Abstract
Thyroid hormone deprivation during fetal life has been implicated in neurodevelopmental morbidity. In humans, poor growth in utero is also associated with fetal hypothyroxinaemia. In guinea pigs, a short period (48 h) of maternal nutrient deprivation at gestational day (gd) 50 results in fetuses with hypothyroxinaemia and increased brain/body weight ratios. Thyroid hormone action is mediated by nuclear thyroid hormone receptors (TRs) and is dependent upon the prereceptor regulation of supply of triiodothyronine (T3) by deiodinase enzymes. Examination of fetal guinea pig brains using in situ hybridization demonstrated widespread expression of mRNAs encoding TRα1, α2 and β1, with regional colocalization of deiodinase type 2 (D2) mRNA in the developing forebrain, limbic structures, brainstem and cerebellum at gd52. With maternal nutrient deprivation, TRα1 and β1 mRNA expression was significantly increased in the male, but decreased in the female fetal hippocampus and cerebellum and other areas showing high TR expression under euthyroid conditions. Maternal nutrient deprivation resulted in elevated D2 mRNA expression in males and females. Deiodinase type 3 (D3) mRNA expression was confined to the shell of the nucleus accumbens, the posterior amygdalohippocampal area, brainstem and cerebellum, and did not change with maternal nutrient deprivation. In conclusion, maternal nutrient deprivation resulted in sex-specific changes in TR mRNA expression and a generalized increase in D2 mRNAs within the fetal brain. These changes may represent a protective mechanism to maintain appropriate thyroid hormone action in the face of fetal hypothyroxinaemia in order to optimize brain development.
Thyroid hormones play an important role in the development of the mammalian central nervous system (CNS), and evidence suggests that this influence extends back into fetal life (Calvo et al. 1990, 2002). Studies in humans have shown that mild maternal hypothyroidism in early pregnancy is associated with adverse neuropsychological outcomes in children (Haddow et al. 1999; Pop et al. 2003), reflecting the sensitivity of the fetal CNS to changes in thyroid status.
Intrauterine growth restriction (IUGR), with fetal brain weight maintained relative to body weight, is often secondary to malplacentation syndromes. IUGR babies contribute significantly to perinatal mortality and childhood morbidity, including neurodevelopmental delay (Gaffney et al. 1994; Kok et al. 1998). Cordocentesis studies show that circulating thyroxine (T4) and triiodothyronine (T3) concentrations are significantly reduced in human fetuses with IUGR (Thorpe-Beeston et al. 1991; Kilby et al. 1998). In this connection, hypothyroxinaemia may be a protective mechanism for maintaining viability in IUGR by reducing metabolic demands, and hence oxygen consumption, at the expense of disrupting neurodevelopment (Thorpe-Beeston & Nicolaides, 1996). Often in severe, early onset intrauterine growth restriction there is vascular redistribution of blood flow, sparing of fetal brain growth and utilization of glycogen stores within the fetal liver. However, CNS abnormalities have been reported, and in animal models of IUGR (Mallard et al. 1998) these abnormalities are similar to those described in thyroid hormone deficiency (Balazs, 1971). This observation has led to the hypothesis that fetal hypothyroxinaemia contributes to the pathogenesis of the neurodevelopmental impairment in IUGR.
Guinea pigs, like humans, show extensive neuroendocrine maturation and rapid brain growth in late fetal life (Dobbing & Sands, 1979; Matthews, 1998). Maternal nutrient deprivation (MND) in guinea pigs results in fetuses with increased brain/body weight ratios and modified fetal endocrine function, including a significant reduction in circulating T4 concentrations (Lingas et al. 1999). In human maternal undernutrition, similar constraints on fetal growth have been reported (Lumey, 1992) and this has been linked to increased cardiovascular, metabolic and endocrine morbidity in adult life (Barker et al. 1989; Barker, 1999). These similarities therefore make the guinea pig a suitable animal model (and human analogue) with which to study the effects of MND on the role of thyroid hormone in fetal brain development.
Thyroid hormone effects are mediated by the expression of thyroid hormone receptors (TRs) and are dependent on the local delivery of the active ligand, T3, determined in part by enzymes that metabolize thyroid hormones, especially the iodothyronine deiodinases (Bianco et al. 2002). The TR isoforms α1, β1 and β2 are nuclear transcription factors which bind T3 to regulate the transcription of thyroid hormone responsive genes (Yen, 2001). Such genes include neurogranin and myelin basic protein, expression of which is reduced in the developing CNS in hypothyroxinaemia (Ibarrola & Rodriguez-Pena, 1997; Dowling & Zoeller, 2000). In addition, the non-ligand binding TRα2 isoform modulates the function of other TRs (Koenig et al. 1989).
The major circulating thyroid hormone is the prohormone, T4, which is converted to T3 by deiodinase type 1 (D1) or type 2 (D2) (Visser et al. 1983). Deiodinase type 3 (D3) is responsible for the inactivation of T4 and T3 to inactive metabolites (Kaplan & Yaskoski, 1980). Deiodinases are prereceptor regulators of local thyroid hormone action, and their actions may potentially compensate for changes in concentrations of circulating thyroid hormone in disease states (Visser et al. 1983; Visser, 1996). Evidence from in vivo and in vitro studies has suggested that D2 and D3 both participate in the homeostatic regulation of local T3 concentrations in the mammalian CNS with findings of D2 up-regulation and D3 down-regulation associated with hypothyroidism (Cavalieri et al. 1986; Ruiz de Ona et al. 1991; Guadano-Ferraz et al. 1999; Tu et al. 1999).
This study was designed to identify thyroid hormone responsive regions in the fetal guinea pig brain and to demonstrate the localization of deiodinase subtypes. This involved partial sequencing of the guinea pig genes for the TR isoforms and the deiodinases. We hypothesized that maternal nutrient deprivation, which is known to induce hypothyroxinaemia and IUGR (Lingas et al. 1999), will result in modification of TR expression and compensatory changes in deiodinase expression in the fetal brain.
Methods
Animals and treatments
Animal breeding and treatments were performed at the University of Toronto according to protocols approved by the Animal Care Committee and in accordance with the Canadian Council for Animal Care as previously described (Lingas et al. 1999). Pregnant guinea pigs were deprived of all food for 48 h on gestational day (gd) 50 (n = 7) or allowed to feed normally (control, n = 5) (term = 70 days). Water was available ad libitum. On gd52 (after 48 h treatment), pregnant guinea pigs were killed by decapitation. Fetuses were quickly removed and the brain dissected and stored at –80°C until processing.
As published previously (Lingas et al. 1999), plasma T4 concentrations were similar in control and deprived mothers but fetal T4 concentrations were significantly lower in the nutritionally deprived group (control 226.7 ± 20.3 ng ml−1; deprived 112.6 ± 13.4 ng ml−1; P < 0.001). We were unable to determine plasma T3 concentrations due to very limited plasma volume. The fetal weights were significantly different (control 44.18 ± 1.22 g; deprived 40.13 ± 0.63 g; P < 0.01) and the brain/body weight ratio was significantly higher in the deprived group (control 0.0438 ± 0.001; deprived 0.0488 ± 0.0006; P < 0.001) while the weights of the thyroid gland were not different. Normal litter size is two to three fetuses, and where possible both male and female fetuses were taken from each litter for subsequent brain analysis (control: male n = 5, female n = 8; deprived: male n = 8, female n = 8).
Gene sequencing and probe design
Partial sequencing of each of the mRNAs encoding the guinea pig TR isoforms and deiodinase subtypes was undertaken using polymerase chain reaction (PCR)-based techniques previously described (McCabe et al. 1999). Briefly, guinea pig tissue (liver, brain striatum or diaphragmatic skeletal muscle; 100 mg) was homogenized, total RNA prepared using a one-step guanidinium phenol–chloroform extraction method (Tri-reagent, Sigma-Aldrich UK) and reverse transcribed using avian myeloblastosis virus (AMV) reverse transcriptase (Promega, Madison, WI, USA), to yield cDNAs for use as templates. Degenerate primers were designed from human sequences, aimed at amplifying regions which included unique sequences of each TR isoform and deiodinase subtype. (Primer Express programs from DNA Star (Madison, USA) and Applied Biosystems (USA) Mac software.)
The products from the PCR performed using Taq DNA polymerase (Roche Diagnostics, Mannheim, Germany) were separated by electrophoresis using 2% Tris-acetate-EDTA (Eppendorf AG, Hamburg, Germany) agarose gels. Fragment sizes were gauged in relation to a 1 kb pair DNA mass ladder (Pharmacia, Uppsala, Sweden). PCR products of the expected sizes for each gene were purified after gel excision and eluted using QIAQuick gel purification kits (Qiagen, Hilden, Germany). The PCR products (50–100 ng) were used in each sequencing reaction, which consisted of the Big Dye cycle sequencing kit (3 μl) containing AmpliTaq (Perkin Elmer Applied Biosystems, Foster City, CA, USA) and 3.2 pmol of the respective forward or reverse primer with annealing temperatures set as for the PCR reactions for each gene. PCR fragments encompassing over 80% of the cDNA were sequenced in both directions on an ABI 377 sequencer and electropherograms analysed using DNAstar software.
The sequenced guinea pig gene fragments showed considerable homology with the human: TRα1 (100%), TRα2 (99%), TRβ1 (90%) and D1 (86%). The resulting partial sequences were used to design a 45 base oligonucleotide probe specific for the guinea pig mRNA for each gene (Sigma-Genosys, Mississuaga, Ontario, Canada) Attempts at sequencing guinea pig D2 and D3 were unsuccessful while TRβ2 was not sequenced. Therefore for these three genes, consensus sequences derived from several different vertebrate species (rat, mouse, chicken, human, killifish, Xenopus; sequences from NCBI PubMed database) were used to design the antisense probes.
A BLAST search (NCBI PubMed) was performed for each oligonucleotide probe sequence to ensure there was no significant resemblance to other sequenced genes. The probe sequences chosen were also highly homologous to other known mammalian sequences for each gene (Table 1). A sense probe with a similar G and C content was used as control. Preliminary experiments on guinea pig tissues known to express specific TRs and deiodinases revealed a positive expression for each probe, while no hybridization was seen with the control probe. (TRα1, TRα2, TRβ1, TRβ2 in the CNS; D1 in kidney and liver; D2 in the anterior pituitary gland; D3 in placenta; not illustrated.)
Table 1.
Probe sequences used for each thyroid hormone receptor (TR) isoform and deiodinase subtype for in situ hybridization and the close sequence homology it has to other species; the periods over which slides of the forebrain and brainstem/cerebellum were exposed to the radiographic film are also indicated
| Percentage comparative homology to | Exposure times required | |||||||
|---|---|---|---|---|---|---|---|---|
| Gene | Bases of coding sequence* | In situ hybridization probe (45 bases) | Human | Rat | Mouse | Pig | Fore-brain | Brainstem/Cerebellum |
| TRα1 | 1136–1180 | TCATGTGGAGGAAGCGGCTGG CGTGGCAGGCCCCGATCATGCGGA | 100 | 100 | 100 | 100 | 3w | 6w |
| TRα2 | 1112–1156 | CTGCCGCTGCCCCCTTGTACAG AATCGAACTCTGCACTTCTCTCT | 100 | 100 | 100 | 100 | 4d | 7d |
| TRβ1 | 493–537 | TCTCTTCTGTTTGGAAGGTCTG AGCACTAGAGATGCTCTGATCAT | 90 | — | 88 | 92 | 4w | 5w |
| TRβ2 | 156–200 | CCAGGGTAACTACAGGTATAAG GCTGATTCACTGCCCAGGCCTGT | 100 | 93 | 100 | — | 6w | — |
| D1 | 215–259 | CAGATGGTGCGCTTCTCTCCTG AGAGGCAAACCACCGAGCAGTCT | 80 | — | 88 | — | — | — |
| D2 | 125–169 | GGTCAGCATGCGCCGCCACTCTCC GCGAGTGGACTTGGAGCGGCT | 100 | 97 | 100 | — | 4w | 6w |
| D3 | 29–73 | GCGGGAACAGCACGAGGCAC GAGGCGGTCTGGGCGCAGAGCCTCA | 97 | 100 | 100 | — | 8w | 6w |
From ORF of human gene.
In situ hybridization (ISH)
The methods used have been described in detail previously (Matthews & Challis, 1995). Coronal cryosections (12 μm) of fetal brain (ranging from the bregma to the posterior hippocampus, and the lambda to the posterior lobe of the cerebellum) were mounted onto poly l-lysine coated slides and postfixed with 4% paraformaldehyde. The probes were labelled using terminal deoxynucleotidyl transferase (Gibco BRL, Burlington or Pharmacia LKB, Ontario, Canada) and 35S-labelled deoxyadenosine 5′-(α-thio)triphosphate (35S-dATP; 1300 Ci mmol−1; Du Pont, Canada) to a specific activity of 0.7–1.0 × 109 c.p.m. μg−1. Labelled probe in hybridization buffer was applied to slides at a concentration of 250–500 c.p.m. μl−1. Slides were incubated overnight in a moist chamber (42°C). After several washes with SSC, sections were dehydrated in ethanol and dried. Slides were exposed to autoradiographic film (Biomax, Kodak) together with sections incubated with the sense probe and 14C-standards (American Radiochemical, MO, USA). The latter were used to ensure that analysis was undertaken in the linear range of the autoradiographic film. Exposure times are listed in Table 1.
Data analysis
Sections from the forebrain and brainstem/cerebellum (BS/CB) were hybridized separately. However, for each gene in either the forebrain or BS/CB, sections were processed simultaneously to allow direct comparison between the control and deprived groups. The relative optical density (ROD) of the signal on autoradiographic film was quantified, after subtraction of background values, using a computerized image analysis system (Imaging Research, St Catherines, Ontario, Canada) (Matthews & Challis, 1995; Lingas et al. 1999).
In the forebrain, measurements were undertaken at the level of the anterior hippocampus (Figs 1 and 2) for the following areas: the cingulate cortex (Cg), cerebral cortex (Cx; all layers measured together, excluding piriform cortex), hippocampus (CA1/2, CA3 and CA4 subfields measured separately), upper blade of the dentate gyrus (DG). The lateral ventricles/shell of the accumbens nuclei (LV/ACBsh) and caudate putamen were measured from more cranial sections. The LV/ACBsh were measured together as they were not distinguishable on film. Measurements of the amygdala (Am) were taken where there was detectable expression. D3 mRNA was only measured in the LV/ACBsh, the capsule of the amygdalohippocampal nucleus (AHiPM) and posteromedial cortical amygdaloid nucleus (PMCo).
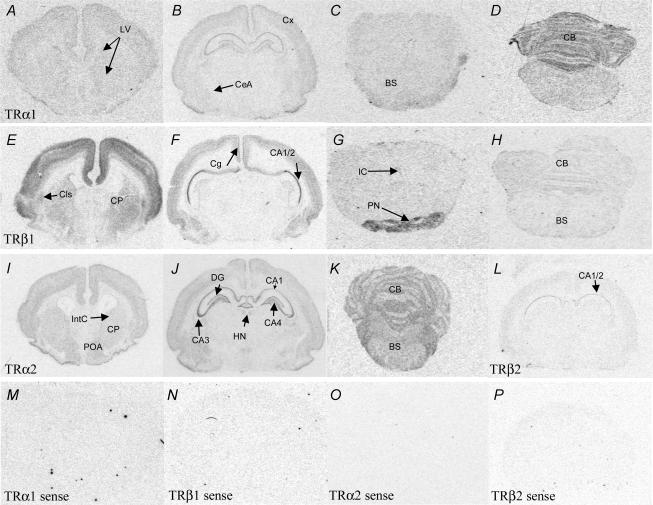
Figure 1. Representative coronal sections illustrating relative expression patterns of mRNA encoding for TRα1 (A–D), TRβ1 (E–H), TRα2 (I–K) and TRβ2 (L) in the brain, following a rostral caudal gradient from the forebrain to the brainstem (note: not all areas illustrated).
TRα1 mRNA expression was detected in the lining of the lateral ventricles (LV), cortex (Cx) hippocampal formation (CA1/2 and DG; see F) and central nucleus of the amygdala (CeA). Very low levels were detected in the brainstem (BS), while relatively high levels were observed in the cerebellum (CB). TRβ1 mRNA was highly expressed in the cerebral cortex, claustrum (Cls) and caudate putamen (CP). Specific expression was detected in the cingulate cortex (Cg) and the CA1/2 subfield of the hippocampus. High expression was observed in the pontine nuclei (PN) with low expression in the rest of the brainstem and cerebellum (BS/CB). TRα2 mRNA was expressed throughout the frontal cortex, including the internal capsule (IntC), caudate putamen (CP) and preoptic areas (POA). High levels were found in the hippocampal formation (CA1–4), dentate gyrus (DG) and habenular nuclei (HN). Low levels were detected in the brainstem (BS) with higher expression in the cerebellum (CB). TRβ2 mRNA was expressed at low levels in the cerebral cortex and the CA1/2 subfield of the hippocampus. As a negative control, sense probes for all thyroid hormone receptors were made. Sections hybridized with sense probes for TRα1 (M), TRβ1 mRNA (N), TRα2 (O) and TRβ2 mRNA (P); no signal was detected.
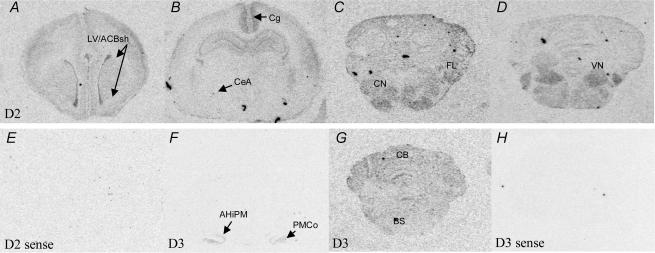
Figure 2. Representative coronal images of brain sections illustrating relative expression patterns of mRNA encoding deiodinase type 2 (D2) following a rostral–caudal gradient from the forebrain to the brainstem (note: not all areas illustrated).
D2 mRNA (A–D) expression was detected in the lining of the lateral ventricles (LV) and shell of the nucleus accumbens (ACBsh), cerebral cortex, cingulate cortex (Cg) and hippocampal formation, with low levels in the central nucleus of amygdala (CeA). D2 mRNA was expressed throughout the brainstem and cerebellum with particularly high levels being detected in the cochlear nuclei (CN), cerebellar flocculus (FL), and vestibular nucleus (VN). Deiodinase type 3 mRNA (D3, F and G) was found specifically in the capsule of the amygdalohippocampal nucleus (AHiPM) and posteromedial cortical amygdaloid nucleus (PMCo) in the forebrain but was more diffusely expressed in the brainstem (BS) and cerebellum (CB). As a negative control, sense probes for the deiodinase subtypes were made. Sections hybridized with sense probes for D2 (E) and D3 mRNA (H); no signal was detected.
In the brainstem and cerebellum, measurements were taken at the inferior colliculus (IC), pontine nuclei (PN), the brainstem as a whole (BS; average of readings at three different levels: caudal to the IC, caudal to the pons, lower medulla at the level of the dorsomotor nucleus of the vagus (DM)) and the cerebellum as a whole (CB; average of readings at the same three levels). In addition, TRα1 and TRα2 signals were quantified in the lateral areas of the brainstem (LatBS), which includes the cochlea nucleus (CN). D2 mRNA levels were also measured in the vestibular nuclei (VN), CN (average of ventral and dorsal areas) and cerebellar flocculus (FL). D3 mRNA was measured in the brainstem and cerebellum, each as a whole, at the level caudal to the pons.
Statistical methods
Group data were statistically analysed using a three-way ANOVA (treatment (MND) × sex × region) followed by Duncan's post hoc analysis. As this revealed differences between the sexes for some genes (detailed in results), we subsequently analysed the data using (1) a two-way ANOVA (sex × region) to detect differences in localization within the control group and (2) a two-way ANOVA (treatment × region) to detect the effects of MND within each sex separately. The ANOVA was followed by Bonferroni's method of all pair wise post hoc multiple comparisons. Group data are presented as means ± s.e.m. and statistical significance was set at P < 0.05.
Results
Localization of TR and deiodinase mRNA in fetal guinea pig brain
Forebrain
There were marked regional differences in the expression of mRNA transcripts encoding all the TR isoforms (P < 0.001) and D2 (P < 0.001). TRα1, TRα2 and TRβ1 mRNAs were widely expressed throughout the cerebral (Cx) and cingulate (Cg) cortices, LV/ACBsh and caudate putamen (CP) (Figs 1 and 3). Overall TRβ2 mRNA expression was more limited, with expression confined to the Cx, Cg and hippocampus. Expression of each TR isoform was highest in the hippocampus. The TRα isoforms were most abundantly expressed in the CA1–3 subfields with moderate expression in CA4 and DG, while the TRβ isoforms were predominantly expressed in CA1/2 with very low expression in the other subfields and DG.
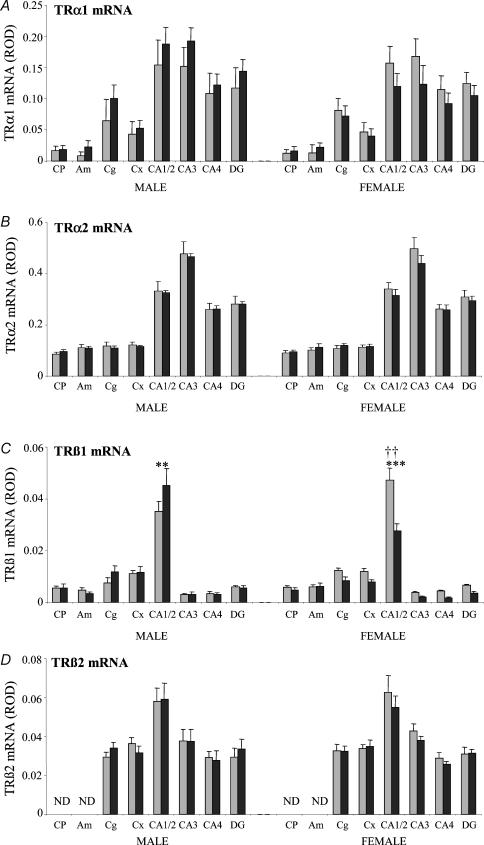
Figure 3. Densitometric analysis of expression of mRNAs encoding the TR isoforms (A: TRα1; B: TRα2; C: TRβ1; D: TRβ2) in the forebrain and limbic structures in control animals and after 48 h of nutrient deprivation in male (control (grey) n = 5, deprived (black) n = 8) and female fetuses (control n = 8, deprived n = 8).
Results are expressed as mean ± s.e.m. relative optical density (ROD). Statistical significance is indicated as: **P < 0.01, ***P < 0.001. †† indicates a significant difference between males and females in the control group. CP, caudate putamen; Am, amygdala; Cg, cingulate cortex; Cx, cerebral cortex; CA1/2, CA3, CA4 (hippocampal subfields); DG, upper blade of the dentate gyrus. ND, expression not detected.
The expression of D2 transcripts was localized to the primary regions of TR expression, though detailed cellular colocalization studies were not undertaken. Highest levels of D2 mRNA were detected in the cingulate cortex (Cg) with more moderate expression in the hippocampus (Figs 2 and 4). D3 expression was confined to the shell of the nucleus accumbens (ACBsh), capsule of the amygdalohippocampal nucleus (AHiPM) and posteromedial cortical amygdaloid nucleus (PMCo) and to the lateral ventricle (LV). No expression was observed in the cortex or hippocampus. There was no D1 mRNA expression in any region of the forebrain.
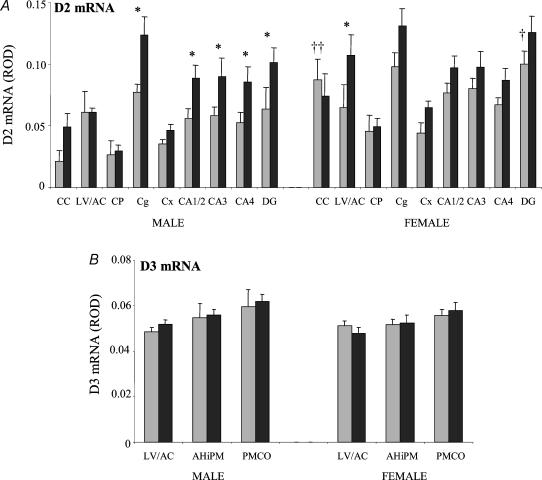
Figure 4. Densitometric analysis of expression of mRNAs encoding the iodothyronine deiodinase type 2 (D2; A) and type 3 (D3; B) in the forebrain and limbic structures in control animals and after 48 h of nutrient deprivation in male (control (grey) n = 5, deprived (black) n = 8) and female fetuses (control n = 8, deprived n = 8).
Results are expressed as mean ± s.e.m. relative optical density (ROD). Statistical significance is indicated as: *P < 0.05. †† indicates a significant difference between males and females in the control group. CC, corpus callosum; LV/AC, lateral ventricle and shell of the nucleus accumbens; CP, caudate putamen; Cg, cingulate cortex; Cx, cerebral cortex; CA1/2, CA3, CA4 are the hippocampal subfields; DG, upper blade of the dentate gyrus; AHiPM, capsule of the amygdalohippocampal nucleus; PMCO, posteriomedial cortical amygdaloid nucleus.
Posterior midbrain, brainstem and cerebellum
There were marked regional differences in the expression of mRNA encoding the TR α1, α2, β1 isoforms (P < 0.001), D2 (P < 0.001) and D3 (P < 0.01). These three TR isoforms were expressed in the inferior colliculus (IC), pontine nuclei (PN), dorsomotor nuclei of the vagus (DM), within various regions of the brainstem and diffusely within the cerebellum, with slightly higher expression in the Purkinje cell layers (Figs 1 and 5).
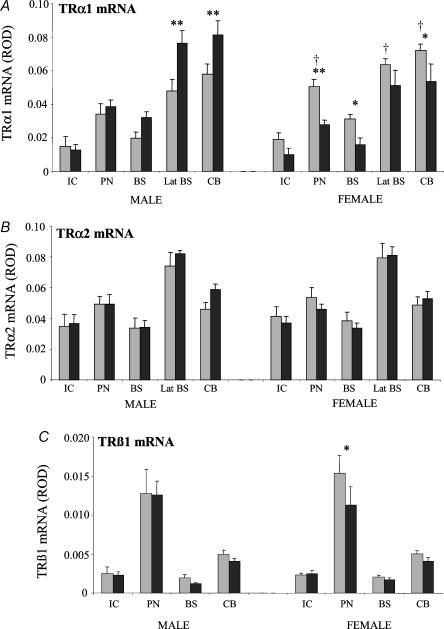
Figure 5. Densitometric analysis of expression of mRNAs encoding the TR isoforms (A: TRα1; B: TRα2; C: TRβ1) in the brainstem and cerebellum in control animals and after 48 h of nutrient deprivation in male (control (grey) n = 5, deprived (black) n = 7) and female fetuses (control n = 8, deprived n = 7).
Results are expressed as mean ± s.e.m. relative optical density (ROD). Statistical significance is indicated as: *P < 0.05, **P < 0.01. † indicates a significant difference between males and females of the control group. IC, inferior colliculus; PN, pontine nuclei; BS, brainstem; Lat BS, lateral regions of the brainstem; CB, cerebellum.
Within the brainstem, the highest levels of TRα1 and α2 transcripts were identified in the lateral regions (Lat BS; which include the cochlea and vestibular nuclei) while TRβ1 mRNA was highest in the pontine nuclei (Figs 1 and 5). There was strong expression of D2 mRNA in the inferior colliculus (IC), pontine nuclei (PN), the ventral and dorsal cochlea nuclei (CN), vestibular nuclei (VN) and the cerebellum (especially the flocculus). D3 mRNA expression was more diffuse throughout the brainstem and cerebellum, with no particular nuclei distinguishable (Figs 2 and 6). There was no TRβ2 or D1 mRNA expression in the BS/CB.
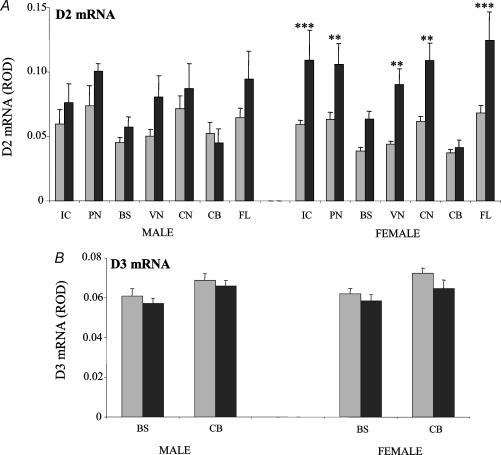
Figure 6. Densitometric analysis of expression of mRNAs encoding the iodothyronine deiodinase type 2 (D2; A) and type 3 (D3; B) in the brainstem and cerebellum in control animals and after 48 h of nutrient deprivation in male (control (grey) n = 5, deprived (black) n = 7) and female fetuses (control n = 8, deprived n = 7).
Results are expressed as mean ± s.e.m. relative optical density (ROD). Statistical significance are indicated as: *P < 0.05, **P < 0.01, ***P < 0.001. IC, inferior colliculus; PN, pontine nuclei; BS, brainstem; VN, vestibular nucleus; CN, cochlea nucleus; CB, cerebellum; FL, flocculus.
The effect of maternal nutrient deprivation (MND) on TR and deiodinase mRNA levels
Thyroid hormone receptors in the forebrain
There was a trend for increased TRα1 mRNA expression in males and reduced expression in females when MND fetuses were compared with controls (three-way ANOVA: effect of sex P < 0.01; Fig. 3A). However, subsequent post hoc analysis revealed that the differences were not regionally specific in either males or females. Overall, MND did not significantly modify TRα2 mRNA levels compared with controls in either male or female fetuses (Fig. 3B). TRβ1 mRNA expression in the control fetuses was higher in females than in males (effect of sex P < 0.01; effect of sex × region P < 0.001) with post hoc analysis revealing significantly higher expression in the CA1/2 subfield of the hippocampus of female control fetuses compared with male control fetuses (P < 0.001; Fig. 3C). MND significantly modified TRβ1 mRNA levels (three-way ANOVA: effect of sex × treatment P < 0.001; Fig. 3C) with different responses observed between male and female fetuses. In male fetuses (two-way ANOVA: effect of treatment not significant), post hoc analysis revealed a significant increase in TRβ1 mRNA in the CA1/2 hippocampal subfield (P < 0.01) with MND. In female fetuses (two-way ANOVA: effect of treatment P < 0.001; effect of treatment × region P < 0.001) there was a significant reduction in TRβ1 mRNA expression in the CA1/2 hippocampal subfield (P < 0.001) with MND compared with control. Maternal nutrient deprivation had no effect on TRβ2 mRNA expression in either male or female fetuses (Fig. 3D).
Deiodinases in the forebrain
D2 mRNA expression was higher in female controls than in male controls (P < 0.001), with significantly greater D2 mRNA expression in the dentate gyrus (DG; P < 0.05) and corpus callosum (P < 0.001) of female controls compared to male control fetuses (Fig. 4A). MND caused a significant widespread increase in D2 mRNA expression in both male and female fetuses (effect of treatment P < 0.001; Fig. 4A). In male fetuses, there was a significant increase of D2 transcripts particularly in the cingulate cortex (Cg; P < 0.01), CA1/2 (P < 0.05), CA3 (P < 0.05), CA4 (P < 0.05) subfields of the hippocampus and dentate gyrus (DG; P < 0.05) with MND compared with control. In female fetuses, significant increases were detected in the region of the lateral ventricle and shell of the nucleus accumbens (LV/ACBsh; P < 0.05) following MND. MND had no effect on D3 mRNA expression in either males or females (Fig. 4B).
Thyroid hormone receptors in the posterior midbrain, brainstem and cerebellum
Basal TRα1 mRNA expression in the control fetuses was higher in females than in males (P < 0.001) with significantly higher TRα1 mRNA expression in the pontine nuclei (P < 0.05), lateral brainstem (P < 0.05) and cerebellum (P < 0.05) in female control fetuses compared with male control fetuses (Fig. 5A). There was increased TRα1 mRNA expression in male fetuses and reduced expression in the females with MND compared with control fetuses (effect of sex × treatment P < 0.00001; Fig. 5A). Post hoc analysis showed a significant increase in TRα1 mRNA expression in the lateral brainstem (Lat BS; P < 0.01) and cerebellum (CB; P < 0.01) in male fetuses, while in female fetuses there was a significant reduction in TRα1 mRNA expression in the pontine nuclei (PN; P < 0.01), the brainstem (BS; P < 0.05) and cerebellum (CB; P < 0.05). Overall, there was a general trend of increased TRα2 mRNA in male fetuses but reduced expression in the females with MND compared with control fetuses (effect of sex × treatment P < 0.05; Fig. 5B) but when male and female fetuses were considered separately, post hoc analysis failed to reveal any statistically significant differences in all the regions measured.
When both sexes were considered together, MND had a significant effect overall on TRβ1 mRNA levels (P < 0.05; Fig. 5C). However, when the sexes were analysed separately, there was no significant differences seen for the effect of MND but post hoc analysis did show a significant reduction in the expression of TRβ1 mRNA in the pontine nuclei (P < 0.05) with MND compared with control in female fetuses only.
Deiodinases in the posterior midbrain, brainstem and cerebellum
Maternal nutrient deprivation resulted in a widespread significant increase in D2 mRNA expression in both male and female fetuses (P < 0.0001; Fig. 6A) with a more marked increase in female fetuses compared with male fetuses (three-way ANOVA: effect of sex × treatment P < 0.01). In male fetuses, even though the overall effect of MND was significant (P < 0.05), no statistically significant differences were found in specific regions. In contrast, in female fetuses, the overall effect of MND was highly significant (P < 0.001) with significant increases in D2 transcripts seen in the inferior colliculus (IC; P < 0.001), pontine nuclei (PN; P < 0.01), vestibular nuclei (VN; P < 0.01), cochlea nuclei (CN; P < 0.01) and flocculus (FL; P < 0.001) in fetuses from nutrient deprived mothers compared with control. Of note, MND had no effect on D2 mRNA expression within the cerebellum in male or female fetuses. Though there was a trend towards a reduction in D3 mRNA levels with MND this failed to reach statistical significance (three-way ANOVA: effect of treatment P= 0.06; two-way ANOVA: not significant for both sexes; Fig. 6B).
Discussion
This is the first study to have simultaneously determined the developmental profile of both the TR isoforms and deiodinases in the fetal brain of any species. This study has localized and defined the relative levels of mRNAs encoding specific TR isoforms and deiodinase subtypes in fetal guinea pig brain during the phase of rapid brain growth (gd50–52). We also describe for the first time sex-specific expression of TRs and deiodinases in the brain during development, as well as profound effects of maternal nutrient deprivation (MND) on their expression.
The widespread yet regionally specific expression patterns of the TR isoforms and deiodinase subtypes in the fetal guinea pig brain indicate they may play important and specific roles in neurodevelopment. The distribution of TR isoforms at gd50 in the guinea pig is consistent with that described in the neonatal rat brain (Mellstrom et al. 1991), which is in line with the notion that maximal brain growth in the guinea pig occurs at gd50, whereas it does not occur until postnatal days 5–8 in the rat (Dobbing & Sands, 1979). As a generalization, the thyroid hormone-responsive areas of the brain (i.e. those expressing TRs) also expressed D2. The D2 enzyme contributes significantly to the local supply of the active ligand, T3, in the CNS (Crantz et al. 1982). These areas also correspond to brain structures implicated in human neurobehavioural deficits (learning and memory (hippocampus), attention (caudate), visuospatial and auditory abilities and cognition (cerebral cortex), locomotor coordination (cerebellum)) observed in children exposed to hypothyroxinaemia early during the course of development (Rovet, 1999).
D2 is the most widely expressed of the three deiodinase subtypes in the fetal guinea pig brain, and this is consistent with reports of widespread expression of D2 transcripts in the neonatal rat brain (Guadano-Ferraz et al. 1997). However, we did not identify high levels of D2 mRNA in the lining of the third ventricle and the median eminence as reported previously in the neonatal (Guadano-Ferraz et al. 1997) and adult (Tu et al. 1997) rat. It is possible that D2 expression in these areas occurs after gd52 in the guinea pig. In this study, we localized D2 and D3 expression within the lateral ventricles, and these enzymes may have a role in the regulation of thyroid hormone supply to the CNS from the circulation via the choroid-plexus (Chanoine et al. 1992; Verhoelst et al. 2004). The highly localized pattern of D3 mRNA expression to specific limbic structures in the fetal guinea pig brain has also been reported in the neonatal rat (Escamez et al. 1999). This pattern of expression contrasts with more extensive D3 mRNA distribution described in the adult rat brain (Tu et al. 1999), perhaps further indicating a targeted protective role for D3 in the developing brain.
The absence of D1 activity in both human fetal and adult brains indicates that this subtype is unlikely to play a significant role in the human CNS (Chan et al. 2002), and the present findings suggest this may also be the case in the guinea pig. In contrast, D1 enzyme activity has been described in the adult rat brain (Visser et al. 1982).
Maternal nutrient deprivation increased mRNA levels for the major transactivating TR isoforms in male fetal brain, whereas expression decreased in females. The areas that expressed the highest amounts of TRα1 mRNA (cerebellum) and TRβ1 mRNA (CA1/2; pontine nuclei) under euthyroid (control) conditions were also the areas which were most affected during periods of nutrient-induced hypothyroxinaemia. Conversely, the expression of the nonligand binding isoform TRα2 and the weakly expressed TRβ2 were largely unaffected.
The mechanisms by which MND alters TR isoform expression are at present unclear but our results suggest that fetal hypothyroxinaemia may play a role. T3 treatment of several CNS cell types in vitro results in an increase in TRβ1 mRNA expression (Lebel et al. 1993; Chan et al. 2003b), with little effect on mRNAs encoding the TRα isoforms. This is likely to be mediated via direct T3 regulation at the two thyroid hormone response elements described in the promoter regions of the TRβ (c-erbAβ) gene (Sakurai et al. 1992). This would explain the decrease in TRβ1 mRNA in response to MND seen in female fetuses. On the other hand, androgens may alter the effect of MND on TR expression in male fetuses resulting in increased TRβ1 mRNA expression. Sexual differentiation of the fetal guinea pig brain occurs at approximately gd50, a time when male androgen levels are known to be elevated (Resko & Roselli, 1997). In this connection, MND also has sex-specific effects on the expression of other steroid hormone receptors within the developing brain, including mineralocorticoid receptors (MR) and glucocorticoid receptors (GR) (Lingas et al. 1999).
In patients with non-thyroidal illness, an increase in TR expression seen in non-CNS cell types may compensate for decreased circulating thyroid hormone levels (Williams et al. 1989). Although no similar studies have been carried out in the brain, it is possible that an up-regulation of TRs may partially compensate for the reduced availability of thyroid hormone, akin to that reported for other steroid hormone receptors (e.g. MR) in the fetal brain (McCabe et al. 2001). However, it is now recognized that during CNS development, TRα1 and β1 play important roles in the basal repression of gene transcription, which helps to regulate the timing of developmental events (Anderson et al. 2003), as well as the conventionally understood transactivating role of inducing thyroid hormone responsive gene transcription. So up-regulation of TR expression may not necessarily afford protection to brain development. Since thus far no clinical study of hypothyroxinaemia has described sex differences in neurodevelopmental outcomes in children, the divergent responses in TR expression to MND in male fetuses compared with female fetuses may represent sex-specific methods of compensation for hypothyroxinaemia or merely result from different developmental profiles at the time of MND. The latter possibility would agree with our findings of significant sex-specific differences in TR expression in specific brain regions under euthyroid conditions.
Fetuses of nutritionally deprived mothers demonstrated increases in D2 mRNA expression throughout the brain. Based on studies in adult rat cerebral cortex which have demonstrated that hypothyroidism has a greater up-regulatory effect on D2 activity than D2 mRNA compared with euthyroid controls (Burmeister et al. 1997), it is likely that in fetal guinea pig brains increased D2 mRNA would reflect increased D2 activity. This would maintain local thyroid hormone concentrations in the face of hypothyroxinaemia, supporting the possibility that D2 plays a crucial part in the homeostatic regulation of local T3 supply. The stimulatory effect that a reduction in T3 has on D2 transcription is likely to be indirect since a negative TRE has not been identified in the D2 promoter (Bianco et al. 2002). One possible mechanism is through the effect of cortisol, the primary glucocorticoid in guinea pigs, which is dramatically up-regulated in fetuses of nutritionally deprived mothers (Lingas et al. 1999). Dexamethasone administration to chick embryos has resulted in increased D2 activity in the brain (Reyns et al. 2005) and synthetic glucocorticoids have been shown to induce the transcription of D2 in a rat pituitary tumour cell line, an effect reduced in the presence of T3 (Kim et al. 1998).
In general, greater increases in D2 transcripts were seen in the forebrain of male fetuses with MND, while in female fetuses greater increases in D2 transcripts were detected in the brainstem. This may reflect a sex difference in compensatory mechanisms aimed at modulating the influence of hypothyroxinaemia in the brain and suggests that sex hormones may also have a role in the regulation of D2 expression. Androgens have been shown to suppress phenolic (outer-ring) monodeiodination activities (probably by D1 and D2) in the pineal gland of Syrian hamsters (Rubio et al. 1996) while oestrogen, but not progesterone and androgens, alters D2 activity in pituitaries of adult rats (Donda et al. 1990; Lisboa et al. 2001). These data suggests that the effects of sex hormones on D2 expression may be different across species, between different tissue types and CNS areas, and during various stages of development. It is notable that the cerebellum, a well-established thyroid hormone responsive organ, did not exhibit an increase in D2 expression except in the flocculus following MND. The lack of a compensatory response in the cerebellum makes it particularly vulnerable to hypothyroxinaemia and this may be associated with findings that fine motor skills are significantly poorer in children with congenital hypothyroidism than in euthyroid controls (Rovet, 1999).
We have previously noted a lack of correlation between changes in D3 transcript levels and D3 activity in fetal cerebral cortex (Chan et al. 2002) and placenta (Chan et al. 2003a) across gestation in normal human development, which is highly suggestive of post-transcriptional regulation but the mechanisms by which this occur are yet to be established. Thus in the present study the lack of change seen in D3 mRNA expression in response to hypothyroxinaemia following MND does not exclude the possibility of a compensatory down-regulation in D3 activity.
Fetuses of nutritionally deprived mothers also represent a model of poor fetal growth. Unlike the findings in this study, we have previously reported that the cerebral cortex and cerebellum of human fetuses with severe IUGR demonstrate a reduction in the expression of all the TR isoform proteins (determined by semiquantitative scoring of immunohistochemical staining) (Kilby et al. 2000). These data were derived from biopsies of cerebral cortex rather than completely intact tissues. Also, male and female fetuses were not analysed separately and were from a wide range of gestational ages. Our current study in guinea pigs is a more comprehensive assessment of TR expression in the CNS (whole brains) associated with, albeit less severe, growth restriction in utero in fetuses of the same gestational age.
Modification of the fetal environment by MND has permanent effects on the hypothalamo–pituitary–adrenal (HPA) axis development in guinea pigs (Lingas et al. 1999). Moreover, the development of GR systems in limbic structures and subsequent disruptions in HPA activity have been linked to thyroid hormone effects on the ascending serotonergic fibres innervating the limbic system (Meaney et al. 1987; Liu et al. 1997). We had previously found that GR mRNA was down-regulated specifically in the paraventricular nucleus (PVN) and CA1/2 region of the hippocampus in both male and female fetuses following MND (Lingas et al. 1999). Interestingly, TRβ1 and D2 mRNA expression were also affected in the CA1/2 region of the hippocampus. However, unlike the case for GR, the effect was strikingly sexually dimorphic for TRβ1 mRNA. Alterations in TR and deiodinase expression in the brainstem of IUGR fetuses and fetuses of nutritionally deprived mothers may also be implicated in the pathogenesis of an abnormal HPA axis.
In conclusion, we have shown that MND dramatically alters transcription of the two major TR isoforms, α1 and β1, in the fetal guinea pig brain in a sex- and region-specific manner, especially in the hippocampus, brainstem and cerebellum. MND-induced changes in mRNA expression, coupled with elevated levels of D2 mRNA may represent a fetal-specific, protective mechanism to maintain local T3 supply and minimize aberrant brain development.
Acknowledgments
Medical Research Council, UK; Royal College of Obstetricians and Gynaecologists, UK; Natural Sciences and Engineering Research Council of Canada; Scientific Projects Committee, University of Birmingham, UK; Society for Endocrinology, UK; Mason Research Trust, UK.
References
- Anderson GW, Schoonover CM, Jones SA. Control of thyroid hormone action in the developing rat brain. Thyroid. 2003;13:1039–1056. doi: 10.1089/105072503770867219. [DOI] [PubMed] [Google Scholar]
- Balazs R. Biochemical effects of thyroid hormones in the developing brain. UCLA Forum Med Sci. 1971;14:273–320. [PubMed] [Google Scholar]
- Barker DJ. Fetal origins of cardiovascular disease. Ann Med. 1999;31(Suppl. 1):3–6. [PubMed] [Google Scholar]
- Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23:38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- Burmeister LA, Pachucki J, St Germain DL. Thyroid hormones inhibit type 2 iodothyronine deiodinase in the rat cerebral cortex by both pre- and posttranslational mechanisms. Endocrinology. 1997;138:5231–5237. doi: 10.1210/endo.138.12.5602. [DOI] [PubMed] [Google Scholar]
- Calvo RM, Jauniaux E, Gulbis B, Asuncion M, Gervy C, Contempre B, Morreale De Escobar G. Fetal tissues are exposed to biologically relevant free thyroxine concentrations during early phases of development. J Clin Endocrinol Metab. 2002;87:1768–1777. doi: 10.1210/jcem.87.4.8434. [DOI] [PubMed] [Google Scholar]
- Calvo R, Obregon MJ, Ruiz De Ona C, Escobar Del Rey F, Morreale De Escobar G. Congenital hypothyroidism, as studied in rats. Crucial role of maternal thyroxine but not of 3,5,3′-triiodothyronine in the protection of the fetal brain. J Clin Invest. 1990;86:889–899. doi: 10.1172/JCI114790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri RR, Gavin LA, Cole R, De Vellis J. Thyroid hormone deiodinases in purified primary glial cell cultures. Brain Res. 1986;364:382–385. doi: 10.1016/0006-8993(86)90852-8. [DOI] [PubMed] [Google Scholar]
- Chan S, Kachilele S, Hobbs E, Bulmer JN, Boelaert K, McCabe CJ, Driver PM, Bradwell AR, Kester M, Visser TJ, Franklyn JA, Kilby MD. Placental iodothyronine deiodinase expression in normal and growth-restricted human pregnancies. J Clin Endocrinol Metab. 2003a;88:4488–4495. doi: 10.1210/jc.2003-030228. [DOI] [PubMed] [Google Scholar]
- Chan S, Kachilele S, McCabe CJ, Tannahill LA, Boelaert K, Gittoes NJ, Visser TJ, Franklyn JA, Kilby MD. Early expression of thyroid hormone deiodinases and receptors in human fetal cerebral cortex. Brain Res Dev Brain Res. 2002;138:109–116. doi: 10.1016/s0165-3806(02)00459-5. [DOI] [PubMed] [Google Scholar]
- Chan S, McCabe CJ, Visser TJ, Franklyn JA, Kilby MD. Thyroid hormone responsiveness in N-Tera-2 cells. J Endocrinol. 2003b;178:159–167. doi: 10.1677/joe.0.1780159. [DOI] [PubMed] [Google Scholar]
- Chanoine JP, Alex S, Fang SL, Stone S, Leonard JL, Korhle J, Braverman LE. Role of transthyretin in the transport of thyroxine from the blood to the choroid plexus, the cerebrospinal fluid, and the brain. Endocrinology. 1992;130:933–938. doi: 10.1210/endo.130.2.1733735. [DOI] [PubMed] [Google Scholar]
- Crantz FR, Silva JE, Larsen PR. An analysis of the sources and quantity of 3,5,3′-triiodothyronine specifically bound to nuclear receptors in rat cerebral cortex and cerebellum. Endocrinology. 1982;110:367–375. doi: 10.1210/endo-110-2-367. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Donda A, Reymond F, Rey F, Lemarchand-Beraud T. Sex steroids modulate the pituitary parameters involved in the regulation of TSH secretion in the rat. Acta Endocrinol (Copenh) 1990;122:577–584. doi: 10.1530/acta.0.1220577. [DOI] [PubMed] [Google Scholar]
- Dowling AL, Zoeller RT. Thyroid hormone of maternal origin regulates the expression of RC3/neurogranin mRNA in the fetal rat brain. Brain Res Mol Brain Res. 2000;82:126–132. doi: 10.1016/s0169-328x(00)00190-x. [DOI] [PubMed] [Google Scholar]
- Escamez MJ, Guadano-Ferraz A, Cuadrado A, Bernal J. Type 3 iodothyronine deiodinase is selectively expressed in areas related to sexual differentiation in the newborn rat brain. Endocrinology. 1999;140:5443–5446. doi: 10.1210/endo.140.11.7244. [DOI] [PubMed] [Google Scholar]
- Gaffney G, Sellers S, Flavell V, Squier M, Johnson A. Case-control study of intrapartum care, cerebral palsy, and perinatal death. BmJ. 1994;308:743–750. doi: 10.1136/bmj.308.6931.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadano-Ferraz A, Escamez MJ, Rausell E, Bernal J. Expression of type 2 iodothyronine deiodinase in hypothyroid rat brain indicates an important role of thyroid hormone in the development of specific primary sensory systems. J Neurosci. 1999;19:3430–3439. doi: 10.1523/JNEUROSCI.19-09-03430.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadano-Ferraz A, Obregon MJ, St Germain DL, Bernal J. The type 2 iodothyronine deiodinase is expressed primarily in glial cells in the neonatal rat brain. Proc Natl Acad Sci U S A. 1997;94:10391–10396. doi: 10.1073/pnas.94.19.10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, O'Heir CE, Mitchell ML, Hermos RJ, Waisbren SE, Faix JD, Klein RZ. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549–555. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- Ibarrola N, Rodriguez-Pena A. Hypothyroidism coordinately and transiently affects myelin protein gene expression in most rat brain regions during postnatal development. Brain Res. 1997;752:285–293. doi: 10.1016/s0006-8993(96)01480-1. [DOI] [PubMed] [Google Scholar]
- Kaplan MM, Yaskoski KA. Phenolic and tyrosyl ring deiodination of iodothyronines in rat brain homogenates. J Clin Invest. 1980;66:551–562. doi: 10.1172/JCI109887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilby MD, Gittoes N, McCabe C, Verhaeg J, Franklyn JA. Expression of thyroid receptor isoforms in the human fetal central nervous system and the effects of intrauterine growth restriction. Clin Endocrinol (Oxf) 2000;53:469–477. doi: 10.1046/j.1365-2265.2000.01074.x. [DOI] [PubMed] [Google Scholar]
- Kilby MD, Verhaeg J, Gittoes N, Somerset DA, Clark PM, Franklyn JA. Circulating thyroid hormone concentrations and placental thyroid hormone receptor expression in normal human pregnancy and pregnancy complicated by intrauterine growth restriction (IUGR) J Clin Endocrinol Metab. 1998;83:2964–2971. doi: 10.1210/jcem.83.8.5002. [DOI] [PubMed] [Google Scholar]
- Kim SW, Harney JW, Larsen PR. Studies of the hormonal regulation of type 2 5′-iodothyronine deiodinase messenger ribonucleic acid in pituitary tumor cells using semiquantitative reverse transcription-polymerase chain reaction. Endocrinology. 1998;139:4895–4905. doi: 10.1210/endo.139.12.6334. [DOI] [PubMed] [Google Scholar]
- Koenig RJ, Lazar MA, Hodin RA, Brent GA, Larsen PR, Chin WW, Moore DD. Inhibition of thyroid hormone action by a non-hormone binding c-erbA protein generated by alternative mRNA splicing. Nature. 1989;337:659–661. doi: 10.1038/337659a0. [DOI] [PubMed] [Google Scholar]
- Kok JH, Den Ouden AL, Verloove-Vanhorick SP, Brand R. Outcome of very preterm small for gestational age infants: the first nine years of life. Br J Obstet Gynaecol. 1998;105:162–168. doi: 10.1111/j.1471-0528.1998.tb10046.x. [DOI] [PubMed] [Google Scholar]
- Lebel JM, L'Herault S, Dussault JH, Puymirat J. Thyroid hormone up-regulates thyroid hormone receptor beta gene expression in rat cerebral hemisphere astrocyte cultures. Glia. 1993;9:105–112. doi: 10.1002/glia.440090203. [DOI] [PubMed] [Google Scholar]
- Lingas R, Dean F, Matthews SG. Maternal nutrient restriction (48 h) modifies brain corticosteroid receptor expression and endocrine function in the fetal guinea pig. Brain Res. 1999;846:236–242. doi: 10.1016/s0006-8993(99)02058-2. [DOI] [PubMed] [Google Scholar]
- Lisboa PC, Curty FH, Moreira RM, Oliveira KJ, Pazos-Moura CC. Sex steroids modulate rat anterior pituitary and liver iodothyronine deiodinase activities. Horm Metab Res. 2001;33:532–535. doi: 10.1055/s-2001-17211. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Lumey LH. Decreased birthweights in infants after maternal in utero exposure to the Dutch famine of 1944–45. Paediatr Perinat Epidemiol. 1992;6:240–253. doi: 10.1111/j.1365-3016.1992.tb00764.x. [DOI] [PubMed] [Google Scholar]
- Mallard EC, Rees S, Stringer M, Cock ML, Harding R. Effects of chronic placental insufficiency on brain development in fetal sheep. Pediatr Res. 1998;43:262–270. doi: 10.1203/00006450-199802000-00018. [DOI] [PubMed] [Google Scholar]
- Matthews SG. Dynamic changes in glucocorticoid and mineralocorticoid receptor mRNA in the developing guinea pig brain. Brain Res Dev Brain Res. 1998;107:123–132. doi: 10.1016/s0165-3806(98)00008-x. [DOI] [PubMed] [Google Scholar]
- Matthews SG, Challis JR. Regulation of CRH and AVP mRNA in the developing ovine hypothalamus: effects of stress and glucocorticoids. Am J Physiol. 1995;268:E1096–E1107. doi: 10.1152/ajpendo.1995.268.6.E1096. [DOI] [PubMed] [Google Scholar]
- McCabe CJ, Gittoes NJ, Sheppard MC, Franklyn JA. Thyroid receptor alpha1 and alpha2 mutations in nonfunctioning pituitary tumors. J Clin Endocrinol Metab. 1999;84:649–653. doi: 10.1210/jcem.84.2.5469. [DOI] [PubMed] [Google Scholar]
- McCabe L, Marash D, Li A, Matthews SG. Repeated antenatal glucocorticoid treatment decreases hypothalamic corticotropin releasing hormone mRNA but not corticosteroid receptor mRNA expression in the fetal guinea-pig brain. J Neuroendocrinol. 2001;13:425–431. doi: 10.1046/j.1365-2826.2001.00649.x. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, Sapolsky RM. Thyroid hormones influence the development of hippocampal glucocorticoid receptors in the rat: a mechanism for the effects of postnatal handling on the development of the adrenocortical stress response. Neuroendocrinology. 1987;45:278–283. doi: 10.1159/000124741. [DOI] [PubMed] [Google Scholar]
- Mellstrom B, Naranjo JR, Santos A, Gonzalez AM, Bernal J. Independent expression of the alpha and beta c-erbA genes in developing rat brain. Mol Endocrinol. 1991;5:1339–1350. doi: 10.1210/mend-5-9-1339. [DOI] [PubMed] [Google Scholar]
- Pop VJ, Brouwers EP, Vader HL, Vulsma T, Van Baar AL, De Vijlder JJ. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clin Endocrinol (Oxf) 2003;59:282–288. doi: 10.1046/j.1365-2265.2003.01822.x. [DOI] [PubMed] [Google Scholar]
- Resko JA, Roselli CE. Prenatal hormones organize sex differences of the neuroendocrine reproductive system: observations on guinea pigs and nonhuman primates. Cell Mol Neurobiol. 1997;17:627–648. doi: 10.1023/A:1022534019718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyns GE, Verhoelst CH, Kuhn ER, Darras VM, Van Der Geyten S. Regulation of thyroid hormone availability in liver and brain by glucocorticoids. General Comp Endocrinol. 2005;140:101–108. doi: 10.1016/j.ygcen.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Rovet JF. Congenital hypothyroidism: long-term outcome. Thyroid. 1999;9:741–748. doi: 10.1089/thy.1999.9.741. [DOI] [PubMed] [Google Scholar]
- Rubio A, Menendez-Pelaez A, Buzzell GR, Vaughan MK, Vaughan GM, Reiter RJ. Sexual differences in 5′-deiodinase activity in the Harderian gland of Syrian hamsters and the effect of pinealectomy: regulation by androgens. J Cell Biochem. 1996;62:397–404. doi: 10.1002/(SICI)1097-4644(199609)62:3%3C397::AID-JCB9%3E3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Ruiz de Ona C, Morreale De Escobar G, Calvo R, Escobar Del Rey F, Obregon MJ. Thyroid hormones and 5′-deiodinase in the rat fetus late in gestation: effects of maternal hypothyroidism. Endocrinology. 1991;128:422–432. doi: 10.1210/endo-128-1-422. [DOI] [PubMed] [Google Scholar]
- Sakurai A, Miyamoto T, DeGroot LJ. Cloning and characterization of the human thyroid hormone receptor beta 1 gene promoter. Biochem Biophys Res Commun. 1992;185:78–84. doi: 10.1016/s0006-291x(05)80957-x. [DOI] [PubMed] [Google Scholar]
- Thorpe-Beeston JG, Nicolaides KH. Maternal and fetal thyroid function in pregnancy. In: Nicolaides KH, editor. Frontiers in Fetal Medicine Series. London: Parthenon Publishing; 1996. pp. 89–92. [Google Scholar]
- Thorpe-Beeston JG, Nicolaides KH, Snijders RJ, Felton CV, McGregor AM. Thyroid function in small for gestational age fetuses. Obstet Gynecol. 1991;77:701–706. [PubMed] [Google Scholar]
- Tu HM, Kim SW, Salvatore D, Bartha T, Legradi G, Larsen PR, Lechan RM. Regional distribution of type 2 thyroxine deiodinase messenger ribonucleic acid in rat hypothalamus and pituitary and its regulation by thyroid hormone. Endocrinology. 1997;138:3359–3368. doi: 10.1210/endo.138.8.5318. [DOI] [PubMed] [Google Scholar]
- Tu HM, Legradi G, Bartha T, Salvatore D, Lechan RM, Larsen PR. Regional expression of the type 3 iodothyronine deiodinase messenger ribonucleic acid in the rat central nervous system and its regulation by thyroid hormone. Endocrinology. 1999;140:784–790. doi: 10.1210/endo.140.2.6486. [DOI] [PubMed] [Google Scholar]
- Verhoelst CH, Darras VM, Roelens SA, Artykbaeva GM, Van Der Geyten S. Type II iodothyronine deiodinase protein in chicken choroid plexus: additional perspectives on T3 supply in the avian brain. J Endocrinol. 2004;183:235–241. doi: 10.1677/joe.1.05743. [DOI] [PubMed] [Google Scholar]
- Visser TJ. Pathways of thyroid hormone metabolism. Acta Med Austriaca. 1996;23:10–16. [PubMed] [Google Scholar]
- Visser TJ, Kaplan MM, Leonard JL, Larsen PR. Evidence for two pathways of iodothyronine 5′-deiodination in rat pituitary that differ in kinetics, propylthiouracil sensitivity, and response to hypothyroidism. J Clin Invest. 1983;71:992–1002. doi: 10.1172/JCI110854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser TJ, Leonard JL, Kaplan MM, Larsen PR. Kinetic evidence suggesting two mechanisms for iodothyronine 5′-deiodination in rat cerebral cortex. Proc Natl Acad Sci U S A. 1982;79:5080–5084. doi: 10.1073/pnas.79.16.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GR, Franklyn JA, Neuberger JM, Sheppard MC. Thyroid hormone receptor expression in the ‘sick euthyroid’ syndrome. Lancet. 1989;2:1477–1481. doi: 10.1016/s0140-6736(89)92930-9. [DOI] [PubMed] [Google Scholar]
- Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81:1097–1142. doi: 10.1152/physrev.2001.81.3.1097. [DOI] [PubMed] [Google Scholar]