66:1806–1816. doi: 10.1152/jappl.1989.66.4.1809. [DOI] [PubMed] [Google Scholar]