Abstract
In standing subjects, we investigated the excitation of quadriceps (Q) motoneurones by muscle afferents from tibialis anterior (TA) and the excitation of semitendinosus (ST) motoneurones by muscle afferents from gastrocnemius medialis (GM). Standing with a backward lean stretches the anterior muscle pair (TA and Q) and they must be cocontracted to maintain balance. Equally, forward lean stretches the posterior muscle pair (GM and ST) and they must be cocontracted. We used these conditions of enhanced lean to increase the influence of γ static motoneurones on muscle spindle afferents, which enhances the background input from these afferents to extrafusal motoneurones. The effects of the conditioning volleys on motoneurone excitability was estimated using the modulation of the on-going rectified EMG and of the H reflex. Stimulation of afferents from TA in the deep peroneal nerve at 1.5–2 × MT (motor threshold) evoked early group I and late group II excitation of Q motoneurones. Stimulation of afferents in the GM nerve at 1.3–1.8 MT evoked only late group II excitation of ST motoneurones. The late excitation produced by the group II afferents was significantly greater when subjects were standing and leaning than when they voluntarily cocontracted the same muscle pairs at the same levels of activation. The early effect produced by the group I afferents was unchanged. We propose that this increase in excitation by group II afferents reflects a posture-related withdrawal of a tonic inhibition that is exerted by descending noradrenergic control and is specific to the synaptic actions of group II afferents.
There is growing evidence that excitation from group II muscle spindle afferents plays a more important role than excitation from group Ia afferents in the control of bipedal stance and gait. Perturbations of stance produce in leg and foot muscles short- and medium-latency responses (SLR and MLR) mediated by Ia and group II afferents, respectively (Schieppati et al. 1995), but, only the MLR has a stabilizing effect and is influenced by the ‘postural set’ (Nardone et al. 1990). During the stance phase of walking, there is no Ia stretch-induced response in the triceps surae after a brisk acceleration of the treadmill (Berger et al. 1984), while group II-mediated responses are consistently observed whatever the nature of the perturbation (Berger et al. 1984; Grey et al. 2001). However, it should be noted that these findings might shed more light on the corrective mechanisms for destabilizing perturbations than they tell us about the control of normal upright stance. The aim of the present investigation was therefore to explore whether changes in transmission in pathways from group II muscle spindle afferents could help to secure the contraction of lower limb muscles involved in the maintenance of unstable upright stance. A contribution of these afferents to the activation of motoneurones has been demonstrated during walking in pathways from soleus to soleus motoneurones (Sinkjær et al. 2000) and from pretibial flexors to quadriceps (Q) motoneurones (Marchand-Pauvert & Nielsen, 2002).
In the cat, a number of interneurones are coactivated by group Ia and group II afferents and such interneurones are particularly numerous in midlumbar segments, rostral to motoneurones (see Jankowska, 1992). Likewise, in humans, group Ia and II afferents have been found to converge onto common excitatory interneurones (Chaix et al. 1997; Bove et al. 2003), which in the following will be referred to as ‘lumbar propriospinal neurones’. These interneurones have also been shown to be located rostral to motoneurones (Chaix et al. 1997; Marque et al. 2005). Besides their homonymous actions (see Schieppati et al. 1995), group II afferents from leg muscles have heteronymous actions to thigh motoneurones. These are particularly potent from afferents in pretibial flexors including tibialis anterior (TA) – in the deep peroneal (DP) nerve – to Q and from gastrocnemius medialis (GM) to semitendinosus (ST) motoneurones (Simonetta-Moreau et al. 1999).
When leaning backward and forward, upright stance is maintained by a weight-bearing cocontraction of the stretched TA and Q, and GM and ST muscles, respectively. Under these circumstances γ static (γs) motoneurones can provide a powerful input to muscle spindles, driving both spindle primary and secondary discharges at high rates (Burke et al. 1978). Because these combinations are those in which the most potent heteronymous group II excitations have been found in humans (see above), the possibility was investigated that group II afferent discharges produced by contractions of stretched leg muscles contribute to the activation of thigh motoneurones in such ‘unstable’ postures. A preliminary report of some of the results has been published in abstract form (Pierrot-Deseilligny, 1999).
Methods
The experiments were carried out on 13 healthy subjects (aged 22–65), all of whom gave written informed consent to the experimental procedures, which were performed in accordance to the Declaration of Helsinki, with the approval of the local ethics committee.
Recordings
EMG activity was recorded by surface electrodes 1 cm apart secured to the skin over the corresponding muscle belly. Silver plates (0.8 cm2) were used to record the on-going EMG from Q (vastus lateralis (VL) and vasto-crureus (VC)), 25–30 and 5–10 cm above the patella, on the lateral and anterior aspects of the thigh, respectively), TA, ST and GM. Differential electrodes DE-2.3 (Delsys Inc., Boston, MA, USA) were used to record Q single units (VL).
Electrical stimulations
Conditioning stimuli
Square shocks of 1 ms duration were delivered through 1.5 cm diameter bipolar (1.5 cm apart) surface electrodes (Ag/AgCl, Niko, UK, or silver plates). The DP nerve was stimulated at the fibular neck, at a position that produced dorsiflexion of the foot without eversion and without paraesthesiae in the territory of the superficial peroneal nerve at motor threshold (1 × MT). The GM nerve was stimulated at the medial and lower part of the popliteal fossa at a position that produced a steep increase in the motor response in the GM muscle without H reflex response in the soleus. The intensity of the nerve stimuli was expressed as a multiple of the threshold for an EMG potential (1 × MT), which was measured in the different postural situations explored. It was checked that the motor response (M) evoked in the target muscle by stimuli above 1 × MT was constant throughout the experiments.
Test stimuli that evoked the H reflex in the Q and ST were applied to the femoral and the sciatic nerve, respectively. The femoral nerve was stimulated unipolarly, with the active cathode in the femoral triangle and the anode on the posterior aspect of the thigh. Double (bipolar) electrodes (3 cm diameter half-balls) were used to stimulate the sciatic nerve on the posterior aspect of the thigh, 20–30 cm above the popliteal fossa.
Assessment of motoneurone excitability
Post-stimulus time histograms (PSTHs) of a voluntarily activated motor unit
In preliminary experiments, the effects of the same DP stimulus were compared on the PSTH, the H reflex and the on-going EMG recorded through the same electrode from the VL (Fig. 1(). PSTHs were recorded for the 20–60 ms following conditioning stimulation using a bin width of 0.5 ms. The EMG potentials of single motor units were converted into standard pulses by a discriminator with variable trigger levels and these were fed to a computer which subsequently triggered the stimulators about once every 0.5 s. Measurements with and without stimulation were randomly alternated within the same sequence. The background firing was subtracted from the conditioned histogram (this accounts for the negative values in Fig. 1B). PSTHs in the VL were constructed after DP and femoral stimulation. PSTHs were also used to estimate the conduction velocity of the fastest group Ia afferents in the DP and femoral nerve: conduction velocity was calculated from the latency of the monosynaptic Ia peaks measured in the PSTH (0.2 ms bin width) of the same unit in the VL and the TA after stimulation of homonymous Ia afferents at two levels (see Hultborn et al. 1987).
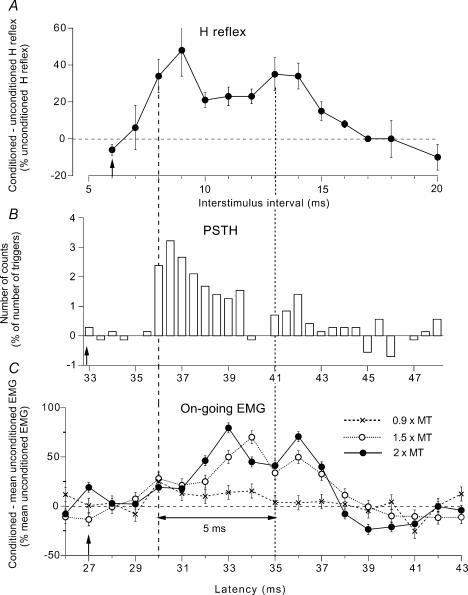
Figure 1. Three measures of modulation of responses of quadriceps (Q) motoneurones following stimulation of the deep peroneal (DP) nerve.
A–C, changes produced in the same subject (same experiment) by DP stimulation (at 1.5 × MT, A, B and C○) during a weak (∼3% of MVC) tonic Q voluntary contraction in the sitting position. A, difference between conditioned and unconditioned H reflexes (expressed as a percentage of unconditioned H reflexes) plotted against the conditioning-test (interstimulus) interval between DP and femoral nerve (FN) stimuli (unconditioned H reflex set at 18% of Mmax). Each symbol represents the mean (± s.e.m.) of 20 responses. B, changes in firing probability of a single motor unit (after subtraction of the background firing, 0.5 ms bin width), with the number of counts expressed as a percentage of the number of triggers, plotted against the latency after DP stimulation. C, changes in the rectified and averaged (mean ± s.e.m. of 150 sweeps) on-going EMG (difference between conditioned and mean unconditioned EMG expressed as a percentage of the mean unconditioned EMG) plotted against the latency after DP stimulation. Results obtained with stimulation at 2 (•) and 0.9 (×) × MT are also shown. Vertical dashed and dotted lines, estimates of the beginning of the early and late facilitation, respectively, separated by 5 ms (double-headed horizontal arrow). Distances from stimulation sites to the spinal cord (L2 vertebra) were 0.70 m (DP) and 0.25 m (FN). At conduction velocities of 67 and 58 ms−1 for DP and FN Ia volleys, respectively, the resulting difference in afferent conduction times for the fastest Ia volleys in the two nerves was 6.1 ms ([0.70/68 = 10.4 ms]–[0.25/58 = 4.3 ms]). Latencies of the Q H reflex 21 ms, and of the homonymous monosynaptic Ia peak in the PSTH 27 ms (because of the trigger delay of the unit, 6 ms). Expected time of arrival of the DP Ia volley at motoneurone level (0 central delay, vertical arrows) 33 ms (27 + 6.1) in the PSTH (B), 27 ms (21 + 6.1) in the on-going EMG (C), and corresponding to the 6 ms interstimulus interval for the H reflex (A).
Post-stimulus averaged on-going EMG activity
On-going EMG activity was recorded using a sampling rate of 1–2 kHz, amplified (× 10 000–20 000), full-wave rectified (for surface analysis) and averaged for 50 ms against the conditioning stimulus. The rectified and integrated EMG activity was displayed on an oscilloscope so that, during a sequence, the subjects could maintain a constant contraction level, expressed as a percentage of the activity measured during a maximal tonic voluntary contraction (MVC) lasting 5 s. Conditioned and unconditioned trials (i.e. trials in which the background EMG activity was measured) were randomly alternated (1 s) during short sequences of 50–100 s to avoid muscular fatigue. The data recorded during three to six sequences were averaged to produce a single run containing at least 100 conditioned responses. The background EMG was measured in the corresponding unconditioned trials and then integrated over 50 ms to provide a fixed measure of baseline EMG within the sequence (mean unconditioned EMG). The difference between the grand average of conditioned values (in a single subject) and the baseline EMG was expressed as a percentage of this baseline. Statistical analysis was confined to the windows corresponding to the different peak(s) or troughs (in the case of the ST) evoked by the conditioning stimulation. For example in Fig. 2A, the integration was done between 30 and 34 ms for the early peak (grey area) and between 34 and 38 ms for the late peak (hatched area). ANOVA and post hoc Scheffé's F-test were used to determine whether the differences between conditioned and unconditioned EMG were significant for each subject. Finally, as shown in Figs 2B and C and 4B and D, the cumulative sum (CUSUM) technique, described for the analysis of PSTHs (Ellaway, 1978), was applied to the analysis of changes in raw EMG. This technique allows a more confident estimate of latency of the different peaks (or troughs) than could reasonably be achieved using the raw EMG, which inevitably contains irregular bin-to-bin fluctuations. To that end, the raw data without amplification (in µV) were used and the unconditioned EMG was subtracted from the conditioned EMG in each bin. The residual values in each bin were summed sequentially (bin 1, then bins 1 + 2, then bins 1 + 2 + 3, etc. …) and plotted against time after the stimulus. In order to avoid contamination by the conditioning stimulus artefact the first bin of the CUSUM was 13 ms after DP or GM stimulation.
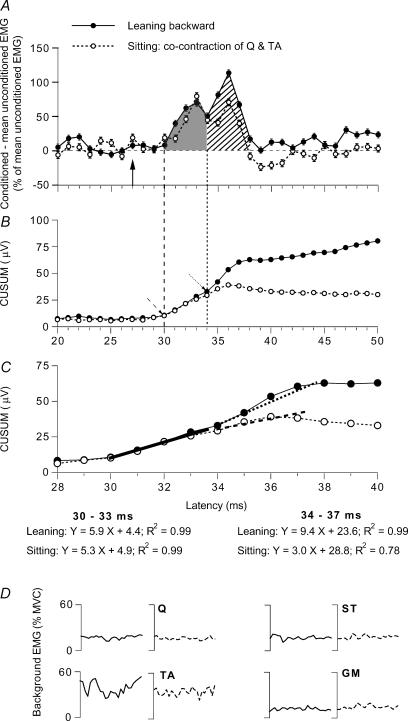
Figure 2. Modulation of the on-going Q EMG while leaning backward and while sitting.
Data for one of the subjects. A–C, changes in the rectified and averaged on-going Q (VC) EMG (A) and corresponding CUSUMs (B and C) after DP stimulation (2 × MT) while leaning backward (•) and during tonic voluntary cocontraction of TA and Q in sitting (○; same subject as in Fig. 1, but stronger Q contraction at 20% of MVC). A, difference between conditioned and mean unconditioned EMG, expressed as a percentage of the mean unconditioned EMG (mean ± s.e.m. of 100 sweeps) plotted against the latency after DP stimulation, with early (grey area) and late (hatched area) excitation. B and C, CUSUMs (in µV, because they were calculated from the raw data without amplification) of the data illustrated in A. B, CUSUM with the same abscissa as in A, i.e. the first bins of the CUSUM (starting 13 ms after DP stimulation, see Methods) are not shown. Dashed and dotted oblique arrow, onset of early and late facilitation, respectively. C, same data as in B with an expanded scale for the abscissa. Linear regressions have been drawn between 30 and 33 ms (thick continuous line) and 34 and 37 ms (dotted line, leaning backward; dashed line, cocontraction in sitting position): the resulting models of the regressions and the coefficients are shown beneath panel C in the two situations (‘leaning’ and ‘sitting’). Vertical arrow (A, 27 ms) and vertical dashed and dotted lines (A and B) as in Fig. 1. D, background rectified and averaged (100 sweeps) on-going EMG in Q, ST, TA and GM (as a percentage of MVC, 25–50 ms after DP stimulation) recorded during the same experiment while leaning backward (continuous lines) and during sitting on a high stool (dotted lines).
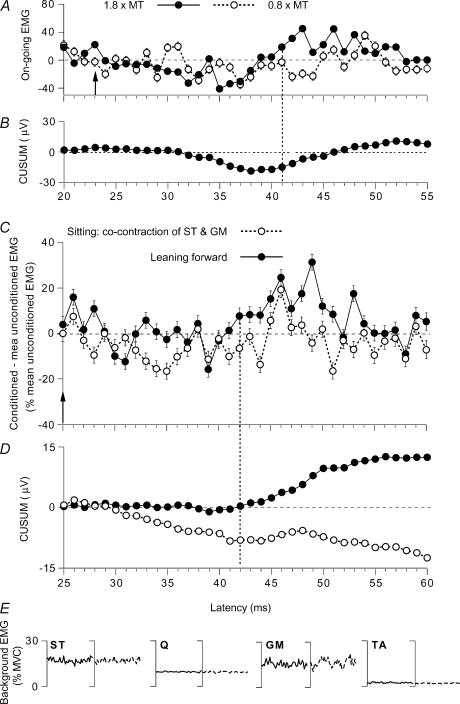
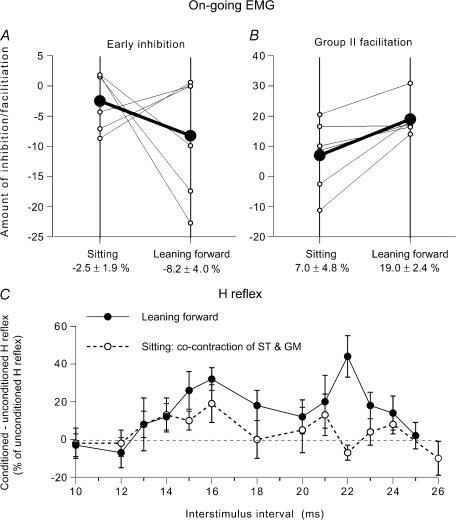
Figure 4. Modulation of the on-going semitendinosus (ST) EMG while leaning forward and while sitting.
Data for individual subjects. A–D, changes in the rectified and averaged on-going ST EMG (A and C, difference between conditioned and mean unconditioned EMG, expressed as a percentage of the mean unconditioned EMG, mean ± s.e.m. of 100 sweeps) and corresponding CUSUMs (B and D, raw data without amplification, in µV) after GM stimulation. A and B and C–E, data from two different subjects. A, effects of GM stimulation set at 1.8 (•) and 0.8 (○) × MT during tonic voluntary contraction (14% of MVC) in sitting position. B, CUSUM of the data illustrated in A after stimulation at 1.8 × MT. C, modulation of the on-going ST EMG by a GM stimulation at 1.3 × MT while leaning forward (•) and during tonic voluntary cocontraction of GM and ST in sitting position (○). D, CUSUMs of the data illustrated in C. Vertical arrow (A, 23 ms; C, 25 ms), expected time of arrival of the GM group I volley at spinal level. Vertical dotted line (A, 41 ms; C, 42 ms), beginning of the EMG facilitation. E, background rectified and averaged (100 sweeps) on-going EMG in ST, Q, GM and TA (as a percentage of MVC, 25–50 ms after GM stimulation) recorded during the same experiment while leaning forward (continuous lines) and during sitting on a high stool (dotted lines).
H reflex studies
Stimulation of the femoral nerve and sciatic nerve evoked H reflexes in Q (VC) and ST, respectively. H reflexes were measured as the peak-to-peak amplitude of the muscle action potentials and expressed as a percentage of the maximal motor response (Mmax) evoked in the same experimental conditions. Because the sensitivity of H reflexes of small size varies with the amplitude of the unconditioned reflex (Crone et al. 1990), the size of the unconditioned reflex was adjusted to be at the same size (between 10 and 30% of Mmax) in the different situations (maintenance of posture and voluntary contraction). For each experimental run, 20 unconditioned and 20 conditioned H reflexes were randomly alternated and different interstimulus (conditioning-test) intervals were investigated. For the analyses, the differences between the conditioned and the unconditioned H reflexes were expressed as a percentage of the unconditioned H reflexes. ANOVA and post hoc Scheffé's F test were used to determine whether the changes evoked by the conditioning stimulations were significant.
Posture and organization of the experiments
The effects of DP volleys on Q motoneurones were tested while the subjects leaned backward (postural coactivation of TA and Q), and those of GM volleys on ST motoneurones while they leaned forward (postural coactivation of GM and ST). The effects of the same conditioning volleys were compared to those obtained in a control situation, during tonic voluntary cocontractions of TA and Q or GM and ST at matched levels of EMG, while the subject was sitting on a high stool. Even though in the daily life such postural situations are usually maintained for only a few seconds, they were explored in tonic conditions so as to keep constant the experimental conditions (conditioning volley, on-going EMG activities, stimulation eliciting the H reflex). Only experiments in which the EMG in each of the four muscles (Q, ST, GM, TA) was not statistically different in the two situations (ANOVA and post hoc Scheffé's F test) were retained for further analysis. In the control situation it was also possible to match the knee and ankle positions observed while leaning backward. However, knee and ankle positions were different in the control situation and while leaning forward: knee 120 deg versus 180 deg, and ankle 135 deg versus 100 deg.
Results
Excitation of quadriceps motoneurones while leaning backward
Investigations using the H reflex and PSTHs of single motor units have shown that DP volleys produce a biphasic excitation in Q motoneurones. The early low threshold (∼0.5–0.6 × MT) group I excitation occurs with a 3 ms central delay (Forget et al. 1989; Marque et al. 1996). The late (appearing 5–6 ms later) and higher threshold ( > 1.2 × MT) excitation has been attributed to slower conduction velocity afferents within the group II range (Simonetta-Moreau et al. 1999). Methods used in these investigations cannot be used during strong contractions (∼20% of MVC) required in unstable stance, because (i) it is then impossible to isolate a single motor unit, and (ii) the modulation of the H reflex is difficult to interpret (Marchand-Pauvert et al. 2002; see below). We have therefore compared the effects of conditioning volleys on the on-going rectified averaged EMG recorded during two motor tasks.
Validation of the method using the on-going EMG modulation
The first step was to ensure that the modulation of the EMG faithfully reflects the group I and group II effects. Thus, during a weak Q tonic voluntary contraction (∼3% of MVC), the effects of a DP nerve volley at 1.5 × MT were compared on different responses recorded in the VL of one subject during the same experimental session: (i) Q H reflex, (ii) PSTH of a voluntarily activated motor unit, and (iii) rectified on-going EMG. In the three cases, there was a biphasic facilitation of the response with the two peaks beginning (vertical dashed and dotted lines) at 8 and 13 ms (H reflex, Fig. 1A), 36 and 41 ms (PSTH, Fig. 1B), and 30 and 35 ms (on-going EMG, Fig. 1C○). The expected time of arrival of the DP Ia volley at the level of Q motoneurones (0 central delay, vertical arrows in Fig. 1) may be estimated by adding the difference between the afferent conduction times of the DP and femoral Ia volleys to the latency of the homonymous monosynaptic Ia facilitation of Q motoneurones. This 0 central delay corresponded to the 6 ms interstimulus interval for the H reflex (A), the 33 ms latency in the PSTH (B), and the 27 ms latency in the on-going EMG (C; see legend of Fig. 1). Figure 1C also shows the modulation of the on-going EMG observed with DP volleys of lower (0.9 × MT, ×) and higher (2 × MT, •) intensity.
Early peak
The threshold of the early peak in the on-going EMG was below 1 × MT (see Fig. 1C, ×; DP, 0.9 × MT), corresponding to a group I effect, as was demonstrated for the early peak of facilitation of the H reflex and the PSTH (Forget et al. 1989; Marque et al. 1996). In both the PSTH and the on-going EMG, the early group I facilitation occurred with a central delay of 3 ms (36–33 in B, and 30–27 in C, ○ and •). The shorter central delay of early facilitation of the H reflex (8–6 = 2 ms, A) reflects that an EPSP elicited by a conditioning volley entering the spinal cord before the test volley may summate with the test Ia EPSP and cause the motoneurone to discharge earlier (see Pierrot-Deseilligny et al. 1981). As discussed previously, the long central delay is not due to a polysynaptic transmission, but to a long intraspinal conduction time to and from propriospinal neurones located rostral to motoneurones (Chaix et al. 1997), which are likely to be responsible for a disynaptically evoked excitation of the motoneurones.
Late peak
The second peak did not appear with stimuli at 0.9 × MT (see Fig. 1C, ×, where only the early peak is present), but had a threshold at ∼1.3 × MT, within the group II range (Simonetta-Moreau et al. 1999). In the three responses, its latency was 5 ms longer than that of the early peak (Fig. 1, double-headed horizontal arrow). Group I and group II afferents converge onto common lumbar propriospinal neurones with the same synaptic linkage (Chaix et al. 1997; Marque et al. 2005), much as in the cat (see Jankowska, 1992). The conduction velocity of group Ia and group II afferents in the DP nerve has been estimated at ∼67 and 42 ms−1, respectively (Simonetta-Moreau et al. 1999). The peripheral conduction time for group I and group II volleys along the 0.70 m from stimulation site to the spinal cord (L2) is therefore of 10.4 ms (0.70/67) and 16.6 ms (0.70/42), respectively. Taking into account that conduction in group II afferents may slow down more than in Ia afferents within the spinal cord and that this can add ∼1.2 ms to the central conduction time (see Schomburg, 1990), the difference between the latencies of the early and the late peak can be explained by the difference in afferent conduction times of group I and group II afferents. Note that the amplitude of the late peak was smaller than that of the early peak. This is probably due to the relatively weak stimulus intensity (1.5 × MT), only slightly higher than the threshold of group II afferents. Accordingly, increasing the conditioning stimulus intensity to 2 × MT increased the amplitude of the late peak in the ongoing EMG (Fig. 1C,•). A similar modulation (time course and threshold) with a biphasic facilitation in response to DP stimulation has been found in eight subjects so explored during weak Q contraction (Marchand-Pauvert et al. 2002).
Conclusion
The biphasic peak of excitation seen in the on-going EMG (Fig. 1C○ and •) may therefore be concluded not to be an ‘artefact of rectification’ with the two portions, positive and negative, of the same EMG potential, but to be the equivalent of the two peaks observed in the modulation of the H reflex (Fig. 1A) or of the PSTH (Fig. 1B) and attributed to group I and group II afferents, respectively. The finding that the two peaks vary independently of each other during the stance phase of walking (Marchand-Pauvert & Nielsen, 2002) or while leaning backward (see below) provides a further argument against an artefact of rectification. The overlap between the two peaks seen in the modulation of the H reflex or of the on-going EMG (Fig. 1A and C), which contrasts with the absence of overlap in single units (Fig. 1B), is probably due to the different conduction velocities for individual motor units and the duration of their EMG potentials contributing to the compound responses.
Modulation of the on-going EMG while leaning backward
In Fig. 2A changes elicited by a DP stimulation at 2 × MT in the on-going Q EMG while leaning backward (•) and in the control situation (tonic and voluntary cocontraction of Q and TA while sitting on a high stool, ○) are compared in one subject (same subject as in Fig. 1). EMG activity was matched in the two situations not only in the Q (∼20% of MVC) but also in the other main flexors and extensors of the leg and of the thigh (Fig. 2D).
Early facilitation
As in Fig. 1C, the early excitation occurred at 30 ms, i.e. with a central delay of 3 ms after the arrival of the DP Ia volley at spinal level (vertical arrow at 27 ms after the conditioning stimulation). The area of this early peak (grey area) assessed within the window of analysis 30–34 ms was similar while leaning backward (48.1 ± 7.1%) and in the control situation (43.1 ± 7.8% of baseline EMG, see Methods).
Late facilitation
At 34 ms, the decline of the early peak was truncated by the occurrence of the late excitation, which peaked at 36 ms and ended at 38 ms. This late peak may be attributed to group II afferents converging onto the same interneurones as those involved in the group I non-monosynaptic facilitation (see above). The area of this group II-mediated excitation (hatched area), assessed within the window 34–38 ms, was greater while leaning backward (81.3 ± 12.3%) than in the control situation (42.9 ± 7.9%; P < 0.05).
CUSUM
Figure 2B and C shows the CUSUMs of the on-going EMG modulations illustrated in Fig. 2A. In both conditions, when leaning backward and during cocontraction of Q and TA in the sitting position, the CUSUM started to increase 30 ms after DP stimulation (dashed arrow in B), and there was a further increase at 34 ms when the subject leaned backward (•, dotted arrow in B). Figure 2C (same data as in B but with an expanded scale for the abscissa) confirms that the slope of the CUSUM was similar in the two situations between 30 and 33 ms (thick continuous line) but steeper between 34 and 37 ms when the subject leaned backward (dotted line) than in the control situation (dashed line). (See also the resulting models of the regressions and the coefficients shown beneath panel C.)
Results with a weaker conditioning volley
To confirm that the difference between the two situations solely involved the excitation evoked by higher threshold afferents, the experiments were repeated with a DP nerve stimulation at 0.9 × MT, that did not activate afferents within the group II range. Figure 3C and D (same subject as in Figs 1 and 2, same data in C and D, but with an expanded scale for the axes in D) shows that the weak DP volley produced only a unique (small) peak of excitation, whose area, assessed within the window 30–36 ms, was similar while leaning backward (•, 16.9 ± 6.6%) and in the control situation (○, 16.4 ± 8.8%).
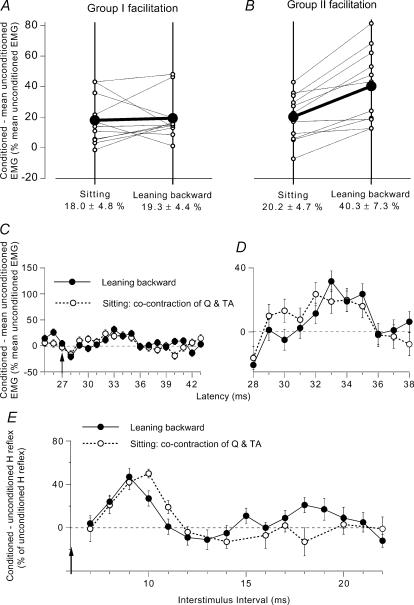
Figure 3. Modulation of the on-going Q EMG (group data) and of the Q H reflex while leaning backward and while sitting.
A and B, group data (11 subjects) on the modulation of the on-going Q EMG by DP stimuli at 2 × MT. Each thin line (and ○) represents one subject and the thick lines (and •) the mean values for the group. The data have been obtained when the subjects were leaning backward (right vertical line) and during voluntary cocontraction (left vertical line), at matched levels of EMG activity. Early group I (A) and late group II (B) facilitation of the Q on-going EMG (difference between conditioned and unconditioned EMG, expressed as a percentage of baseline unconditioned EMG) are calculated within windows corresponding to dotted and hatched areas in Fig. 2A, respectively. Mean (±1 s.e.m.) values in the group are shown beneath each corresponding vertical line. C and D, effects of a weak (0.9 × MT) DP stimulation on the on-going Q EMG (mean ± 1 s.e.m. of 100 sweeps, same subject as in Fig. 1). Difference between conditioned and baseline unconditioned EMG, expressed as a percentage of baseline unconditioned EMG, plotted against the latency after DP stimulation while leaning backward (•) and during tonic voluntary cocontraction of TA and Q while sitting (○). C, same ordinate as in Fig. 2A; vertical arrow as in Fig. 1. D, same data as in C but with expanded scale for the two axes. E, changes in the Q H reflex produced by DP stimulation (2 × MT; difference between conditioned and unconditioned H reflexes, expressed as a percentage of unconditioned H reflexes (set at 25–30% of Mmax)), plotted against the DP–FN interstimulus interval. Results are compared (at matched level of EMG activity, ∼20% of MVC in Q) while leaning backward (•) and during a strong tonic voluntary cocontraction of TA and Q while sitting on a high stool (○). Each symbol represents the mean (± s.e.m.) of 20 responses (same subject as in Figs 1 and 2).
Group data
A similar twofold facilitation of the on-going Q EMG was observed in all 11 subjects tested while using a DP stimulation at 2 × MT. The matched background Q EMG activity was ∼20% of MVC. The averaged central delay of the early peak was 3.4 ± 0.6 ms and the second peak occurred on average 4.7 ± 0.4 ms later. The area of the group I facilitation was assessed from its onset up to its truncation by the occurrence of the second peak and that of the group II facilitation from its onset up to its end (cf. grey and hatched areas in Fig. 2A). Group data are shown in Fig. 3A and B (with a different scale from that in Fig. 2A, because the mean facilitation assessed over 5 ms (area) is, not surprisingly, smaller than the peak of maximal facilitation). Each thin line (between ○) corresponds to one subject and the thick line (between •) to the mean amount of facilitation observed during voluntary cocontraction in sitting position (left vertical line) and while leaning backward (right vertical line). There was no change in the amount of early facilitation when comparing the two situations (Fig. 3A; decrease in 4 subjects, increase in 5 subjects, and no change in 2 subjects) and the mean group values were similar (18.0 ± 4.8% in sitting versus 19.3 ± 4.4% while leaning backward; P > 0.6 Wilcoxon matched-pairs signed-ranks test). Conversely, the group II facilitation was increased in all the subjects while leaning backward (Fig. 3B), and, on average, was significantly greater than during sitting (20.2 ± 4.7% in sitting versus 40.3 ± 7.3 while leaning backward; P < 0.01 Wilcoxon matched-pairs signed-ranks test). Accordingly, when tested with the Mann-Whitney U test, the difference in the amount of facilitation between the two situations was significantly larger for the late than for the early peak (P < 0.01).
Modulation of the H reflex while leaning backward
During a weak (3% MVC) Q voluntary contraction, modulations of the H reflex and on-going EMG exhibit a similar biphasic facilitation (Fig. 1A and C). In striking contrast, during a stronger voluntary contraction (∼20% of MVC), the end of the early reflex facilitation (after 10 ms) was truncated, and the second peak of reflex facilitation was replaced by inhibition at 12–18 ms ISIs (Fig. 3E○, same subject as in Fig. 1A, see below the reason for the discrepancy). However, the late facilitation of the on-going EMG increased with the strength of the contraction and was larger in Fig. 2A than in Fig. 1C (even when, as in Fig. 2A, the conditioned EMG was normalized to the enhanced level of the on-going unconditioned EMG). This discrepancy indicates the existence of an inhibitory mechanism gating effects of the afferent volley of the test reflex. The nature of this mechanism was clarified in experiments involving PSTHs of single Q motor units (Marchand-Pauvert et al. 2002). The suppression of the H reflex was shown to be due to the convergence of joint and/or cutaneous inputs in the conditioning peroneal volley and group I input in the test femoral volley onto interneurones mediating the non-reciprocal group I inhibition (autogenetic ‘Ib inhibition’) to Q motoneurones. Figure 3E compares the modulation of the Q H reflex during a voluntary contraction in sitting position (○) and while leaning backward (•), at matched level of Q contraction (∼20% of MVC). At short interstimulus intervals (8–13 ms), the modulation of the H reflex was much the same in the two situations: early facilitation appearing at 8 ms, peaking at 9 or 10 ms, and then declining rapidly to be replaced with suppression at 13–14 ms. However, at longer intervals (15–20 ms), a second peak of facilitation appeared while leaning backward, cutting short the reflex suppression observed during voluntary contraction. In contrast with the results observed at intervals between 11 and 14 ms during weak Q contraction (Fig. 1A), the early peak of reflex facilitation was replaced with suppression in the two situations compared in Fig. 3E. The finding that the suppression had then the same extent in the two situations indicates a similar gating of the test reflex volley while leaning backward (Fig. 3E, •) as during strong voluntary cocontraction of Q and TA (Fig. 3E○, see above). This suggests that at longer interstimulus intervals (15–20 ms), the DP-induced group II excitation while leaning backward is superimposed on a suppression of equal magnitude as during strong voluntary contraction. It can be assumed that, in the absence of such a suppression of the test reflex, the amount of late reflex facilitation would be as great as that of the on-going EMG. Superimposition of a facilitated group II excitation on a suppression of the test reflex might account for the discrepancy between the timing of the late peak of excitation of the Q H reflex in Fig. 1A and 3E (•): (i) because of the initial reflex suppression, the onset of the excitation would be delayed (15 versus 13 ms); but (ii) because group II excitation is facilitated (and produced by a stronger DP stimulation, 2 versus 1.5 × MT) it would probably last longer, finishing at 20 ms instead of 16 ms.
Group data
Similar results were observed in the six subjects so explored. There was a similar amount of early facilitation in the two situations (mean values at the peak: 31 and 30 ± 4%) and suppression following it immediately (mean values at the intervals 13–14 ms: –17 and –15 ± 5%). At longer intervals (15–20 ms) while leaning backward, a late significant facilitation appeared (mean value at its peak: 28 ± 2%), which contrasted with the absence of facilitation in the control situation during sitting (−3 ± 4%; P < 0.05, Wilcoxon matched-pairs signed-ranks test). It should be noted that the mean values of the late and early peaks of H reflex facilitation while leaning backward were similar in the group (30 versus 28%), although this was not the case for the subject illustrated in Fig. 3E.
Excitation of semitendinosus motoneurones while leaning forward
As a counterpart of the TA–Q synergy while leaning backward, GM and ST are coactivated while leaning forward. Investigations using the H reflex and PSTHs of single motor units have revealed that this corresponds to a strong heteronymous group II excitation from GM to ST motoneurones (Simonetta-Moreau et al. 1999). However, because, here again, methods used in these investigations cannot be used during unstable stance, we have compared the effects of GM conditioning volleys on the on-going rectified averaged EMG of the ST recorded during two motor tasks.
Validation of the method of using the on-going ST EMG modulation
Figure 4A shows the modulation of the on-going EMG of the ST after a conditioning GM stimulation at 0.8 (○) and 1.8 (•) × MT during a tonic voluntary contraction (sitting position). The latency of the ST H reflex was 19 ms, and the arrival of the GM group I volley at the S1 spinal level could be expected 4 ms later, at 23 ms (vertical arrow; see Simonetta-Moreau et al. 1999). At 1.8 × MT (•), a late facilitation appeared at 41 ms, but its real onset was masked by an earlier inhibition. However, its peak occurred ∼20 ms after the arrival of the conditioning volley at spinal level, much as the late group II-mediated facilitation of the ST H reflex at rest and of the PSTHs of single units (Simonetta-Moreau et al. 1999). Both the early inhibition and the late facilitation are more apparent in the CUSUM (Fig. 4B). After the conditioning stimulus intensity had been decreased to 0.8 × MT (i.e. was within the group I range), the late facilitation disappeared, while a trend to inhibition persisted between 32 and 44 ms (Fig. 4A, ○). A similar low-threshold GM-induced inhibition of ST motoneurones (group I and possibly cutaneous in origin) has been described in a previous study, particularly when the responsible pathway is facilitated by corticospinal volleys (Marchand-Pauvert et al. 1999; their Fig. 6). Thus, here again, similar results (late and high-threshold excitation, consistent with a group II effect) are observed with the modulation of the EMG and of the H reflex or PSTHs of single units.
Modulation of the on-going ST EMG while leaning forward
In Fig. 4C (another subject), the GM modulation of the ST on-going EMG is compared in the control situation (during tonic voluntary cocontraction of GM and ST in sitting position) and while leaning forward. In order to enhance the facilitation, the conditioning stimulation was set at 1.3 × MT, i.e. just above the threshold of group II afferents (at higher intensities, the facilitation may be truncated by feedback inhibition of the relevant interneurones; see Marchand-Pauvert et al. 1999). It produced mainly a suppression in the control situation (○): facilitation only appeared between 45 and 47 ms, and was absent within the window 42–51 ms (−2.5 ± 4.1% of the baseline EMG). In contrast, under conditions of leaning forward (•), there was a facilitation within the window 42–51 ms (18.3 ± 4.7%), which was statistically significant (P < 0.05). The differences between the dominant suppression during voluntary cocontraction and the dominant facilitation while leaning forward are even more apparent in the corresponding CUSUMs illustrated in Fig. 4D. The CUSUM also shows that the late peak at 53 ms while leaning forward is probably a simple prolongation of the preceding excitation. Here also, EMG activity was matched in the two situations (Fig. 4E). In all six subjects so tested the GM nerve-induced late and high-threshold facilitation of the ST on-going EMG was greater in the postural task than that in the control situation. The group data are illustrated in Fig. 5B (same legend as in Fig. 3A and B). On average, the amount of group II facilitation was of 7.0 ± 4.8% of the mean unconditioned EMG in sitting position and of 19.0 ± 2.4% while leaning forwards; the difference between the two motor tasks was significant (P < 0.05; Wilcoxon matched-pairs signed-ranks test).
Figure 5. Modulation of the semitendinosus (ST) on-going EMG (group data) and of the ST H reflex while leaning forward and while sitting.
A and B, group data (6 subjects) on the modulation of the on-going ST EMG by a GM volley at 1.3 × MT. Each thin line (and ○) represents one subject and the thick lines (and •) the mean values in the group. Early group I inhibition (A) and late group II (B) facilitation of the ST on-going EMG (difference between conditioned and baseline unconditioned EMG, expressed as a percentage of baseline unconditioned EMG) are compared while leaning forward (right vertical line) and during voluntary cocontraction (left vertical line) at matched level of EMG activity. Mean (±1 s.e.m.) values in the group are shown beneath each corresponding vertical line. C, changes in the ST H reflex produced by GM stimulation (1.5 × MT; difference between conditioned and unconditioned H reflexes, expressed as a percentage of unconditioned H reflexes (set at 15% of Mmax)), plotted against the conditioning-test (interstimulus) interval between GM and sciatic nerve stimulations. Results are compared while leaning forward (•) and during a tonic voluntary cocontraction of GM and ST during sitting (○), at matched level of EMG activity (∼18% of MVC in ST). Each symbol represents the mean (± s.e.m.) of 20 responses in a single subject.
Modulation of the ST H reflex while leaning forward
In two subjects, it was possible to compare the effects of GM stimulation on the amplitude of the ST H reflex in the two motor tasks. Figure 5C illustrates the result in one subject, in whom the simultaneous arrival of conditioning and test Ia volleys at spinal level was expected at the 5 ms ISI. During leaning forward, GM stimulation (1.5 × MT) evoked a large facilitation of the H reflex between 14 and 24 ms interstimulus intervals (•). The onset of the reflex facilitation corresponds to the delay required for GM group II volleys to reach ST motoneurones (Simonetta-Moreau et al. 1999). During voluntary cocontraction of GM and ST by the sitting subject (○), at matched levels of EMG activity (18% of MVC in the ST), the H reflex facilitation was significantly smaller than that observed during leaning forward (P < 0.05). A similar result was observed in the other subject.
Discussion
The main finding of the present study is that the late high-threshold facilitation from TA to Q, and from GM to ST motoneurones, is stronger while leaning backward and forward, respectively, than during tonic voluntary cocontractions at matched levels of EMG activity. It is argued below that the difference could be due to tuning of excitatory actions of muscle spindle secondaries in leg muscles.
Evidence for contribution of group II muscle afferents
Several lines of evidence show that the late high-threshold heteronymous excitation of Q and ST motoneurones investigated here is mediated through spinal pathways from group II afferents. Firstly, the long latency is compatible with activation of peripheral afferents of slower conduction velocity than group I fibres, the ratio between the conduction velocity of afferents responsible for the late excitation and of group Ia afferents being ∼0.65 (Simonetta-Moreau et al. 1999), much as the ratio between conduction velocities of group II and of group Ia afferents in the cat (see Matthews, 1972). The ratio between the electrical thresholds of these two types of afferents (∼2.1, Simonetta-Moreau et al. 1999) is also similar to that between the electrical thresholds of group II and of group Ia afferents in the cat. Secondly, tizanidine, the α2 adrenergic receptor agonist, selectively blocks transmission from group II effects both in the cat (Bras et al. 1989) and in humans (Corna et al. 1995; Marque et al. 2005; see below), and selectively suppresses only the late and high-threshold DP-induced excitation of Q motoneurones (Maupas et al. 2004).
Afferents within the group II range activated by conditioning volleys may primarily originate from spindle secondaries, but a contribution from cutaneous and/or joint afferents must also be considered. It is therefore important that stimuli mimicking the non-painful cutaneous sensation, when electrical stimuli at 1.3–2 × MT were applied to the DP or the GM nerves, failed to produce the late excitation of either Q or ST motoneurones (Marque et al. 1996; Simonetta-Moreau et al. 1999). This is consistent with animal data that tizanidine depresses responses of dorsal horn neurones to noxious skin stimuli, but does not modify responses to innocuous skin stimuli (Davies et al. 1984). It is therefore likely that the late excitation suppressed by tizanidine was not related to an effect transmitted by cutaneous afferents mediating non-painful tactile sensation (see Marque et al. 2005). A contribution from joint afferents to the late excitation appears also negligible. The threshold of joint afferents in the human DP nerve is ∼1 × MT, i.e. between those for group I and group II afferents, much as has been described for joint afferents in the cat (MacLennan, 1972), and their effect on the Q H reflex is maximal with stimulus intensities at 1.4 × MT (Marchand-Pauvert et al. 2002). In contrast, the threshold of the late excitations investigated here is ∼1.3 × MT, and the maximal effect on the target motoneurones is at 1.8–2 × MT (Simonetta-Moreau et al. 1999). In addition, in the cat, a few non-spindle group II afferents can be activated by stimulation of the GM nerve, but they are in the high-threshold range (Lundberg et al. 1987a). It is therefore likely that stimuli applied to the nerve branch of the GM, at intensities corresponding to the threshold of group II afferents (1.3 × MT; Fig. 4C and D), activate mainly if not exclusively spindle afferents (if one excludes cutaneous afferents beneath the electrode which fail to produce the late excitation of ST motoneurones, see above).
In the cat, little spatial or temporal facilitation of transmission has been found in group II excitatory pathways, where occlusion is the rule (Lundberg et al. 1987b). Occlusion at interneuronal level between the conditioning volley and the strong spindle discharge from stretched leg muscles due to the γs-assisted weight-bearing contractions in unstable stance appears therefore likely. As a result, the increase in group II excitation, as assessed in the present experiments, is probably underestimated.
Possible mechanisms underlying the increased group II excitation in unstable stance
Peripheral conditions
Stronger group II excitation during unstable stance than under voluntary muscle cocontractions of sitting subjects may be related to central rather than peripheral influences, since ‘peripheral conditions’ were matched as much as possible in the two situations: (i) same EMG activity in the different muscles, (ii) similar cutaneous discharge from about the same area of the foot sole (posterior part during voluntary TA contraction and leaning backward; anterior part during voluntary triceps surae contraction and leaning forward), and (iii) similar ankle and knee position during voluntary contraction and leaning backward. However, knee and ankle positions were different in voluntary cocontraction of GM and ST and while leaning forward (see Methods) and, in any case, there was an extra cutaneous input during voluntary contraction due to the contact of the buttock with the stool.
Changes in the excitability of the interneurones related to unstable stance?
Similar degree of excitation of motoneurones, as revealed by matched EMG activity, does not exclude different subthreshold excitation of interneurones. Interneurones more readily responding to group II input might be coexcited by neurones responding to signals during leaning backward or forward, e.g. from the labyrinths, neck receptors or trunk. In this respect, in the cat, many interneurones with group II input are potently activated by vestibulo- and reticulo-spinal tract fibres (Davies & Edgley, 1994) and in conjunction with neck reflexes (Suzuki et al. 1985; Yates et al. 1989). If facilitation (or disinhibition) of the relevant lumbar propriospinal neurones by descending tract neurones, or as an element of postural reflexes evoked from the vestibular, neck receptors or from the trunk, was stronger than during voluntary contractions, excitation of motoneurones from group II would be more potent during maintenance of posture. Vestibulospinal tract neurones might affect group II interneurones both directly and indirectly – secondary to the selective activation of γs motoneurones (Grillner et al. 1969) and the resulting enhancement of muscle spindle secondaries (see Jankowska, 1992).
However, the same interneurones may mediate excitation of motoneurones from group I and group II afferents (Chaix et al. 1997; Bove et al. 2003) and disynaptic excitation from group I afferents might be expected to be facilitated under the same increased γs drive and descending facilitation of the interneurones. The finding that the early DP-induced group I excitation of Q motoneurones was unchanged while the late group II excitation was enhanced (Figs 2A and B, and 3A and B) may be considered as an argument against the above proposed explanation. Nevertheless, group II interneurones are more effectively activated by group II than by group I afferents, let alone those without group I input (see Jankowska, 1992). We consider this argument as not critical and, in spite of this reservation, favour a mechanism affecting transmission through group II interneurones.
Mechanisms acting selectively on transmission from group II afferents
Two so far investigated neural modulatory systems have been found to be involved in selective depression of transmission from group II afferents: interneurones mediating presynaptic inhibition of group II afferents associated with primary afferent depolarization (PAD; Jankowska et al. 2002), referred to as ‘PAD interneurones’, and noradrenaline-releasing neurones (Jankowska et al. 2000).
PAD interneurones
These interneurones are fed by group II afferents themselves and are activated from the reticular formation, locus coeruleus and raphe nuclei in the medulla (see Jankowska & Riddell, 1998). If the descending activation of PAD interneurones is weaker during unstable stance than during voluntary contraction, this would produce weaker presynaptic inhibition of group II afferents and more potent group II excitation.
Noradrenaline-releasing neurones
These neurones are in the locus coeruleus/subcoeruleus in the brainstem, and selectively suppress transmission from group II afferents to midlumbar interneurones (Bras et al. 1989; Jankowska et al. 2000). Weakening of this gating would accordingly enhance group II excitation without modifying the early group I excitation. It has been argued (see Schieppati et al. 2001; Bove et al. 2003) that this mechanism could explain why, under various normal and pathological conditions, the homonymous stretch-induced group II-mediated medium latency response (MLR) of leg muscles is changed, but not the Ia-mediated short-latency response (SLR): the MLR, but not the SLR, is markedly reduced when subjects support themselves by holding onto a stable frame, i.e. when the responses are no longer required to ensure the equilibrium (Nardone et al. 1990). This MLR suppression is not related to the stabilized posture itself but originates from the descending command leading to a transition to a new stabilized ‘postural set’ (Schieppati & Nardone, 1995). Thus, when the MLR is no longer required to ensure the control of upright stance, there would be a reduction in a descending inhibitory control of the locus coeruleus, leading to increased activity from the locus, and thereby to increased gating of group II volleys. The finding that the same selective suppression of the MLR is observed after administration of tizanidine, a noradrenergic agonist (Corna et al. 1995), suggests that descending projections from the locus coeruleus/subcoeruleus probably also exist in human subjects. In parkinsonian patients, in whom there is a significant cell loss in the locus coeruleus (German et al. 1992), this adaptation is lost and there is a failure to suppress the MLR when stability is assisted (Schieppati & Nardone, 1991). In contrast, when balance is unstable, transmission in group II pathways would be tuned up by decreased activity in this monoaminergic control system. While leaning backward or forward, the coactivated γs drive may be expected to produce strong activation of spindle secondaries in stretched leg muscles. The resulting group II discharges may also be expected to be tuned up by the noradrenergic control system. Through the potent heteronymous group II connections linking leg and thigh muscles, the resulting group II excitation might contribute to the required cocontraction of leg and thigh muscles in each postural task.
Functional implications
Unstable stance when leaning backward or forward is a circumstance where muscles in the ventral (TA and Q) or the dorsal (GM and ST) lower limb aspects are weight-bearing and contract while stretched, with strong discharges from both spindle primaries and secondaries due to the coactivated γs drive. Such contractions, initiated by descending activation of motoneurones, could be maintained by the effect of spindle secondaries activated by descending excitation of γs motoneurones (group II part of the ‘FRA hypothesis’, cf. Lundberg et al. 1987c). The γs-driven group II excitation of motoneurones, tuned up by the noradrenergic control system, could be reinforced by at least two additional mechanisms. Firstly, the excitation of γ motoneurones by group II afferents may assist in sustaining their activation by positive feedback (Gladden et al. 1998), and this would provide further support for the active α motoneurones. Secondly, the γs-driven Ia discharge would reinforce the group II excitation because of the convergence of group Ia and group II afferents on the relevant interneurones (Chaix et al. 1997; Bove et al. 2003). The reinforcing effect of Ia afferents explains why, even in the absence of an overt increase in the discharge of motoneurones at Ia latency, ischaemic blockade of group I afferents is able to suppress DP-induced group II excitation to Q motoneurones during the early stance phase of walking (Marchand-Pauvert & Nielsen, 2002) and to delay the homonymous group II excitation of soleus motoneurones (Sinkjær et al. 2000).
Heteronymous group II excitation links one group of muscles to antagonists operating at another joint (e.g. group II afferents from pretibial flexors influence both Q and hamstring motoneurones, Simonetta-Moreau et al. 1999). The selection of the appropriate heteronymous group II pathway for a given postural task (e.g. Q but not hamstrings while leaning backward) might be ensured by the parallel activation of inhibitory pathways preventing activation of muscles not required in this task. Several neural pathways may contribute to such a focusing action: PAD interneurones and noradrenaline-releasing neurones activated from the brainstem (see above), corticospinal activation of feedback inhibitory interneurones inhibiting lumbar propriospinal neurones (Marchand-Pauvert et al. 1999), and selective control of heteronymous recurrent inhibition (Barbeau et al. 2000).
Acknowledgments
This work was supported by grants from Institut pour la Recherche Médicale (IRME) and Assistance Publique-Hôpitaux de Paris (AP-HP). Philippe Marque was supported by grant from Hôpitaux de Toulouse and AP-HP.
References
- Barbeau H, Marchand-Pauvert V, Meunier S, Nicolas G, Pierrot-Deseilligny E. Posture-related changes in heteronymous recurrent inhibition from quadriceps to ankle muscles in humans. Exp Brain Res. 2000;130:345–361. doi: 10.1007/s002219900260. [DOI] [PubMed] [Google Scholar]
- Berger W, Dietz V, Quintern J. Corrective reactions to stumbling in man: neuronal coordination of bilateral leg muscle activity during gait. J Physiol. 1984;405:1–37. doi: 10.1113/jphysiol.1984.sp015492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove M, Nardone A, Schieppati M. Effects of leg muscle tendon vibration on group Ia and group II reflex responses to stance perturbation in humans. J Physiol. 2003;550:617–630. doi: 10.1113/jphysiol.2003.043331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bras H, Cavallari P, Jankowska E, McCrea D. Comparison of effects of monoamines on transmission in spinal pathways from group I and II muscle afferents in the cat. Exp Brain Res. 1989;76:27–37. doi: 10.1007/BF00253620. [DOI] [PubMed] [Google Scholar]
- Burke D, Hagbarth KE, Löfstedt L. Muscle spindle activity in man during shortening and lengthening contractions. J Physiol. 1978;277:131–142. doi: 10.1113/jphysiol.1978.sp012265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaix Y, Marque P, Meunier S, Pierrot-Deseilligny E, Simonetta-Moreau M. Further evidence for non-monosynaptic group I excitation of motoneurones in the human lower limb. Exp Brain Res. 1997;115:35–46. doi: 10.1007/pl00005683. [DOI] [PubMed] [Google Scholar]
- Corna S, Grasso M, Nardone A, Schieppati M. Selective depression of medium-latency leg and foot muscle responses to stretch by an α2-agonist in humans. J Physiol. 1995;484:803–809. doi: 10.1113/jphysiol.1995.sp020705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Mazières L, Morin C, Nielsen J, Pierrot-Deseilligny E. Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size: a study in man and the cat. Exp Brain Res. 1990;81:35–45. doi: 10.1007/BF00230098. [DOI] [PubMed] [Google Scholar]
- Davies HE, Edgley SA. Inputs to group II-activated midlumbar interneurones from descending motor pathways in the cat. J Physiol. 1994;479:463–473. doi: 10.1113/jphysiol.1994.sp020310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J, Johnston SE, Hill DR, Quilliam JE. Tizanidine (Ds 103-282), a centrally acting muscle relaxant, selectively depresses excitation of feline dorsal horn neurons to noxious peripheral stimuli by an action at α2-adrenoreceptors. Neurosci Lett. 1984;48:197–202. doi: 10.1016/0304-3940(84)90019-3. [DOI] [PubMed] [Google Scholar]
- Ellaway PH. Cumulative sum technique and its application to the analysis of peristimulus time histogram. EEG Clin Neurophysiol. 1978;45:302–304. doi: 10.1016/0013-4694(78)90017-2. [DOI] [PubMed] [Google Scholar]
- Forget R, Pantieri R, Pierrot-Deseilligny E, Shindo M, Tanaka R. Facilitation of quadriceps motoneurones by group I afferents from pretibial flexors in man. 1. Possible interneuronal pathway. Exp Brain Res. 1989;78:10–20. doi: 10.1007/BF00230681. [DOI] [PubMed] [Google Scholar]
- German DC, Manaye KF, White CL, Woodward DJ, McIntire DD, Smith WK, Kalaria RN, Mann DM. Disease-specific patterns of locus coeruleus cell loss. Ann Neurol. 1992;32:667–676. doi: 10.1002/ana.410320510. [DOI] [PubMed] [Google Scholar]
- Gladden MH, Jankowska E, Czarkowska-Bauch J. New observations on coupling between group II muscle afferents and feline γ-motoneurones. J Physiol. 1998;512:507–520. doi: 10.1111/j.1469-7793.1998.507be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey MJ, Ladouceur M, Andersen JB, Nielsen JB, Sinkjær T. Group II muscle afferents probably contribute to the medium latency soleus stretch reflex during walking in humans. J Physiol. 2001;534:925–933. doi: 10.1111/j.1469-7793.2001.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S, Hongo T, Lund S. Descending monosynaptic and reflex control of gamma-motoneurones. Acta Physiol Scand. 1969;75:592–613. doi: 10.1111/j.1748-1716.1969.tb04414.x. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Meunier S, Morin C, Pierrot-Deseilligny E. Assessing changes in presynaptic inhibition of Ia fibres: a study in man and the cat. J Physiol. 1987;389:729–756. doi: 10.1113/jphysiol.1987.sp016680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol. 1992;38:335–378. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Chojnicka B, Heden CH. Effects of monoamines on interneurons in four spinal reflex pathways from group I and/or group II muscle afferents. Eur J Neurosci. 2000;12:701–714. doi: 10.1046/j.1460-9568.2000.00955.x. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Riddell JS. Neuronal systems involved in modulating synaptic transmission from group II muscle afferents. In: Rudomin P, Romo R, Mendell L, editors. Presynaptic Inhibition and Neural Control. New York: Oxford University Press; 1998. pp. 315–328. [Google Scholar]
- Jankowska E, Slawinska U, Hammar I. On organization of a neuronal network in pathways from group II muscle afferents in feline lumbar spinal segments. J Physiol. 2002;542:301–314. doi: 10.1113/jphysiol.2001.014076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Reflex pathways from group II muscle afferents. 1. Distribution and linkage of reflex actions to alpha-motoneurones. Exp Brain Res. 1987a;65:271–281. doi: 10.1007/BF00236299. [DOI] [PubMed] [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Reflex pathways from group II muscle afferents. 2. Functional characteristics of reflex pathways to α-motoneurones. Exp Brain Res. 1987b;65:282–293. doi: 10.1007/BF00236300. [DOI] [PubMed] [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Reflex pathways from group II muscle afferents. 3. Secondary spindle afferents and the FRA: a new hypothesis. Exp Brain Res. 1987c;65:294–306. doi: 10.1007/BF00236301. [DOI] [PubMed] [Google Scholar]
- MacLennan C. The behaviour of receptors of extramuscular and muscular origin with afferent fibers contributing to the group I and group II of the cat tibialis anterior muscle nerve. J Physiol. 1972;222:90–91P. [PubMed] [Google Scholar]
- Marchand-Pauvert V, Nicolas G, Burke D, Pierrot-Deseilligny E. Suppression of the H reflex in humans by disynaptic autogenetic inhibitory pathways activated by the test volley. J Physiol. 2002;542:963–976. doi: 10.1113/jphysiol.2002.021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand-Pauvert V, Nielsen JB. Modulation of non-monosynaptic excitation from ankle dorsiflexor afferents to quadriceps motoneurones during human walking. J Physiol. 2002;538:647–657. doi: 10.1113/jphysiol.2001.012675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand-Pauvert V, Simonetta-Moreau M, Pierrot-Deseilligny E. Cortical control of spinal pathways mediating group II excitation to human thigh motoneurones. J Physiol. 1999;517:301–313. doi: 10.1111/j.1469-7793.1999.0301z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marque P, Nicolas G, Simonetta-Moreau M, Pierrot-Deseilligny E, Marchand-Pauvert V. Group II excitations from plantar foot muscles to human leg and thigh motoneurones. Exp Brain Res. 2005;161:486–501. doi: 10.1007/s00221-004-2096-6. [DOI] [PubMed] [Google Scholar]
- Marque P, Pierrot-Deseilligny E, Simonetta-Moreau M. Evidence for excitation of the human lower limb motoneurones by group II muscle afferents. Exp Brain Res. 1996;109:357–360. doi: 10.1007/BF00231793. [DOI] [PubMed] [Google Scholar]
- Matthews PBC. Mammalian Muscle Spindles and Their Central Action. London: Arnold; 1972. [Google Scholar]
- Maupas E, Marque P, Roques CF, Simonetta-Moreau M. Modulation of the transmission in group II heteronymous pathways by tizanidine in spastic hemiplegic patients. J Neurol Neurosurg Psychiatry. 2004;75:130–135. [PMC free article] [PubMed] [Google Scholar]
- Nardone A, Giordano A, Corrà T, Schieppati M. Responses of leg muscles in humans displaced while standing. Effects of types of perturbation and of postural set. Brain. 1990;113:65–84. doi: 10.1093/brain/113.1.65. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E. Heteronymous group II pathways in the human lower limb: spinal organization, cortical control and possible functional role. J Physiol. 1999;518.P:27S. [Google Scholar]
- Pierrot-Deseilligny E, Morin C, Bergego C, Tankov N. Pattern of group I fibre projections from ankle flexor and extensor muscles in man. Exp Brain Res. 1981;42:337–350. doi: 10.1007/BF00237499. [DOI] [PubMed] [Google Scholar]
- Schieppati M, Nardone A. Free and supported stance in Parkinson's disease. The effect of posture and postural set on leg muscle responses to perturbation, and its relation to the severity of the disease. Brain. 1991;4:749–755. doi: 10.1093/brain/114.3.1227. [DOI] [PubMed] [Google Scholar]
- Schieppati M, Nardone A. Time course of ‘set’-related changes in muscle response to stance perturbation in humans. J Physiol. 1995;487:787–796. doi: 10.1113/jphysiol.1995.sp020918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieppati M, Nardone A, Corna S, Bove M. The complex role of spindle afferent input, as evidenced by the study of posture control in normal subjects and patients. Neurol Sci. 2001;22(Suppl. 1):S15–S20. [Google Scholar]
- Schieppati M, Nardone A, Siliotto R, Grasso M. Early and late stretch responses of human foot muscles induced by perturbation of stance. Exp Brain Res. 1995;105:411–422. doi: 10.1007/BF00233041. [DOI] [PubMed] [Google Scholar]
- Schomburg ED. Spinal sensorimotor systems and their supraspinal control. Neurosci Res. 1990;7:265–340. doi: 10.1016/0168-0102(90)90008-3. [DOI] [PubMed] [Google Scholar]
- Simonetta-Moreau M, Marque P, Marchand-Pauvert V, Pierrot-Deseilligny E. The pattern of excitation of human lower limb motoneurones by probable group II muscle afferents. J Physiol. 1999;517:287–300. doi: 10.1111/j.1469-7793.1999.0287z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkjær T, Andersen JB, Ladouceur M, Christensen LOD, Nielsen JB. Major role for sensory feedback in soleus EMG activity in the stance phase of walking in man. J Physiol. 2000;523:817–827. doi: 10.1111/j.1469-7793.2000.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki I, Timerick SJB, Wilson VJ. Body position with respect to the head or body position in space is coded by lumbar interneurons. J Neurophysiol. 1985;54:123–133. doi: 10.1152/jn.1985.54.1.123. [DOI] [PubMed] [Google Scholar]
- Yates BJ, Kasper J, Wilson VJ. Effects of muscle and cutaneous afferents on L4 neurons whose activity is modulated by neck rotation. Exp Brain Res. 1989;77:48–56. doi: 10.1007/BF00250566. [DOI] [PubMed] [Google Scholar]