Abstract
Hereditary disorders of magnesium homeostasis comprise a heterogenous group of diseases mainly affecting the renal conservation of magnesium. In the past few years, genetic studies in affected individuals disclosed the first molecular components of epithelial magnesium transport: the tight junction protein paracellin-1 (claudin-16) was discovered as a key player in paracellular magnesium and calcium reabsorption in the thick ascending limb of Henle's loop and the γ-subunit was identified as a component of renal Na+–K+-ATPase critical for transcellular magnesium reabsorption in the distal convoluted tubule. However, the molecular identity of proteins directly involved in cellular magnesium transport remained largely unknown until a series of recent studies highlighted the critical role of two members of the transient receptor potential (TRP) family, for body magnesium homeostasis. TRPM6 and TRPM7 belong to the melastatin-related TRPM subfamily of TRP channels whose eight members exhibit a significant diversity in domain structure as well as cation selectivity and activation mechanisms. Both proteins share the unique feature of an atypical kinase domain at their C-terminus for which they have been termed ‘chanzymes’ (channels plus enzymes). Whereas electrophysiological and biochemical analyses identified TRPM7 as an important player in cellular magnesium homeostasis, the critical role of TRPM6 for epithelial magnesium transport emerged from the discovery of loss-of-function mutations in patients with a severe form of hereditary hypomagnesaemia called primary hypomagnesaemia with secondary hypocalcaemia or HSH. The aim of this review is to summarize the data emerging from molecular genetic, biochemical and electrophysiological studies on these fascinating two new proteins combining ion channel and enzyme functions/properties.
Magnesium is the dominant divalent intracellular cation and is essential for a tremendous variety of cellular processes such as enzyme function, DNA and protein synthesis, and the regulation of ion channels. Magnesium homeostasis primarily depends on the balance between intestinal absorption and renal excretion. However, little is known about the molecular nature of the proteins involved in transepithelial transport of magnesium in these organs. Therefore, genetic studies in rare hereditary diseases affecting magnesium metabolism offer a unique opportunity to study the mechanisms of cellular magnesium transport at the molecular level.
Recently, the genetic analysis of patients with hypomagnesaemia with secondary hypocalcaemia (HSH), a disorder characterized by a combined defect of intestinal and renal magnesium transport, resulted in the discovery of TRPM6 as a crucial component of epithelial magnesium transport (Schlingmann et al. 2002; Walder et al. 2002). Before, a close homologue of TRPM6, the ubiquitiously expressed TRPM7, was functionally characterized as a constitutively active ion channel permeable for a variety of cations including calcium and magnesium and regulated by intracellular concentrations of magnesium and/or magnesium–nucleotide complexes (Nadler et al. 2001; Runnels et al. 2001). TRPM7 was shown to play a crucial role in cellular magnesium homeostasis as targeted gene deletion of TRPM7 in cell lines led to intracellular magnesium deficiency and growth arrest (Schmitz et al. 2003).
Both TRPM6 and TRPM7 are closely related members of the TRPM transient receptor potential ion channel family named after its founding member melastatin. The eight proteins of the TRPM family harbour an ion channel moiety with six transmembrane domains and a putative pore region between the fifth and sixth transmembrane domain and contain a 25-amino acid TRP domain of unknown function located C-terminally of the ion channel domain common to all TRP channels. TRPM channels differ from other TRP subfamilies in exhibiting a long conserved N-terminus of unknown function as well as an extended C-terminus. Three members of the TRPM family are characterized by a C-terminal enzyme domain for which these proteins have been termed ‘chanzymes’ (Montell, 2003). TRPM6 and TRPM7 share the feature of unique C-terminal serine/threonine protein kinase domains resembling those of elongation factor 2 (eEF-2) kinase and other α-kinases (Ryazanova et al. 2001).
TRPM6 mutations found in HSH patients are the only naturally occurring human knock-out for a member of the TRPM family described so far. Therefore, the mutational analysis of affected individuals together with a functional characterization of mutant TRPM6 represents an attractive approach to gain further insight into TRPM ion channel function.
Hypomagnesaemia with secondary hypocalcaemia (HSH)
Primary hypomagnesaemia with secondary hypocalcaemia is a rare autosomal-recessive disease characterized by extremely low serum magnesium levels (∼0.2 mmol l−1) accompanied by hypocalcaemia (∼1.6 mmol l−1). It was first described by Paunier et al. (1968). Hypocalcaemia results from an inhibition of parathyroid hormone synthesis and release from the parathyroid gland in the presence of profound hypomagnesaemia (Anast et al. 1972). It is typically manifested in affected children during the first months of life with generalized convulsions or signs of increased neuromuscular excitability like muscle spasms or tetany. Relief of clinical symptoms and complete normalization of calcium homeostasis is assured by an immediate administration of magnesium usually via the intravenous route followed by lifelong substitution with high oral doses of magnesium (Shalev et al. 1998). In contrast, serum magnesium levels usually fail to reach normal values under oral substitution and remain in the subnormal range (around 0.5–0.6 mmol l−1). Delay in diagnosis can lead to severe neurological deficits or may even be fatal. Daily oral magnesium doses greatly vary between patients with a mean dose of around 1 mmol (kg body wt)−1 day−1 which is equivalent to four times the recommended daily allowance. Adolescent patients usually tolerate oral magnesium to a lesser extent than infants and younger children and on average receive larger amounts per kilogram body weight. The main side-effect of high oral magnesium supplementation is pronounced diarrhoea which is observed in a considerable number of patients.
Contrasting with all other hereditary disorders of magnesium homeostasis, pathophysiological studies in affected patients using radioactive magnesium isotopes pointed to a primary defect in intestinal magnesium absorption (Lombeck et al. 1975; Milla et al. 1979). The presence of an additional renal magnesium leak in HSH was controversially discussed (Milla et al. 1979; Stromme et al. 1981; Matzkin et al. 1989). At last, renal magnesium wasting in HSH patients was clearly demonstrated by using magnesium loading tests (Walder et al. 2002). Whereas magnesium excretions tend to be low during initial presentation, the renal magnesium leak becomes apparent with rising serum magnesium concentrations indicating a lowered renal threshold for magnesium. Physiologically, the kidney aims at preserving magnesium by lowering fractional excretions below 0.5–1% during hypomagnesaemia with virtually absent renal magnesium excretion when serum magnesium decreases below ∼0.7 mmol l−1 (Rodriguez-Soriano et al. 1987). In contrast, HSH patients exhibit high fractional excretions of around 3% in the face of continuous hypomagnesaemia (see above) (Schlingmann et al. 2002).
By using a DNA pooling strategy, Walder and co-workers had mapped a gene locus for HSH on chromosome 9q22 (Walder et al. 1997). A positional candidate gene approach then identified mutations in TRPM6 as the underlying genetic defect in HSH (Schlingmann et al. 2002; Walder et al. 2002). The gene product TRPM6 had initially been identified following a screening for homologues of elongation factor-2 kinase and was originally termed kidney kinase or channel kinase-2 (ChaK2) (Ryazanova et al. 2001). Expression studies detected the presence of TRPM6 along the entire gastrointestinal tract as well as in kidney, predominantly in the distal convoluted tubule (DCT) (Schlingmann et al. 2002). Immunohistochemistry confirmed the expected apical expression in the distal convoluted tubule and showed a complete co-localization with the sodium–chloride co-transporter (NCCT) and also with parvalbumin and calbindin-D28K, two cytosolic proteins that putatively act as intracellular magnesium buffers (Voets et al. 2004).
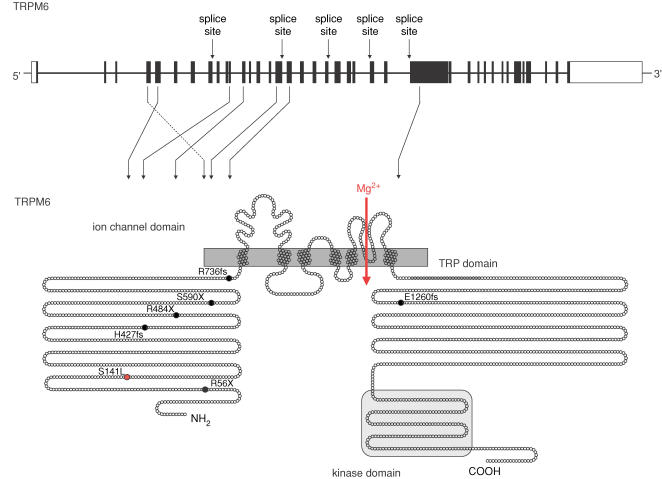
The TRPM6 gene comprises 39 exons and codes for a large protein of up to 2022 amino acids (Fig. 1). A variety of splice variants has been identified including three alternative first exons (Chubanov et al. 2004). The biological relevance of these variants, however, remains unknown. The spectrum of mutations identified in HSH so far comprises stop mutations, splice site mutations, frame shift mutations, deletions of exons, as well as a single point mutation (Schlingmann et al. 2002; Walder et al. 2002). Mutations mostly lead to a truncated TRPM6 protein. The only point mutation, S141L, affects a highly conserved amino acid residue in the N-terminus of the TRPM6 protein. Mutations leading to premature stops of translation are distributed over the entire TRPM6 gene.
Figure 1. TRPM6 gene and grotein.
TRPM6 consists of 39 exons spanning 167 kb of genomic sequence and coding for a protein of 2022 amino acids. The TRPM6 protein harbours an ion channel region with six transmembrane domains and a putative pore region between the fifth and sixth transmembrane domain, a long N-terminus conserved within the TRPM family, the TRP domain of unknown function located C-terminally of the ion channel domain, and a C-terminal kinase domain with sequence similarity to the atypical α kinases. The mutations identified in HSH patients by Schlingmann et al. (2002) and Walder et al. (1997) are indicated. Several splice site mutations were detected, indicated at the corresponding TRPM6 exon in the upper part of the figure. Diverse premature stop mutations and single base pair deletions leading to shifts of the reading frame and a premature stop of translation (black) as well as one single point mutation S141L (red) were identified. For these the consecutive change in TRPM6 amino acid sequence is shown in the lower part of the figure.
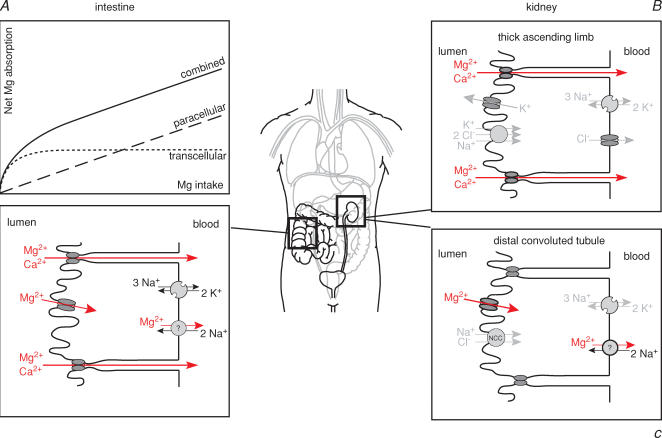
What are the pathophysiological consequences of a TRPM6 defect for magnesium transport in intestine and distal convoluted tubule? In the intestine, physiological studies indicate two different transport systems for magnesium (Fig. 2): an active transcellular transport and a passive paracellular pathway (Fine et al. 1991; Kerstan & Quamme, 2002). The saturable transcellular uptake consists of an apical entry into the epithelial cell through a specific ion channel and an unknown basolateral extrusion mechanism which probably couples magnesium export to sodium influx. Whereas at relatively low, physiological intraluminal concentrations magnesium is primarily taken up via the active transcellular route, passive paracellular magnesium absorption linearly increases with rising intraluminal concentrations. Together the two transport processes yield a curvilinear kinetic for intestinal magnesium absorption (Fig. 2). By increasing oral magnesium intake and consecutively enhancing passive paracellular absorption, HSH patients with defective transcellular magnesium transport are able to achieve relief of symptoms and at least subnormal serum magnesium levels.
Figure 2. Epithelial magnesium transport in intestine and kidney.
A, intestinal absorption follows a curvilinear kinetic resulting from two transport mechanisms: a saturable transcellular transport (dotted line) which is of functional importance at low intraluminal concentrations and a paracellular passive transport (dashed line) linearly rising with intraluminal magnesium concentrations. TRPM6 is a component of the active transcellular pathway as HSH patients are able to compensate for their genetic defect by high oral magnesium intake. B, in the thick ascending limb magnesium is reabsorbed via the paracellular route. Here, a specific tight juntion protein, paracellin-1 or claudin-16, permits the selective paracellular flux of calcium and magnesium. Defects in paracellin-1 lead to combined calcium and magnesium wasting. C, the distal convoluted tubule reabsorbs magnesium in a transcellular fashion, consisting of an apical entry into the DCT cell through a magnesium-selective ion channel, probably consisting of TRPM6/TRPM7 heterotetramers, and a basolateral extrusion of unknown molecular identity.
In the kidney, different transport pathways for magnesium exist along the nephron (Fig. 2) (de Rouffignac & Quamme, 1994). The vast majority of filtered magnesium is reabsorbed in the thick ascending limb of Henle's loop via the paracellular route. This process is mediated by a specific pore-forming protein called claudin-16 or paracellin-1 (Simon et al. 1999). Mutations in claudin-16 lead to familial hypomagnesaemia with hypercalciuria and nephrocalcinosis, a combined urinary magnesium and calcium wasting which almost invariably leads to progression to end stage renal disease (Weber et al. 2001).
Only around 10% of filtered magnesium is reabsorbed in the distal convoluted tubule (DCT). However, the DCT determines the final urinary magnesium concentration as no reabsorption takes place beyond this segment (Quamme, 1997). Magnesium reabsorption in the DCT is active and transcellular in nature.
The renal magnesium leak in HSH patients together with the expression pattern argue for an important role of TRPM6 for active transcellular magnesium reabsorption in the DCT. The current data suggest that TRPM6, probably in cooperation with TRPM7, comprises the apical magnesium channel responsible for magnesium uptake from urine into DCT cells. In HSH patients, renal magnesium wasting not only contributes to the development of hypomagnesaemia in the postnatal period but also prevents an adequate conservation of the absorbed magnesium under supplementation.
Functional characterization of TRPM6
The discovery of TRPM6 mutations in hereditary hypomagnesaemia together with the functional data generated for TRPM7 called for functional characterization of TRPM6. Unfortunately, results of different functional characterizations of TRPM6 vary substantially. One group succeeded in the heterologous expression of TRPM6 in HEK293 cells and described large outwardly rectifying whole-cell currents strongly resembling the currents observed for TRPM7 by Nadler and colleagues (Voets et al. 2004). In the presence of millimolar concentrations of calcium and magnesium, currents show a reversal potential near 0 mV without change in the current–voltage relationship upon removal of either extracellular sodium or chloride. As, under extracellular divalent-free conditions, large inward currents develop most probably indicating sodium permeability, the authors assumed that the outwardly rectifying current–voltage profile of TRPM6-induced channels reflects small inward fluxes of divalent cations at negative potentials and large outward flux of sodium at positive voltages (Voets et al. 2004).
The permeation characteristics with inward currents exclusively carried by divalent cations with a higher affinity for magnesium than calcium would support the role of TRPM6 as the apical magnesium influx pathway. Furthermore, Voets and colleagues demonstrated that TRPM6-induced currents – in analogy to TRPM7 – exhibit a marked sensitivity to intracellular magnesium (Voets et al. 2004).
In contrast, our group failed to observe measurable currents upon TRPM6 expression. Instead, heterologous expression studies of TRPM6 pointed to an intracellular retention of the TRPM6 protein (Fig. 3). As heteromultimerization had already been demonstrated for several members of the TRP family, in particular for the classic or canonical TRPC channels (Hofmann et al. 2002), a functional interaction between TRPM6 and TRPM7 appeared possible. Studies on TRPC heteromultimerization indicate that TRP subunits solely interact with highly homologous partners, e.g. heteromultimerization only occurs within the TRPC1/4/5 and TRPC3/6/7 subgroups. The phylogenetic analysis of the TRPM subfamily based on amino acid sequence similarities indicates that TRPM6 and TRPM7 fall into one of four distinct groups composed of the eight family members (Vennekens et al. 2002). Therefore, a functional interaction between the two chanzymes appeared quite plausible.
Figure 3. Immunolocalization of transiently expressed TRPM6 and TRPM7 in HEK 293 cells.
TRPM7 (M7) and TRPM6 (M6) were expressed alone and co-expressed (M6 + M7). In co-expression experiments a cDNA ratio of 1: 3 (TRPM6/TRPM7) was used. Transfected cells were fixed and stained using either TRPM7-(M7) or TRPM6-(M6 and M6 + M7) specific antisera. Cells were examined by confocal microscopy. Images of FITC fluorescence (FITC) and overlays (FITC/DIC Overlay) of FITC and the corresponding differential interference-contrast images (DIC) are shown. The arrows indicate the plasma membrane. Typical examples of three independent experiments are presented.
In fact, we could provide several lines of evidence that support the critical role of TRPM6/7 heteromultimerization for TRPM6 function (Chubanov et al. 2004). First, RT-PCR studies on microdissected rat nephron segments confirmed the overlapping expression patterns of TRPM6 and TRPM7 along the nephron with marked expression of both proteins in the distal convoluted tubule. Heterologous expression either in the Xenopus oocyte expression system or in mammalian cells consistently proved that the TRPM6 protein alone is retained intracellularly.
In contrast, co-expression with TRPM7 resulted in incorporation of TRPM6 into channel complexes at the plasma membrane detected by a TRPM6-specific polyclonal antibody or by using GFP-fusion constructs. Furthermore, a direct and specific protein–protein interaction was confirmed by a static FRET (fluorescence resonance energy transfer) strategy.
Electrophysiological data on TRPM6/7 heteromultimerization originated from two-electrode voltage clamp experiments in the Xenopus oocyte expression system. Co-expression of TRPM6 and TRPM7 resulted in a significant amplification of TRPM7-induced currents (Chubanov et al. 2004). Electrophysiological recordings from Xenopus oocytes were also supported by manganese quench experiments. In HEK293 cells, co-expression of TRPM6 and TRPM7 results in a marked enhancement of manganese entry compared with manganese entry in cells expressing TRPM7 alone (Chubanov et al. 2004).
Finally, these results are also confirmed by functional analysis of the TRPM6S141L mutation observed in HSH. The TRPM6S141L mutation observed in an HSH patient was found to abrogate the interaction between TRPM6 and TRPM7 and the mutant TRPM6S141L is retained within the cell (Fig. 3). The failure of TRPM6S141L to form heteromultimers with TRPM7 was also demonstrated using the FRET technique. Furthermore, the mutant failed to increase TRPM7-induced currents as does wild-type TRPM6 in the Xenopus oocyte system (Chubanov et al. 2004). Therefore, the S141L mutation is assumed to interfere with TRPM heteromultimerization probably in the endoplasmic reticulum. In addition, these findings might point to a critical role of the long N-terminus of TRPM channels for the assembly of tetrameric channel complexes.
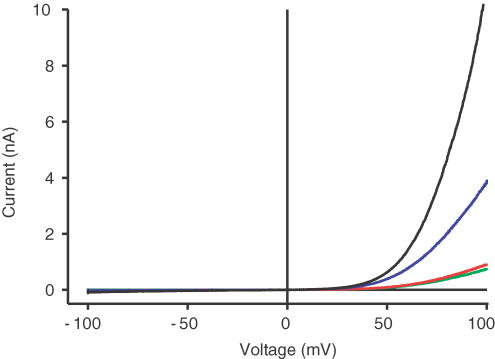
We also replicated the electrophysiological results obtained in the Xenopus oocyte system in a mammalian cell system (Fig. 4). For this purpose, TRPM6 and TRPM7 were stablely transfected into HEK293 cells by using an ecdysone-inducible gene expression system (Invitrogen). Uninduced cells displayed only minor endogenous currents probably indicating endogenous TRPM7 (Fig. 4, green curve). In line with the results obtained in Xenopus oocytes, induction of stably transfected TRPM6 with Ponasterone results in currents not significantly different from endogenous currents (Fig. 4, red curve). In contrast, Ponasterone induction of a stably transfected TRPM7 construct leads to an induction of currents with the typical features observed for TRPM7 in mammalian cells with pronounced outward rectification at positive potentials (Fig. 4, blue curve). These currents are again significantly amplified by transient co-expression of TRPM6 (Fig. 4, black curve).
Figure 4. Patch-clamp analysis of stably transfected TRPM6/7 in HEK293 cells.
TRPM6 and TRPM7 were each stably transfected into HEK293 cells by using an ecdysone inducible gene expression system (Invitrogen) that allows the tightly controlled expression of transfected constructs by induction with Ponasterone A (5 μm). Whole-cell patch clamp recordings were performed 18–24 h after induction. Without induction, small endogenous TRPM7 currents are observed (green curve). Induction of stably transfected TRPM6 does not result in a significant increase in currents (red curve), whereas induction of a stably transfected TRPM7 results in the development of large currents with outward rectification at positive potentials (blue curve) as described before (Runnels et al. 2001; Nadler et al. 2001). Additional transient expression of TRPM6 (Fugene6, Roche) in the Ponasterone-induced TRPM7 cell line leads to a significant amplification of TRPM7-like currents (black curve).
Taken together, these data convincingly suggest that TRPM6 interacts with its homologue TRPM7 to form functional channel complexes at the plasma membrane, although this assumption is based on data obtained in heterologous over-expression systems and TRPM6 channel characteristics may vary in vivo. Furthermore, information on pore properties and ion selectivity is still lacking for TRPM6/7 channels. To ultimately establish the role of TRPM6/7 heterotetramers as an essential component of the epithelial magnesium uptake machinery, future studies will have to provide deeper insight into functional characteristics and regulation of TRPM6/7 channel complexes. In this context, the functional analysis of further TRPM6 single amino acid substitutions observed in HSH patients presents an attractive approach.
Another intriguing question is whether a putative heteromeric TRPM6/7 channel complex exhibits distinct biophysical properties compared with TRPM7 homotetramers. Whereas TRPM7 putatively serves as an ubiquitious cellular magnesium uptake mechanism, the hypomagnesaemic phenotype of HSH patients together with the expression pattern of TRPM6 suggest that TRPM6 – probably in co-operation with TRPM7 – accomplishes the uptake of magnesium through the epithelial barrier in intestine and kidney. One can easily imagine that this specialized function requires distinct qualities in activation and regulation of the channel complex.
The isolated TRPM6 kinase domain has already been shown to exhibit phosphotransferase activity with properties nearly identical to those of the TRPM7 kinase domain (Ryazanova et al. 2001). Recent data demonstrate a critical role of the kinase domain for TRPM7 ion channel function. While phosphotransferase activity is obviously not required for channel activation, deletion of the kinase domain leads to a suppression of ion channel activation at physiological intracellular magnesium levels (Schmitz et al. 2003). The authors conclude that the channel inhibition by intracellular magnesium is influenced by the co-ordinated action of ion channel and kinase domains. The ion channel domain probably modulates phosphotransferase activity either by increasing intracellular magnesium or directly via conformational changes induced by gating of the channel. The kinase might then phosphorylate yet unidentified substrates providing real time information on channel activity or cellular magnesium status. Therefore, TRPM7 could potentially serve both as a magnesium uptake mechanism and a magnesium sensor (Schmitz et al. 2004). In contrast to the observations reported above, TRPM7 was recently found to be completely inactive upon deletion of the entire kinase domain, although the expression level of the C-terminally truncated protein was very similar to that of wild-type TRPM7 (Matsushita et al. 2005). Further experimentation is required to reconcile these discrepant findings.
A scenario similar to that of TRPM7 is well conceivable for TRPM6. Truncation of TRPM6 prior to the kinase domain leads to a loss of TRPM6 ion channel function as observed in HSH (Schlingmann et al. 2002). Unfortunately, mutations directly affecting the kinase domain and impairing phosphotransferase activity have not been identified yet. Potentially, abrogating phosphotransferase activity does not abolish TRPM6 ion channel function while deleting the kinase structure destroys ion channel activity as observed for TRPM7.
Very recently, Ryazanov's group identified annexin 1 as a first substrate of TRPM7 kinase (Dorovkov & Ryazanov, 2004). Annexin 1 is member of the ‘annexin’ family of calcium- and phospholipid-binding proteins, that was originally identified as an endogenous mediator of the anti-inflammatory actions of glucocorticoids, but was also implicated in the regulation of cell growth and apoptosis (Perretti & Solito, 2004; Rescher & Gerke, 2004). Phosphorylation occurs specifically at a conserved serine residue (Ser5) in the N-terminus of annexin 1, that is thought to mediate interactions of annexin 1 with protein ligands and membranes. Though phosphorylation of annexin 1 at different threonine and serine residues has been described before, phosphorylation of Ser5 is possibly specific for TRPM7 protein kinase (Dorovkov & Ryazanov, 2004). As both, annexin 1 and TRPM7, have been linked to processes of cell survival and cell growth (Aarts et al. 2003; Perretti & Solito, 2004), the authors speculate that the regulation of cell death and proliferation by TRPM7 involves the phosphorylation of annexin 1 by TRPM7 kinase. It will be interesting to see if this specific phosphorylation can also be mediated by TRPM6 kinase or is specific for TRPM7. As phosphorylation of annexin 1 at its N-terminus possibly alters its membrane binding abilities, it is intriguing to speculate if phosphorylation of annexin 1 in turn influences the integrity of the TRPM6/7 channel complex at the cell surface. The discovery of the substrates of TRPM6 and TRPM7 kinases will certainly be key in understanding the physiological role and regulation of these molecules in vivo.
In conclusion, the identification of TRPM6 and TRPM7 for the first time provides insight into cellular magnesium transport. The functional properties and cellular role of TRPM7, the phenotype of HSH patients with defective TRPM6, and the functional link between both TRPM proteins point to a crucial role of TRPM6/7 channel complexes for epithelial magnesium uptake. The specific functional characteristics and the regulation of the supposed heterotetramer as a prerequisite for transepithelial magnesium transport are intriguing subjects of future investigations. For this purpose, the combined genetic and functional analysis of defective TRPM6 identified in HSH patients provides a favourable opportunity.
References
- Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, et al. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–877. doi: 10.1016/s0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- Anast CS, Mohs JM, Kaplan SL, Burns TW. Evidence for parathyroid failure in magnesium deficiency. Science. 1972;177:606–608. doi: 10.1126/science.177.4049.606. [DOI] [PubMed] [Google Scholar]
- Chubanov V, Waldegger S, Mederos y Schnitzler M, Vitzthum H, Sassen MC, Seyberth HW, et al. Disruption of TRPM6/TRPM7 complex formation by a mutation in the TRPM6 gene causes hypomagnesemia with secondary hypocalcemia. Proc Natl Acad Sci U S A. 2004;101:2894–2899. doi: 10.1073/pnas.0305252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rouffignac C, Quamme G. Renal magnesium handling and its hormonal control. Physiol Rev. 1994;74:305–322. doi: 10.1152/physrev.1994.74.2.305. [DOI] [PubMed] [Google Scholar]
- Dorovkov MV, Ryazanov AG. Phosphorylation of annexin I by TRPM7 channel-kinase. J Biol Chem. 2004;279:50643–50646. doi: 10.1074/jbc.C400441200. [DOI] [PubMed] [Google Scholar]
- Fine KD, Santa Ana CA, Porter JL, Fordtran JS. Intestinal absorption of magnesium from food and supplements. J Clin Invest. 1991;88:396–402. doi: 10.1172/JCI115317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann T, Schaefer M, Schultz G, Gudermann T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc Natl Acad Sci U S A. 2002;99:7461–7466. doi: 10.1073/pnas.102596199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstan D, Quamme G. Physiology and pathophysiology of intestinal absorption of magnesium. In: Massry SG, Morii H, Nishizawa Y, editors. Calcium in Internal Medicine. London: Springer-Verlag; 2002. pp. 171–183. [Google Scholar]
- Lombeck I, Ritzl F, Schnippering HG, Michael H, Bremer HJ, Feinendegen LE, Kosenow W. Primary hypomagnesemia. I. Absorption studies. Z Kinderheilkd. 1975;118:249–258. doi: 10.1007/BF00492330. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Kozak JA, Shimizu Y, McLachlin DT, Yamaguchi H, Wei FY, et al. Channel function is dissociated from the intrinsic kinase activity and autophosphorylation of TRPM7/CHAK1. J Biol Chem. 2005 doi: 10.1074/jbc.M413671200. [DOI] [PubMed] [Google Scholar]
- Matzkin H, Lotan D, Boichis H. Primary hypomagnesemia with a probable double magnesium transport defect. Nephron. 1989;52:83–86. doi: 10.1159/000185588. [DOI] [PubMed] [Google Scholar]
- Milla PJ, Aggett PJ, Wolff OH, Harries JT. Studies in primary hypomagnesaemia: evidence for defective carrier-mediated small intestinal transport of magnesium. Gut. 1979;20:1028–1033. doi: 10.1136/gut.20.11.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C. Mg2+ homeostasis: the Mg2+nificent TRPM chanzymes. Curr Biol. 2003;13:R799–801. doi: 10.1016/j.cub.2003.09.048. [DOI] [PubMed] [Google Scholar]
- Nadler MJ, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ, et al. LTRPC7 is a Mg. ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- Paunier L, Radde IC, Kooh SW, Conen PE, Fraser D. Primary hypomagnesemia with secondary hypocalcemia in an infant. Pediatrics. 1968;41:385–402. [PubMed] [Google Scholar]
- Perretti M, Solito E. Annexin 1 and neutrophil apoptosis. Biochem Soc Trans. 2004;32:507–510. doi: 10.1042/BST0320507. [DOI] [PubMed] [Google Scholar]
- Quamme GA. Renal magnesium handling: new insights in understanding old problems. Kidney Int. 1997;52:1180–1195. doi: 10.1038/ki.1997.443. [DOI] [PubMed] [Google Scholar]
- Rescher U, Gerke V. Annexins – unique membrane binding proteins with diverse functions. J Cell Sci. 2004;117:2631–2639. doi: 10.1242/jcs.01245. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Soriano J, Vallo A, Garcia-Fuentes M. Hypomagnesaemia of hereditary renal origin. Pediatr Nephrol. 1987;1:465–472. doi: 10.1007/BF00849255. [DOI] [PubMed] [Google Scholar]
- Runnels LW, Yue L, Clapham DE. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science. 2001;291:1043–1047. doi: 10.1126/science.1058519. [DOI] [PubMed] [Google Scholar]
- Ryazanova LV, Pavur KS, Petrov AN, Dorovkov MV, Ryazanov AG. Novel type of signaling molecules: protein kinases covalently linked with ion channels. Mol Biol. 2001;35:271–283. [PubMed] [Google Scholar]
- Schlingmann KP, Weber S, Peters M, Niemann Nejsum L, Vitzthum H, Klingel K, et al. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet. 2002;31:166–170. doi: 10.1038/ng889. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Perraud AL, Fleig A, Scharenberg AM. Dual-function ion channel/protein kinases: novel components of vertebrate magnesium regulatory mechanisms. Pediatr Res. 2004;55:734–737. doi: 10.1203/01.PDR.0000117848.37520.A2. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, Penner R, et al. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell. 2003;114:191–200. doi: 10.1016/s0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- Shalev H, Phillip M, Galil A, Carmi R, Landau D. Clinical presentation and outcome in primary familial hypomagnesaemia. Arch Dis Child. 1998;78:127–130. doi: 10.1136/adc.78.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, et al. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 1999;285:103–106. doi: 10.1126/science.285.5424.103. [DOI] [PubMed] [Google Scholar]
- Stromme JH, Steen-Johnsen J, Harnaes K, Hofstad F, Brandtzaeg P. Familial hypomagnesemia – a follow-up examination of three patients after 9–12 years of treatment. Pediatr Res. 1981;15:1134–1139. doi: 10.1203/00006450-198108000-00012. [DOI] [PubMed] [Google Scholar]
- Vennekens R, Voets T, Bindels RJ, Droogmans G, Nilius B. Current understanding of mammalian TRP homologues. Cell Calcium. 2002;31:253–264. doi: 10.1016/s0143-4160(02)00055-6. [DOI] [PubMed] [Google Scholar]
- Voets T, Nilius B, Hoefs S, van der Kemp AW, Droogmans G, Bindels RJ, Hoenderop JG. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem. 2004;279:19–25. doi: 10.1074/jbc.M311201200. [DOI] [PubMed] [Google Scholar]
- Walder RY, Landau D, Meyer P, Shalev H, Tsolia M, Borochowitz Z, et al. Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat Genet. 2002;31:171–174. doi: 10.1038/ng901. [DOI] [PubMed] [Google Scholar]
- Walder RY, Shalev H, Brennan TM, Carmi R, Elbedour K, Scott DA, et al. Familial hypomagnesemia maps to chromosome 9q, not to the X chromosome: genetic linkage mapping and analysis of a balanced translocation breakpoint. Hum Mol Genet. 1997;6:1491–1497. doi: 10.1093/hmg/6.9.1491. [DOI] [PubMed] [Google Scholar]
- Weber S, Schneider L, Peters M, Misselwitz J, Ronnefarth G, Boswald M, et al. Novel paracellin-1 mutations in 25 families with familial hypomagnesemia with hypercalciuria and nephrocalcinosis. J Am Soc Nephrol. 2001;12:1872–1881. doi: 10.1681/ASN.V1291872. [DOI] [PubMed] [Google Scholar]