Abstract
The carotid body (CB) chemoreceptors participate in the ventilatory responses to acute and chronic hypoxia (CH). Arterial hypoxaemia increases breathing within seconds, and CB chemoreceptors are the principal contributors to this reflex hyperventilatory response. Acute hypoxia induces depolarization of CB chemoreceptors by inhibiting certain K+ channels, but the role of these channels in CH, as in high-altitude acclimatization, is less known. Here we explored the effects of prolonged (24–48 h) hypoxic exposure of rabbit CB chemoreceptor cells in primary cultures on the voltage-dependent K+ currents and on their response to acute hypoxia. We found that CH induces a decrease in the amplitude of outward K+ currents due to a reduction in a fast-inactivating BDS- and highly TEA-sensitive component of the current. In spite of this effect, acute hypoxic inhibition of K+ currents is increased in CH cultures, as well as hypoxia-induced depolarization. These data suggest that downregulation of this component (that does not contribute to the oxygen-sensitive K+ current (IKO2)) participates in the hypoxic sensitization. Pharmacological, immunocytochemical and quantitative PCR (qPCR) experiments demonstrate that CH-induced decrease in outward K+ currents is due to a downregulation of the expression of Kv3.4 channels. Taken together, our results suggest that CH sensitization in rabbit CB could be achieved by an increase in the relative contribution of IKO2 to the outward K+ current as a consequence of the decreased expression of the oxygen-insensitive component of the current. We conclude that acute and chronic hypoxia can exert their effects acting on different molecular targets.
The peripheral arterial chemoreceptors of the carotid body (CB) participate in the ventilatory responses to hypoxia and hypercapnia, the arousal responses to asphyxial apnoea, and the acclimatization to high altitude. CB chemoreceptor cells respond to hypoxia by releasing neurotransmitters that increase the activity of the afferent chemosensory fibres (Gonzalez et al. 1994). Hypoxia depolarizes chemoreceptor cells by inhibiting K+ channels, leading to the activation of voltage-dependent Ca2+ channels, Ca2+ influx and exocytosis. Although this seems to be a general mechanism present in all oxygen-sensitive tissues (Youngson et al. 1993; Weir & Archer, 1995), there are interspecies differences in the molecular nature of the oxygen-sensitive K+ channels (KO2), and even in the same species, more than one type of KO2 may be present (Peers & Buckler, 1995; Pérez-García & López-López, 2000; Patel & Honore, 2001; Peers & Kemp, 2001). The first KO2 was found in rabbit CB chemoreceptor cells, where a transient outward K+ current was reported to be reversibly inhibited by hypoxia (López-Barneo et al. 1988), which also induced changes in its kinetic properties (Ganfornina & López Barneo, 1992). In this same preparation, we have identified three fast inactivating Kv subunits (Kv3.4, Kv4.1, and Kv4.3) expressed in chemoreceptor cells (Sanchez et al. 2002). The cellular distribution of the Kv4 subunits, their electrophysiological properties and the effect of Kv4 dominant-negative constructs, led us to suggest that Kv4 channels are the molecular correlate of the KO2 (Pérez-García et al. 2000; Sanchez et al. 2002; Lopez-Lopez et al. 2003).
Chronic hypoxia (CH) increases the CB sensitivity to acute hypoxia, contributing to acclimatization process (Bisgard, 2000), and produces profound morphological and neurochemical changes in CB (Wang & Bisgard, 2002). However, our knowledge about the mechanisms of these changes is limited, since most of the data on acclimatization to hypoxia have been obtained in whole animals. The CB is a heterogeneous structure in which different cell types interact in the process of chemoreception (Gonzalez et al. 1994). Besides, due to its high vascularization, intraorgan haemodynamic changes during hypoxia may also influence chemoreception. In this regard, primary cultures of chemoreceptor cells represent a simpler model to study the effect of CH in the process of hypoxic chemoreception.
It is well documented that CH induces changes in the expression of proteins involved in the hypoxic transduction, such as tyrosine hydroxylase (TH), the rate-limiting enzyme in catecholamine synthesis. Increased TH transcription during CH contributes to the augmented secretory response of CB (Czyzyk Krzeska et al. 1992). However, as the chemotransduction cascade begins with the inhibition of KO2, a change in the expression levels or in the oxygen sensitivity of KO2 could also contribute to the increased response. In fact, CH has been shown to remodel the expression of ion channels in the rat CB (Stea et al. 1995; Wyatt et al. 1995).
In this report we investigated the effects of CH on the transient K+ currents of cultured rabbit CB chemoreceptor cells, since they are key elements in hypoxic chemotransduction. Using electrophysiological techniques, we have explored the effects of CH on the functional expression of the currents and on their response to acute hypoxia. We found that CH decreases the amplitude of the transient K+ currents and potentiates the effect of acute hypoxia on K+ channel inhibition. Membrane potential measurements indicate that this K+ channel remodelling increases cell excitability. Finally, combining pharmacological, immunocytochemical and real-time RT-PCR techniques, we demonstrate that CH induces a downregulation in the expression of Kv3.4 channels. These results indicate that acute and chronic hypoxia modulate a different set of Kv channels.
Methods
Dissociation and culture of CB chemoreceptor cells
Adult New Zealand rabbits (1.5–2 kg) were anaesthetized with intravenous application of sodium pentobarbital (40 mg kg−1) through the lateral vein of the ear. After tracheostomy, carotid artery bifurcations were dissected and animals were killed by intracardiac injection of sodium pentobarbital. The protocols were approved by the Institutional Care and Use Committee of the University of Valladolid. The CBs were enzymically dispersed as previously described (Sanchez et al. 2002). Dispersed cells were plated onto poly-l-lysine-coated coverslips with 2 ml of growth medium (DMEM:F12 with 5% FBS), and maintained in culture at 37°C in humidified incubators containing either 5% CO2/air (control culture) or 5% CO2/5% O2/90% N2 (chronic hypoxia culture) up to 72 h. An oxygen electrode mounted at the rear of the incubator chamber constantly monitored and adjusted the oxygen level by injecting N2 gas.
Electrophysiological methods
Ionic currents were recorded at room temperature (20–25°C) using the whole-cell configuration of the patch-clamp technique (Hamill et al. 1981). Whole-cell current recordings and data acquisition from CB chemoreceptor cells were made as previously described (Pérez-García et al. 2004). The coverslips with the attached cells were placed at the bottom of a small recording chamber (0.2 ml) on the stage of an inverted microscope, and perfused by gravity with the bath solution. This solution was connected to ground via a 3 m KCl agar bridge and an Ag–AgCl electrode. Patch pipettes had resistances ranging from 1.5 to 3 MΩ when filled with the internal solution. The composition of the bath solution was (mm): 141 NaCl, 4.7 KCl, 1.2 MgCl2, 1.8 CaCl2, 10 glucose, 10 Hepes (pH 7.4 with NaOH), and the pipette was filled with a solution containing (mm): 125 KCl, 4 MgCl2, 10 Hepes, 10 EGTA, 5 MgATP; (pH 7.2 with KOH). Whole-cell currents were recorded using an Axopatch 200 patch-clamp amplifier, filtered at 2 kHz (−3dB, 4-pole Bessel filter), and sampled at 10 kHz. When leak-subtraction was performed, an online P/4 protocol was used. Recordings were digitized with a Digidata 1200 A/D interface, driven by CLAMPEX 8 software (Axon Instruments) in a Pentium clone computer.
Hypoxia was achieved by bubbling the reservoir that fed the perfusion chamber with 100% N2, obtaining a final partial pressure of oxygen (PO2) level in the perfusion chamber of ≈15 mmHg. Oxygen levels were measured with small needle PO2 electrodes (Diamond General Development Corporation, Michigan, USA) placed in the vicinity of the cells. To exclude possible artifacts by prolonged bubbling (mainly changes in osmolarity and/or temperature), additional controls were used: in all experiments, the effects of N2-equilibrated solutions during different time intervals and the perfusion of the same solutions through different perfusion lines within the same experiment were tested. Also, the effect of air-bubbling the control solution was compared in selected experiments, with no differences in the results obtained. Additionally, all experiments in which the effect of hypoxia did not recover upon return to normoxic solutions were excluded from analysis. Full steady-state inactivation curves were obtained applying 500 ms depolarizing pulses to +40 mV preceded by 6.42 s prepulses to voltages between −100 and +30 mV in 10 mV increments. The holding potential was −80 mV. Recovery of inactivation between pulses was allowed applying pulses at 0.03 Hz (López-López et al. 1993). In most cells, the amplitude of the transient outward current was studied with the same protocol but applying either no prepulse, to measure the full current, or a prepulse to 0 mV, to inactivate the transient current. Currents obtained with the pulse in the two conditions were subtracted to obtain the fraction of the outward current that inactivates.
The inactivation time course of the transient current was best fitted to a biexponential function:
where A1, A2, τ1 and τ2 are free parameters, t is time, and C is fixed to the amplitude of the fully inactivated current (current at +40 mV following the prepulse to 0 mV).
The Kv3.4-specific toxin BDS-I (Alomone Laboratories, Israel) was prepared by following the manufacturer's instructions. The other reagents and drugs for electrophysiological experiments were obtained from Sigma.
Membrane potential measurements were performed at 37°C using the perforated-patch technique to avoid dialysis of intracellular medium. Pipette tips were briefly dipped into a solution containing (mm): 40 KCl, 95 potassium glutamate, 8 CaCl2, 10 Hepes, pH 7.2, and backfilled with the same solution containing amphotericin B (250 μg ml−1). After obtaining a high-resistance seal, electrical access to cell cytoplasm was assessed by monitoring the increase in cell capacitance. At this point, the amplifier was switched to current-clamp mode, and membrane potential was continuously recorded.
Electrophysiological data analyses were performed with the CLAMPFIT subroutine of the PCLAMP software and with ORIGIN 7.0 software (Microcal, Inc.). Pooled data are expressed as means ± standard error of the mean (s.e.m.). Statistical comparisons between groups of data were carried out with two-tailed Student's t test for paired or unpaired data, and values of P < 0.05 were considered statistically different.
Immunofluorescence in CB cells
Immunocytochemical procedures were carried out as previously described (Pérez-García et al. 2004). Briefly, CB cells were fixed with 4% paraformaldehyde in phosphate buffer, pH 7.5, washed in PBTx (PBS, 0.1% Triton X-100), and blocked with PBTx/10 mg ml−1BSA/2% normal goat serum. The primary antibodies, rabbit polyclonal anti-Kv3.4 (1:100; Alomone Laboratories) and mouse monoclonal anti-TH (1:2000, Abcam) were diluted in blocking solution and incubated with the cells for 60 min at room temperature. After washes in PBTx, cells were incubated with secondary antibodies (1:1000) for 30 min. The fluorescence-labelled secondary antibodies used were Alexa 488/597-conjugated goat antirabbit/mouse secondary antibodies (Molecular Probes). After washes in PBS, the coverslips were mounted with Vectashield H-1000 (Vector Laboratories), and the cells were examined with the appropriate filters for immunofluorescence. Images were processed with PaintShop Pro 7.0 (Jasc Software, Inc.)
qPCR methods
The levels of mRNA encoding for the transient outward K+ currents present in CB chemoreceptor cells were studied by quantitative PCR with TaqMan probes. Control experiments included the detection of L18 ribosomal protein (as an internal control to correct for the amount of mRNA in the sample) (Sanchez et al. 2002) and TH (as a control of the CH application).
qRT-PCR experiments were carried out with two different protocols. In one of them, we used single chemoreceptor cells, in which electrophysiological studies were performed, as the source of RNA. This group of experiments was designed to look for correlations between the electrophysiological properties of the individual cells and the transient outward K+ channel mRNA expression profile. In another set of experiments, total RNA was extracted from pooled chemoreceptor cells kept in culture during 48 h in control or CH. In all cases, the holder was either baked for 1 hour at 200°C or treated with RNAse ZAP solution (Ambion), and the capillary glass used was heated overnight at 200°C. Sterile gloves were worn during the procedure to minimize RNAse contamination. When mRNA from single chemoreceptor cells was used, the recording electrode was filled with 7 μl of nominally RNAse-free pipette solution. After recording, the cell was aspirated, the electrode was removed from the holder and its content was ejected into a 0.2 ml eppendorf tube containing 1 μl of RNAsin (20 u μl−1; Applied Biosystems) and kept at −80°C till the RT was performed. For the RT reaction, the contents of each tube (either the cytoplasm of the single cell or the purified mRNA obtained from the pooled cells) were transferred to another 0.2 ml Eppendorf containing 1 μl of random hexamers (50 μm) and, when necessary, DEPC water was added to keep the final volume to 9 μl. The tubes were heated to 70°C for 5 min in a thermal cycler (GeneAmp 9700; Applied Biosystems) and incubated at 20°C for 10 min. During this time, a master mix containing 2 μl of 10× PCR buffer, 4 μl of 25 mm MgCl2, 4 μl of mixed dNTPs (10 mm) and 1 μl of MulVRT (5000 u ml−1) was added to each tube and single-strand cDNA was synthesized in 1 h incubation at 42°C. The reaction was terminated by heating the mixture at 70°C for 10 min and then icing it. All reagents were obtained from Applied Biosystems. Aliquots of 5–9 μl of this reaction were used for each qPCR determination.
For collecting pooled chemoreceptor cells, the electrode was filled with 7 μl of RNAlater (Ambion), and the tip was broken to facilitate the aspiration of multiple cells. After collecting 80–150 chemoreceptor cells, the contents of the pipette were ejected into a 0.2 ml Eppendorf tube and processed to extract total mRNA by using RNAqueous micro (Ambion) following manufacturer instructions. The RT reaction was carried out as above, but in a final volume of 40 μl, and 5–7 μl was used in each qPCR assay.
Unique PCR primers and fluorescence-labelled probes used for amplification of fragments of rabbit Kv3.4, Kv4.1, Kv4.3, L18 and TH were designed from GenBank sequences of these genes, using the Primer Express software (Applied Biosystems) according to the specifications of the TaqMan protocol (Winer et al. 1999). In all cases, primers were designed in intron-spanning regions to overcome potential DNA contamination of the samples. Primer and probe sequences, as well as labelling fluorochromores and GenBank accession numbers, are detailed in Table 1. All plasmid cDNAs except Kv4.3 (a gift of Dr J. Rae) were cloned from PCR fragments in our laboratory. Primers were obtained from Genotek (Bonsai Technologies Group) and used at a final concentration of 500 nm, whereas the probes were synthesized and purified by Eurogentec, and used at a final concentration of 250 nm. Amplification was done in a 25 μl final volume, under the following cycling conditions: 10 min at 95°C, and then 40 cycles of 95°C for 15 s followed by 60°C for 1 min. Two different real-time thermal cyclers were used: an Mx4000 (Strategene) for the single-cell experiments, and a RotorGene 5 (Corbbet Research) for the pooled-cell qPCR experiments. Although the probes for the Kv channels (labelled with FAM), TH (labelled with Yakima yellow) and L18 (labelled with Cy5) were designed to allow multiplex analysis, each primer–probe set was amplified in separate wells. Standards were amplified in triplicate and samples were amplified in duplicate. In all experiments, no template controls (NTC) were included to exclude nonspecific amplification or sample contamination. The standard curves for each Kv channel, TH and L18 endogenous control were constructed from the respective mean critical threshold (Ct) value and the sample Ct was calculated by extrapolation into the standard curve. Then the expression levels of each transcript were normalized to L18 levels. As the efficiency of the PCR reaction for endogenous control (L18) and the different genes under study was similar (differences were less than 0.1), the ΔΔCt method (Livak & Schmittgen, 2001) was also used for studying variations in the expression levels of the Kv channel transcripts in the two conditions tested (control and CH), without any differences in the final results. In this latter case, the fold change in expression (2−ΔΔCt) was calculated from ΔΔCt values obtained with the expression:
where the GOI is the gene of interest (TH or Kv subunits) and HKG is the housekeeping gene (L18). The value of the Ct in the duplicate samples was averaged before performing the 2−ΔΔCt calculations. In order to do statistical comparisons, ΔCt values obtained in each control sample (Ct,GOI−Ct,HKG)Control were subtracted from the mean ΔCt to provide the s.e.m. of the control group.
Table 1.
Primer and probe sequences for qPCR
| mRNA | Primer sequence | TaqMan probe sequence (5′–3′ labelling) | Amplicon size (bp) | GenBank accession no. |
|---|---|---|---|---|
| Rb Kv3.4 | F: TCACCACCTTCTGCCTGGA | CGAGGCCTTCAACATCGACCGC) | 68 | AF493545 |
| R: CCGGTGGATCTCAGTCACGT | (5′ FAM –3′ TAMRA) | |||
| Rb Kv4.1 | F: TGAGGACAACGGCAGTGGA | AGGAGCAGGCGCTGTGTGTCAGAAA | 66 | AF493548 |
| R: TGCTGTTCAAAAGCAGACCG | (5′ FAM –3′ TAMRA) | |||
| Rb Kv4.3 | F: ACAAGGACCGCAAGAGGGA | AACGCCGAGCGCCTCATGGAC | 64 | AF198445 |
| R: TGGTTGTTCTCCGAGTCGTTG | (5′ FAM–3′ TAMRA) | |||
| Rb L18 | F: CAACAAGGACAGGAAGGTTCG | CGCAAGGAGCCCAAGAGCCAGG | 64 | AY764298 |
| R: CAGCAGCCGCAGGTAGATG | (5′ Cy5–3′ ElleQuencher) | |||
| Rb TH | F: AGCCTTCCAGTACAAGCATGG | AGCCCCATCCCCCGCGTG | 63 | AF493546 |
| R: CAATCTCCTCGGCCGTGT | (5′YakimaYellow–3′ ElleQuencher) |
All primer and probe sequences are given as 5′–3′. F, forward primer; R, reverse primer.
Results
Effects of CH on the amplitude and kinetics of the transient outward K+ currents
CH has been reported to induce remodelling of ion channels expression in different chemosensitive tissues, including pheochromocytoma cells (Conforti & Millhorn, 1997; Taylor & Peers, 1999), vascular smooth muscle cells from pulmonary arteries (Platoshyn et al. 2001; Pozeg et al. 2003) and rat CBs (Stea et al. 1995; Wyatt et al. 1995). However, there has been no study on the changes in the electrophysiological properties of rabbit CB chemoreceptor cells under long-term hypoxic exposure, and their effects on the acute hypoxic response.
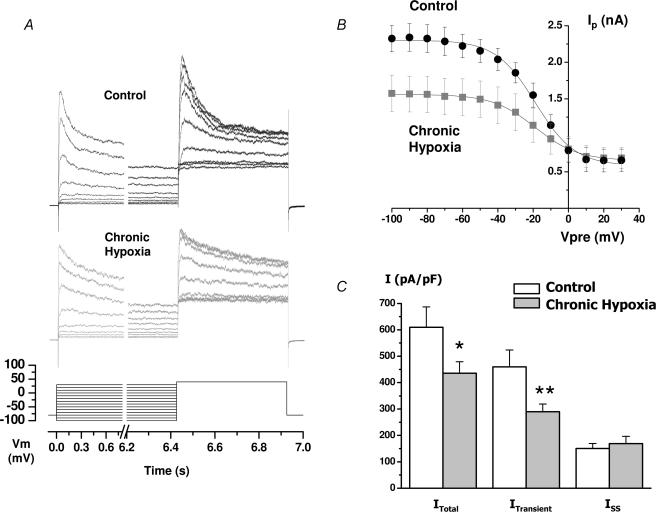
Application of a 500 ms depolarizing pulse to +40 mV from a holding potential of −80 mV elicited outward K+ currents with an inactivating component both in the control and in CH exposed cells. However, outward K+ currents obtained in CH cells consistently showed an overall reduction of the current amplitude and an apparent slowdown in the inactivation of the transient component as compared with control cells. The changes in current amplitude were characterized by analysing the steady-state inactivation curves, obtained as described in Methods, both in the control and in CH exposed cells (Fig. 1A). Boltzmann fit of the data (Fig. 1B) clearly showed that the decrease of the current amplitude in CH cells was due to a decrease of the inactivating component, without changes in the non-inactivating fraction of the current. There was also an apparent (although non significant) shift in the membrane potential at 50% inactivation (V0.5) of inactivation towards more hyperpolarized potentials under CH (from −17.8 ± 1.9 mV in control to −25.0 ± 4.5 mV in CH). As modifications in cell size induced by CH have been reported in other studies (Stea et al. 1992), changes in current amplitude may not sustain when data are expressed as current density. However, we did not see significant changes in cell size (cell capacitance was 4.04 ± 0.36 pF in control versus 3.58 ± 0.2 pF in CH), so that differences remain when current amplitude is expressed as pA pF−1 (Fig. 1C). Neither did we observe any change in the input resistance of the cells (calculated by measuring the holding current at −80 mV, data not shown).
Figure 1. Analysis of the outward K+ currents of control and chronic hypoxia (CH) chemoreceptor cells.
A, steady-state inactivation curves were obtained with a two-pulse protocol, in which a family of 6.42 s depolarizing prepulses from −100 to +30 mV was followed by a 500 ms pulse to +40 mV, as indicated in the bottom part of the figure. The figure shows representative traces (corresponding to the prepulses from −60 mV to +20 mV) in a control cell (upper traces) and a CH cell (lower traces). B, mean ± s.e.m. values obtained from 7–9 cells in each condition with the same protocol as in A. Peak current amplitude during the 500 ms depolarizing pulse to +40 mV is plotted against the voltage of the prepulse in control and CH cells. The continuous lines show the Boltzmann fit of the data. C, mean current density of the total current (ITotal), the transient component (ITransient) and the non-inactivating fraction of the current (Iss) in the two conditions. ITotal was obtained from the peak current at +40 mV after the −100 mV prepulse, Iss represents the value at the end of the +40 mV pulse after the +0 mV prepulse, and ITransient was calculated as the difference between ITotal and Iss. The values are the means ± s.e.m. of 22 control cells and 37 CH cells. *P < 0.05; **P < 0.01.
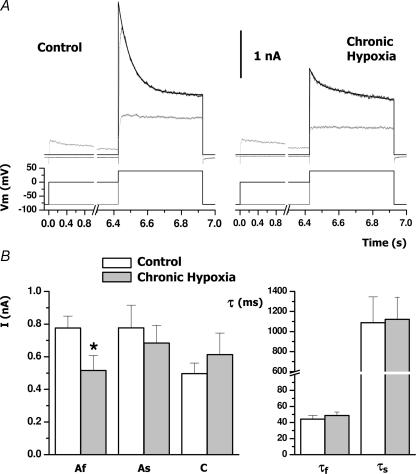
The effects of CH on the inactivation properties of the transient currents were characterized in more detail by determining the time course of inactivation as explained in Methods and depicted in Fig. 2. Both in control and in CH conditions, the time course of inactivation of the transient component was best fitted to a biexponential function with time constants (τ1 and τ2) that were not significantly different between the two groups (Fig. 2B). However, CH induced a significant decrease in the amplitude of the fast component of the transient current, which changed from 775 ± 73 pA in control cells to 515 ± 92 pA in CH cells (P < 0.05). There were not significant changes in the amplitude of the slow component or the non-inactivating fraction of the currents.
Figure 2. Time course of inactivation of the transient outward currents of control and CH cells.
A, representative traces obtained from one cell in each condition. Full K+ current (black traces) was elicited with a 500 ms depolarizing pulse to +40 mV, whilst the non inactivating fraction of the current (grey traces) was obtaining by preceding the pulse with a 6.24 s prepulse to 0 mV. The inactivation course of the currents was fitted to a two-exponential decay function (I=Af exp(−t/τf) +As exp(−t/τs) +C) in which C was fixed to the value of the current amplitude obtained after the 0 mV depolarizing prepulse. B, means ± s.e.m. of the values obtained from the two-exponential fit of 12–13 cells in each group. The left panel shows the values for the amplitude of the fast (Af) and the slow (As) components and the non-inactivating fraction (C), and the right panel represents the values for the two time constants. *P < 0.05.
Effect of CH on the response to acute hypoxia
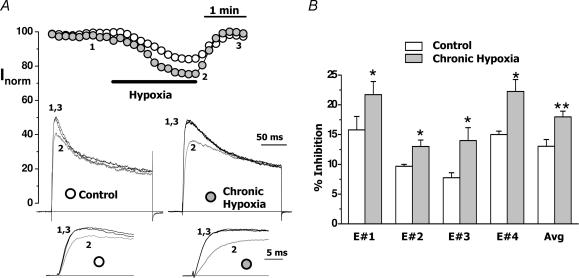
The above observations could be explained if CH induces a remodelling of the expression or, most unlikely, changes the kinetic properties of K+ channels underlying the transient outward current of rabbit CB chemoreceptor cells. However, as it is well established that acute hypoxic exposure produces a reversible decrease in the amplitude of the transient component of the outward K+ current (López-Barneo et al. 1988; López-López et al. 1989), we further characterized the effect of CH exploring the hypoxic sensitivity of CH cells compared with control cells. Figure 3A shows the normalized peak current amplitude (expressed as a percentage of current amplitude at the beginning of the whole-cell recording) obtained with depolarizing pulses to +40 mV from a holding potential of −80 mV in a control cell and a CH cell from the same culture. When indicated, the bath solution was replaced with an N2-equilibrated solution, and a reduction in the amplitude of the current was observed in both cases (15% in the control cell and 24% in the CH cell). The effect of acute hypoxia was consistently obtained in all the cultures studied, and in spite of wide variations in the amount of hypoxic inhibition from culture to culture, a significant increase in the magnitude of hypoxic inhibition was consistently observed in CH cells (Fig. 3B). Hypoxic inhibition of the transient K+ current averaged 13.1 ± 1.1% in control cells and 18.0 ± 0.9% in the CH cells. This sensitization of the hypoxia-induced inhibition of K+ currents by CH certainly contributes towards explaining the well-known sensitization that has been described in the in vivo experiments.
Figure 3. Effect of acute hypoxic exposure on the current amplitude of control and CH cells.
A, peak current plot of normalized outward currents recorded in a CB chemoreceptor cell from control (open circles) and CH (grey circles) cultures after application of depolarizing steps to +40 mV every 10 s. During the time indicated by the bar, the cells were bathed with a N2-equilibrated solution. In the insets, traces from the control and the CH cells at times 1, 2 and 3 are shown both at their full length and at a larger scale to appreciate hypoxic inhibition. B, plot of the average (mean ± s.e.m.) percentage inhibition of the current amplitude in control (white bars) and CH (grey bars) cells from four different cultures (E#1–E#4) and from all cells studied (Avg). Control amplitude value in each individual cell was considered as 100%, and was calculated as the mean of the peak current amplitude before and after hypoxic application. n = 3–6 cells for the four individual experiments and 18–25 cells in the Avg group. *P < 0.05; **P < 0.01.
Functional and molecular identification of the channels involved in changes induced by CH
We have previously identify three Kv subunits (Kv3.4, Kv4.1 and Kv4.3) as the molecular correlates of the transient outward K+ current of rabbit CB chemoreceptor cells (Sanchez et al. 2002), and we have speculated that the pattern of distribution in chemoreceptor cells of Kv4 channels, along with their electrophysiological properties, makes it conceivable that channels of this family, as homo- or heteromultimers, could be the molecular correlate of the oxygen-sensitive K+ currents. The above-described effects of CH (a decrease in the amplitude of the transient component of the outward K+ current together with an increase in the effect of low oxygen) could be explained simply if CH cells downregulate channels that contribute to the transient current but not to the oxygen-sensitive current increasing the contribution of oxygen-sensitive channels to the total K+ current.
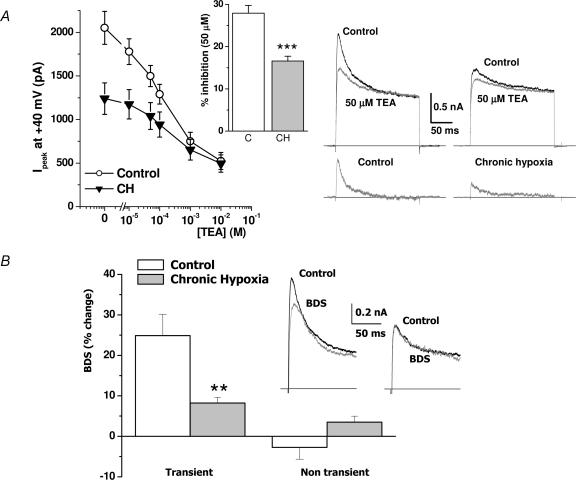
To test this hypothesis, we studied the pharmacological profile of the transient currents of control and CH cells using tetraethylammonium ions (TEA). Although TEA is a nonselective blocker of many K+ channels, at low (μm) concentrations only K+ channels of the Kv3 subfamily are blocked by TEA (Coetzee et al. 1999; Pérez-García et al. 2004). We tested the effect of TEA on the native CB chemoreceptor cells by analysing the reduction in the peak current amplitude in 200 ms depolarizing pulses to +40 mV in the presence of different TEA concentrations (from 10 μm to 10 mm) in the bath solution. Figure 4A shows the mean reduction in the current amplitude in pooled data obtained from 9–12 cells in each condition (control and CH) as a function of TEA concentration. As illustrated, TEA inhibition can be observed with TEA concentrations as low as 10 μm, these low concentrations being more effective in inhibiting K+ currents in control than in CH cells. On the contrary, at millimolar doses, the effect of TEA in both groups was similar. These differences in the effect of TEA are more evident when expressed as the percentage of reduction of the current amplitude. As an example, 10 μm TEA produced a 12.6 ± 1.3% reduction of the current amplitude in control cells and a 5.1 ± 3.0% reduction of the currents in CH cells (P < 0.001, data not shown). Similar differences can be observed with 50 μm TEA, as shown in the inset (Fig. 4A): inhibition of the K+ currents in control cells (27.6 ± 1.8%) was significantly larger than in CH cells (16.6 ± 1.1%). The representative traces in Fig. 4A show that the component blocked by this TEA concentration is a fast transient K+ current with an inactivation time constant of about 20 ms. These results indicate that CH reduces the amplitude of the transient K+ current by affecting a component highly sensitive to TEA. As Kv3.4 currents are blocked by TEA in the micromolar range, this pharmacological profile suggests that CH decreases the amplitude of the transient K+ current by reducing the expression of Kv3.4. To further confirm this observation, we studied the effect of BDS-I, a selective blocker of Kv3.4 channels (Diochot et al. 1998), in the two groups. Accordingly, we have previously described that BDS-I application in rabbit chemoreceptor cells inhibits a transient component with a τ of inactivation around 20 ms (Sanchez et al. 2002). BDS-I toxin was applied to the bath at a final concentration of 1 μm, and its effect on the transient current was assessed by using the two-pulse protocol previously described (see Fig. 2). The inset in Fig. 4B shows the effect of the toxin in a control cell and a CH cell from the same culture. In this example, BDS-I produced a 30% reduction in the amplitude of the transient K+ current in the control cell but no changes in the CH cell. The effect of BDS was explored in 5–6 cells in each condition obtained from two different cultures, and the averaged results are also shown in Fig. 4B. BDS-I inhibited only the transient component of the current in the two conditions tested, with no significant changes in the non-inactivating fraction. While in control cells the reduction in the transient current amplitude was around 25%, BDS-I only modestly decreased the transient currents in CH cells (8.2 ± 1.4%, P < 0.01).
Figure 4. Pharmacological characterization of the K+ currents of control and CH chemoreceptor cells.
A, effect of different TEA concentrations on the current amplitude of normoxic and chronically hypoxic cells. Peak current amplitude at +40 mV of 9–12 cells in each group is plotted against TEA concentration in the bath solution. The bar plot shows the percentage inhibition of the current amplitude by 50 μm TEA in control and CH cells. As in Fig. 3, current amplitude in the absence of TEA was taken as 100%. Data are means ± s.e.m., ***P < 0.001, n = 9. Sample traces showing the effect of 50 μm TEA in cells from control and CH cultures are depicted on the right; the bottom currents were obtained by subtracting TEA traces from control traces. B, effect of 1 μm BDS-I in control and CH chemoreceptor cells. In this case, transient outward currents were elicited (under control and toxin application conditions) by depolarizing steps to +40 mV with or without applying a 6.42 s prepulses to 0 mV (see legend to Fig. 2). The inset shows representative traces of the transient currents (obtained by subtracting the current obtained with and without prepulse) before and during BDS-I application in a control cell (left) and a CH cell (right). The bar plot represents the percentage reduction in the amplitude of both transient and nontransient components calculated as in Fig. 1, in the presence of 1 μm BDS-I for control cells (open bars) and CH cells (filled bars). Data are means ± s.e.m. of 5–6 cells in each group. **P < 0.01.
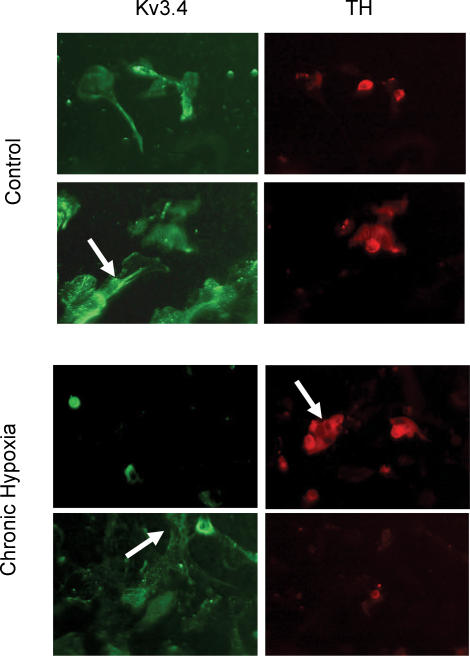
These data demonstrate a decrease in the functional contribution of Kv3.4 channels to the transient K+ current in CH rabbit chemoreceptor cell cultures. Kv3.4 channnels are expressed in several cells types of the CB, although their expression in chemoreceptor cells can be revealed by double immunocytochemical identification of TH and Kv3.4 (Sanchez et al. 2002). This type of experiment, carried out in CH cells, showed a clear decrease in the proportion of chemoreceptor cells (TH+) labelled with the anti-Kv3.4 antibody, as expected if the expression of these channels decreases in CH. On the contrary, there are no apparent differences in Kv3.4 labelling of TH(−) cells, suggesting a specific effect of CH on chemoreceptor cells (Fig. 5).
Figure 5. Immunofluorescence double-labelling of cultured normoxic (control) and CH rabbit carotid body (CB) cells.
Upper panels show colocalization of Kv3.4 and tyrosine hydrolase (TH) in chemoreceptor cells in control cultures. Both chemoreceptor cells TH(+) and other TH(−) cell types, some of them with a smooth muscle morphology (arrow), are found to express Kv3.4. In CH cultures (lower panels), most TH(+) cells do not show Kv3.4 immunostaining (arrow in right panel), although Kv3.4 labelling can be observed in nonchemoreceptor cells (arrow in lower left panel).
Effect of CH on Kv channels genes encoding transient K+ currents
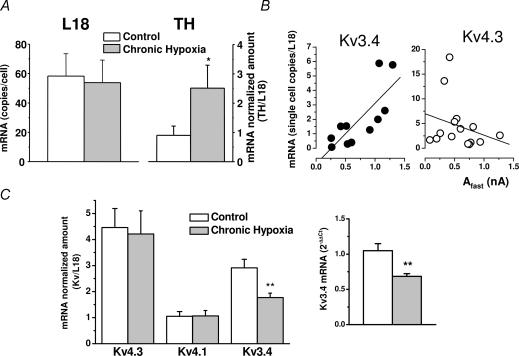
In order to explore if the reduction of Kv3.4 expression by CH is due to a downregulation of mRNA levels or alternatively occurs at a post-transcriptional level, we used quantitative real-time PCR to measure the expression levels of the transient Kv channel genes in rabbit chemoreceptor cells. Single-cell real-time PCR experiments were designed in order to establish a relationship between the electrophysiological and pharmacological profile of the transient K+ currents and the mRNA levels for Kv4.3, Kv4.1 and Kv3.4. As a previous step, we have selected an endogenous control (ribosomal protein L18) to normalize for the total amount of mRNA in the samples and another control (TH) to identify chemoreceptor cells and to confirm CH administration. Single-cell real-time PCR showed detectable amounts of L18 mRNA in all cells studied (Fig. 6A), and also validated this gene as an endogenous control, since no variation in its expression was detected between the two groups studied (control and CH). In selected cells in each experiment, TH mRNA levels were also quantified and, as shown in Fig. 6A, CH induced a significant increase in the levels of expression of the gene. When we studied the expression of the Kv channel genes, we were not able to detect Kv4.1 mRNA at the single-cell level, and regarding Kv4.3 and Kv3.4, the data showed so much variability that no significant changes between the two experimental groups could be established. However, we did see a positive correlation between the levels of mRNA encoding for Kv3.4 channels in a single cell and the amplitude of the fast inactivating current in the cell, and this correlation could not be confirmed for Kv4.3 mRNA quantity (Fig. 6B). To circumvent the problem of low mRNA amount and consequently high dispersion of the data, we quantified the expression of the channels in pooled chemoreceptor cells from control and CH cultures. The results from 5–7 independent experiments (with 3–6 pools of control and CH cells each) are shown in Fig. 6C, in which the normalized amounts of mRNA for each channel (copies of Kv mRNA/copies of L18) in both conditions are plotted as means ± s.e.m. While Kv4.3 and Kv4.1 levels did not show variations in response to CH treatment, Kv3.4 mRNA was significantly reduced (P < 0.01) in this latter condition by 40% (from 2.91 ± 0.33 to 1.77 ± 0.17 copies of Kv3.4/copies of L18). The same results were obtained when the fold change in the amount of Kv3.4 mRNA was calculated with the ΔΔCt method; in this case 2−ΔΔCt was 1.05 ± 0.1 in control cells and decreased to 0.68 ± 0.04 in the CH cells (inset in Fig. 6C). These results indicate that the reduced expression of Kv3.4 protein upon CH is at least in part a consequence of the decrease in the transcription of Kv3.4 gene in CB chemoreceptor cells.
Figure 6. Quantification of mRNA levels in chemoreceptor cells by real-time PCR.
A, average values (means ± s.e.m.) for L18 and TH mRNA levels in single chemoreceptor cells from normoxic (open bars) and chronically hypoxic (grey bars) cells. The number of copies of L18 per cell was calculated by extrapolation of critical threshold (Ct) values into a standard curve constructed with known amounts of rabbit L18 plasmid (see Methods). n = 12 cells in each group. The levels of TH in single cells were normalized to the number of copies of L18 in the same cell. Data are means ± s.e.m. of 6 cells in each group; *P < 0.05. B, expression levels of Kv3.4 (•) and Kv4.3 (○) mRNA in single cells were also normalized to L18. Electrophysiological recording of the cells before cytoplasm suction was performed with the protocol shown in Fig. 2 (see legend) and the inactivation time course of the currents was fitted to a biexponential function. The figure shows the normalized amount of Kv3.4 or Kv4.3 plotted against the amplitude of the fast component of the current. The dotted lines represent the fit of the data to a linear regression function. The regression coefficient (R) was 0.78 for Kv3.4 and 0.25 for Kv4.3. C, quantification of Kv mRNA levels in pooled chemoreceptor cells from normoxic and chronically hypoxic cultures. mRNA was obtained from 80–150 chemoreceptor cells aspirated with a recording microelectrode. In all cases, a 5 μl aliquot of the mRNA sample was used to quantify L18 expression, as described in Fig. 6 (in duplicate), and 7 μl aliquots were used for the determination of the indicated Kv channels, also in duplicate. The expression levels of Kv4.3 and Kv4.1 and Kv3.4 mRNA obtained from control (open bars) and CH cultures (grey bars) were expressed as copies of the channel per copies of L18 in the sample. Expression levels of Kv3.4 mRNA were also calculated using the ΔΔCt method. Data are means ± s.e.m. of 7–14 individual determinations in each group obtained from at least 5 different cultures. **P < 0.01.
Effect of CH on chemoreceptor cell excitability
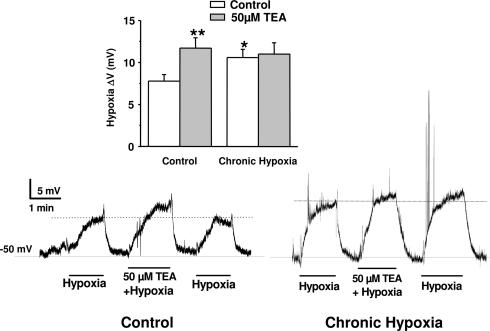
In order to confirm that we had CH sensitization in our in vitro model, and that downregulation of Kv3.4 was implicated in the sensitization, we explored: (1) the effect of CH treatment on the resting membrane potential of the cells under current-clamp conditions and on low PO2-induced depolarization, and (2) the effect of pharmacological blockade of Kv3.4 currents (using micromolar concentrations of TEA) on the magnitude of hypoxia-induced depolarization. The resting membrane potential measured in 19 control cells in the perforated-cell configuration was −49.2 ± 2.9 mV, not differing from the membrane potential found in CH cells (−46.0 ± 3.2 mV; n = 17). These data suggest that Kv3.4 channels do not participate in controlling resting membrane potential, in agreement with the reported kinetic properties of these channels (Coetzee et al. 1999; Pérez-García et al. 2004). Figure 7 shows two representative experiments (in a control cell and a CH cell) in which the effect of hypoxia alone or in the presence of 50 μm TEA on resting membrane potential was explored. Decrease in PO2 by perfusion with an N2-equilibrated solution induced a significant depolarization that was often accompanied by repetitive action potential firing. In the control cell, hypoxia-induced depolarization (8 mV in the example shown) was readily reversible upon returning to normoxic bath solution. In this same cell, perfusion with a N2-equilibrated solution containing 50 μm TEA produced a larger depolarization (13 mV). Similar results were obtained in nine more cells, in which the average depolarization obtained was 7.77 ± 0.78 mV with low PO2 alone, and 11.71 ± 1.24 with low PO2 and TEA 50 μm (see inset). When a similar experiment was performed in a CH cell we observed a larger depolarization in response to low PO2 and no changes in the magnitude of the response in the presence of TEA. The experiment shown is again representative of nine cells (see Fig. 7 inset).
Figure 7. Effect of CH on chemoreceptor cell excitability.
Current-clamp experiments were performed at 37°C in the perforated-patch configuration. The figure shows continuous recording of the membrane potential obtained in one control cell and one CH cell. Both cells were subjected to three consecutive applications of N2-equilibrated solutions, one of them containing 50 μm TEA, during the time indicated by the lines under the traces. The average depolarizations obtained in 10 cells in each group studied with the same protocol are shown in the inset. *P < 0.05 and **P < 0.01 as compared with the hypoxic depolarization in control, untreated cells.
Discussion
The findings reported here indicate that in vitro chronic hypoxia is able to induce changes in the expression profile of Kv channels of rabbit CB chemoreceptor cells that lead to an increase of hypoxic sensitivity of K+ currents. We show that exposure of CB primary cultures to low levels of oxygen for 24–72 h (5%; ∼33 mmHg) produces a specific downregulation of Kv3.4 channel (both at the protein and mRNA levels) that accounts for the decrease in the fast transient outward K+ current observed in the whole-cell experiments. Importantly, as the oxygen-sensitive K+ current (IKO2) in this preparation has been identified as belonging to the Kv4 family, downregulation of Kv3.4 gene leads to a decrease of the oxygen-insensitive component of the K+ current. This results in a more predominant role of IKO2 in the total outward current, with the subsequent relative increase in the magnitude of acute low PO2-induced inhibition. Under these conditions, acute hypoxia induced a significantly larger depolarization of resting membrane potential and consequently increased excitability, indicating that this ionic remodelling could explain CH sensitization. In addition, the involvement of Kv3.4 downregulation in the sensitization process is demonstrated by the fact that its blockade by low TEA concentrations induces significant potentiation of the hypoxia-induced depolarization in control cells (P < 0.01), but has no effect on CH cells. These results represent a novel mechanism of long-term modulation of ion channels by hypoxia and provide a molecular basis for the reported adaptation of the CB during sustained hypoxia.
The ventilatory acclimatization to hypoxia is almost exclusively dependent on CB (Smith et al. 1986; Bisgard, 2000), where CH exposure induces profound morphological and neurochemical changes together with increased chemosensitivity. However, in spite of intensive studies, adaptation of the CB and other chemosensitive tissues remains poorly understood, mainly because of the lack of knowledge of the mechanism of chemotransduction and the complexity of the transmission process in the organ. The use of molecular biology and electrophysiological techniques that allow us to characterize oxygen-sensitive processes and adaptive mechanisms at the cellular and molecular level is a necessary step to elaborate integrative physiological data. Using this approach, it has become clear that CH is able to induce changes in the expression pattern of many proteins within the CB, including ion channels. There are several studies in the literature characterizing the remodelling of ion channel expression induced by CH in the CB, but most of the studies were performed after in vivo application of hypoxia during long (2–4 weeks) periods of time. Under those conditions, CB chemoreceptors cells exhibited a marked hyperplasia that correlated with the morphological changes reported in the whole organ (Wyatt et al. 1995), making it difficult to assess whether the changes in the expression of ion channels are a primary consequence of CH stimulation or secondary to the morphological and neurochemical modifications induced by hypoxia. The increased size of CB chemoreceptor cells accompanying ion channel expression changes has also been reported in vitro (Stea et al. 1992, 1995), and both effects have been linked to an increase in the levels of cAMP, as this agent is able to mimic these two effects on normoxic cells. However, increased CB chemoreceptor cell size is not observed in shorter hypoxic periods, while some other changes in gene expression induced by hypoxia are reported. As an example, TH upregulation, which is a well-established specific marker of CH stimulation of the CB, can be already observed within a few hours of hypoxia (Gonzalez et al. 1979; Czyzyk Krzeska et al. 1992). This observation is in agreement with the fact that ventilatory acclimatization to hypoxia has a fast onset, and suggests that hypoxic regulation of gene expression in the CB could be already noted at these early stages, in which the observed changes can be attributed to a primary effect of hypoxia on its target genes. Accordingly, several recent works using cell lines, some of them derived from chemosensitive organs, report induction or repression of several ion channels upon hours of hypoxic exposure (Taylor & Peers, 1999; Colebrooke et al. 2002; Hartness et al. 2003; Del Toro et al. 2003). In this same framework, the present study represents the first characterization at the molecular level of the effects of CH on ion channel expression in rabbit CB chemoreceptor cells. Our results suggest that CH-induced downregulation of Kv3.4 channels in rabbit CB cells is an early event in the hypoxic adaptation process, before morphological changes are detected (we did not see changes in cell size with the CH treatment). Also, the regulation of Kv3.4 gene by CH seems to be fairly specific, since all changes in the transient K+ current can be explained by a decrease in Kv3.4 expression. Even the small change in the V0.5 of inactivation is concordant with a decrease in Kv3.4 expression, which shows a more positive value of V0.5 than Kv4 channels (Coetzee et al. 1999). No other differences in outward K+ currents were noticed: there was no modification in the magnitude of the non-inactivating component and no changes in the holding current or in the input resistance of the cells. Nevertheless, we cannot rule out the possibility of modulation by CH of the expression of other ion channels (such as voltage-dependent Ca2+ and Na+ channels) whose presence has been described in rabbit CB type I cells (Ureña et al. 1989). The same considerations apply to the current-clamp measurements, because even though the demonstrated downregulation of Kv3.4 channels under chronic hypoxia can account for the lack of effect of TEA on the hypoxia-induced depolarization in the CH cells, we cannot exclude the presence of other channels that could be contributing to the effect of TEA on membrane potential.
Reports concerning the effects of prolonged hypoxia on the acute hypoxic sensitivity of the CB are mixed, and some discrepancies might be accounted for by species-dependent rates of adaptation of the CB to CH. Long-term exposure to hypoxia leads to an enhancement of acute hypoxic reactivity that within some time (weeks to years, depending on species) changes into a blunting of the response to acute hypoxia. Although the regulation of the functional expression of ion channels (and particularly K+ channels) by prolonged hypoxia in CB has been documented in several studies in rat CB, the CH exposure was selected so as to mimic the conditions in which the acute hypoxic response of the cells is diminished (Wyatt et al. 1995; Carpenter et al. 1998). Moreover, due to the intrinsic difficulties of the preparation (small size of the organ, large heterogeneity), no molecular characterization studies have been performed. To circumvent these problems, the study of the molecular correlates of oxygen-sensitive K+ currents and the modulation of their expression by CH has been addressed more recently using cell lines, some of which were derived from chemosensitive tissues, such as H146 cells or PC12 cells (Conrad et al. 2001; Colebrooke et al. 2002). In the latter preparation, the effect of CH on the expression pattern of K+ currents has been studied, and upregulation of the oxygen-sensitive K+ current (Kv1.2) has been proposed as the mechanism of hypoxic sensitization. A similar process (i.e. changes in the expression of the oxygen-sensitive components of the K+ current) has been implicated in the loss of hypoxic sensitivity of pulmonary artery smooth muscle cells upon exposure to prolonged hypoxia (Platoshyn et al. 2001; Pozeg et al. 2003). In contrast, the results presented here reveal a different mechanism that results in modification of hypoxic sensitivity by CH without affecting the expression of oxygen-sensitive K+ channels. An analogous finding has been previously reported by Carpenter et al., although they could not reconcile their results with the assumption that acute hypoxic response in their experimental conditions was abolished; however, neither this presumed blunting of hypoxic response nor the comparison of the degree of K+ channel inhibition by acute hypoxia was directly studied in their work (Wyatt et al. 1995; Carpenter et al. 1998).
Our findings imply that both acute and chronic hypoxia are able to modulate ion channels in rabbit CB chemoreceptor cells, but that they exert their effects by acting through different molecules: while acute exposure to hypoxia decreases the activity of Kv4 channels, prolonged hypoxia downregulates Kv3.4 channels, and doing so increases the relative contribution of Kv4 channels to the total outward current. We can not rule out an additional effect of CH on the intrinsic oxygen sensitivity of the oxygen-sensing channels, but taking into account that the average reduction in the peak current amplitude under CH (around 29%, see Fig. 1) is very similar to the average increase in acute hypoxic inhibition under this condition (28% more, see Fig. 3), the sole diminution of Kv3.4 currents could explain the increase in the hypoxic inhibition.
Although these results need to be confirmed with more physiological approaches (whole-organ or in vivo experiments), the data presented in this paper provide new mechanisms to explain CH sensitization of CB chemoreceptor cells, demonstrating that CH induces functional changes in the K+ channel expression pattern. Whilst this mechanism may participate in adaptive responses such as high-altitude acclimatization, such altered channel expression may also contribute to the onset of pathological disorders.
Acknowledgments
We want to thank Esperanza Alonso and Bianca Hildebrand for excellent technical assistance. We also thank Dr C. Gonzalez for helpful discussions and critical reading of the manuscript. This work was supported by Instituto de Salud Carlos III grant G03/011 (Red Respira); Instituto de Salud Carlos III Grant G03/045 (Red Heracles) and DGICYT Grant BFI2001-1691 to J.R.L.L.; MCYT Programa de Acciones Integradas Grant HA02-44 and DGICYT grant BFU2004-05551 to M.T.P.G., and BMBF grants 01GS0109, 01GS0499, 01GI0204, and DAAD Acciones Integradas D/0246266 to S.K.
References
- Bisgard GE. Carotid body mechanisms in acclimatization to hypoxia. Resp Physiol. 2000;121:237–246. doi: 10.1016/s0034-5687(00)00131-6. [DOI] [PubMed] [Google Scholar]
- Carpenter E, Bee D, Peers C. Ionic currents in carotid body type I cells isolated from normoxic and chronically hypoxic adult rats. Brain Res. 1998;811:79–87. doi: 10.1016/s0006-8993(98)00962-7. [DOI] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz dM, Rudy B. Molecular diversity of K+ channels. Ann N Y Acad Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- Colebrooke RL, Smith IF, Kemp PJ, Peers C. Chronic hypoxia remodels voltage-gated Ca2+ entry in a human airway chemoreceptor cell line. Neurosci Lett. 2002;318:69–72. doi: 10.1016/s0304-3940(01)02479-x. [DOI] [PubMed] [Google Scholar]
- Conforti L, Millhorn DE. Selective inhibition of a slow-inactivating voltage-dependent K+ channel in rat PC12 cells by hypoxia. J Physiol. 1997;502:293–305. doi: 10.1111/j.1469-7793.1997.293bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad PW, Conforti L, Kobayashi S, Beitner-Johnson D, Rust RT, Yuan Y, Kim HW, Kim RH, Seta K, Millhorn DE. The molecular basis of O2-sensing and hypoxia tolerance in pheochromocytoma cells. Comp Biochem Physiol B Biochem Mol Biol. 2001;128:187–204. doi: 10.1016/s1096-4959(00)00326-2. [DOI] [PubMed] [Google Scholar]
- Czyzyk Krzeska MF, Bayliss DA, Lawson EE, Millhorn DE. Regulation of tyrosine hydroxylase gene expression in the rat carotid body by hypoxia. J Neurochem. 1992;58:1538–1546. doi: 10.1111/j.1471-4159.1992.tb11376.x. [DOI] [PubMed] [Google Scholar]
- Del Toro R, Levitsky KL, Lopez-Barneo J, Chiara MD. Induction of T-type calcium channel gene expression by chronic hypoxia. J Biol Chem. 2003;278:22316–22324. doi: 10.1074/jbc.M212576200. [DOI] [PubMed] [Google Scholar]
- Diochot S, Schweitz H, Beress L, Lazdunski M. Sea anemone peptides with a specific blocking activity against the fast inactivating potassium channel Kv3.4. J Biol Chem. 1998;273:6744–6749. doi: 10.1074/jbc.273.12.6744. [DOI] [PubMed] [Google Scholar]
- Ganfornina MD, López Barneo J. Gating of O2-sensitive K+ channels of arterial chemoreceptor cells and kinetic modifications induced by low PO2. J Gen Physiol. 1992;100:427–455. doi: 10.1085/jgp.100.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994;74:829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Kwok Y, Gibb J, Fidone S. Effects of hypoxia on tyrosine hydroxylase activity in rat carotid body. J Neurochem. 1979;33:713–719. doi: 10.1111/j.1471-4159.1979.tb05216.x. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch clamp techniques for high-resolution current recordings from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hartness ME, Brazier SP, Peers C, Bateson AN, Ashford MLJ, Kemp PJ. Post-transcriptional control of human maxiK potassium channel activity and acute oxygen sensitivity by chronic hypoxia. J Biol Chem. 2003;278:51422–51432. doi: 10.1074/jbc.M309463200. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(–Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- López-Barneo J, López-López JR, Ureña J, Gonzalez C. Chemotransduction in the carotid body: K+ current modulated by PO2 in type I chemoreceptor cells. Science. 1988;241:580–582. doi: 10.1126/science.2456613. [DOI] [PubMed] [Google Scholar]
- López-López JR, De Luis DA, Gonzalez C. Properties of a transient K+ current in chemoreceptor cells of rabbit carotid body. J Physiol. 1993;460:15–32. doi: 10.1113/jphysiol.1993.sp019456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-López JR, Gonzalez C, Ureña J, López-Barneo J. Low pO2 selectively inhibits K+ channel activity in chemoreceptor cells of the mammalian carotid body. J Gen Physiol. 1989;93:1001–1015. doi: 10.1085/jgp.93.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lopez JR, Perez-Garcia MT, Sanz-Alfayate G, Obeso A, Gonzalez C. Functional identification of Kvalpha subunits contributing to the O2-sensitive K+ current in rabbit carotid body chemoreceptor cells. Adv Exp Med Biol. 2003;536:33–39. doi: 10.1007/978-1-4419-9280-2_4. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honore E. Molecular physiology of oxygen sensitive potassium channels. Eur Respir J. 2001;18:221–227. doi: 10.1183/09031936.01.00204001. [DOI] [PubMed] [Google Scholar]
- Peers C, Buckler KJ. Transduction of chemostimuli by the type I carotid body cell. J Membr Biol. 1995;144:1–9. doi: 10.1007/BF00238411. [DOI] [PubMed] [Google Scholar]
- Peers C, Kemp P. Acute oxygen sensing: diverse but convergent mechanisms in airway and arterial chemoreceptors. Resp Res. 2001;2:145–149. doi: 10.1186/rr51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-García MT, Colinas O, Miguel-Velado E, Moreno-Dominguez A, López-López JR. Characteriztion of the Kv channels of mouse carotid body chemoreceptor cells and their role in oxygen-sensing. J Physiol. 2004;557:457–471. doi: 10.1113/jphysiol.2004.062281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-García MT, López-López JR. Are Kv channels the essence of O2 sensing. Circ Res. 2000;86:490–491. doi: 10.1161/01.res.86.5.490. [DOI] [PubMed] [Google Scholar]
- Pérez-García MT, López-López JR, Riesco AM, Hoppe UC, Marban E, Gonzalez C, Johns DC. Viral gene transfer of dominant-negative Kv4 construct suppresses an O2-sensitive K+ current in chemoreceptor cells. J Neurosci. 2000;20:5689–5695. doi: 10.1523/JNEUROSCI.20-15-05689.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platoshyn O, Yu Y, Golovina VA, McDaniel SS, Krick S, Li L, Wang JY, Rubin L, Yuan JXJ. Chronic hypoxia decreases KV channel expression and function in pulmonary artery myocytes. Am J Physiol Lung Cell Mol Physiol. 2001;280:L801–812. doi: 10.1152/ajplung.2001.280.4.L801. [DOI] [PubMed] [Google Scholar]
- Pozeg ZI, Michelakis ED, McMurtry MS, Thebaud B, Wu XC, Dyck JRB, Hashimoto K, Wang S, Moudgil R, Harry G, Sultanian R, Koshal A, Archer SL. In vivo gene transfer of the O2-sensitive potassium channel Kv1.5 reduces pulmonary hypertension and restores hypoxic pulmonary vasoconstriction in chronically hypoxic rats. Circulation. 2003;107:2037–2044. doi: 10.1161/01.CIR.0000062688.76508.B3. [DOI] [PubMed] [Google Scholar]
- Sanchez D, López-López JR, Pérez-García MT, Sanz-Alfayate G, Obeso A, Ganfornina MD, Gonzalez C. Molecular identification of Kvalpha subunits that contribute to the oxygen-sensitive K+ current of chemoreceptor cells of the rabbit carotid body. J Physiol. 2002;542:369–382. doi: 10.1113/jphysiol.2002.018382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, Bisgard GE, Nielsen AM, Daristotle L, Kressin NA, Forster HV, Dempsey JA. Carotid bodies are required for ventilatory acclimatization to chronic hypoxia. J Appl Physiol. 1986;60:1003–1010. doi: 10.1152/jappl.1986.60.3.1003. [DOI] [PubMed] [Google Scholar]
- Stea A, Jackson A, Macintyre L, Nurse CA. Long-term modulation of inward currents in O2 chemoreceptors by chronic hypoxia and cyclic AMP in vitro. J Neurosci. 1995;15:2192–2202. doi: 10.1523/JNEUROSCI.15-03-02192.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stea A, Jackson A, Nurse CA. Hypoxia and N6,O2′-dibutyryladenosine 3′,5′-cyclic monophosphate, but not nerve growth factor, induce Na+ channels and hypertrophy in chromaffin-like arterial chemoreceptors. Proc Natl Acad Sci U S A. 1992;89:9469–9473. doi: 10.1073/pnas.89.20.9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SC, Peers C. Chronic hypoxia enhances the secretory response of rat phaeochromocytoma cells to acute hypoxia. J Physiol. 1999;514:483–491. doi: 10.1111/j.1469-7793.1999.483ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ureña J, López-López J, Gonzalez C, López-Barneo J. Ionic currents in dispersed chemoreceptor cells of the mammalian carotid body. J Gen Physiol. 1989;93:979–999. doi: 10.1085/jgp.93.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZY, Bisgard GE. Chronic hypoxia-induced morphological and neurochemical changes in the carotid body. Microsc Res Tech. 2002;59:168–177. doi: 10.1002/jemt.10191. [DOI] [PubMed] [Google Scholar]
- Weir EK, Archer SL. The mechanism of acute hypoxic pulmonary vasoconstriction: the tale of two channels. FASEB J. 1995;9:183–189. doi: 10.1096/fasebj.9.2.7781921. [DOI] [PubMed] [Google Scholar]
- Winer J, Jung CK, Shackel I, Williams PM. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem. 1999;270:41–49. doi: 10.1006/abio.1999.4085. [DOI] [PubMed] [Google Scholar]
- Wyatt CN, Wright C, Bee D, Peers C. O2-sensitive K+ currents in carotid body chemoreceptor cells from normoxic and chronically hypoxic rats and their roles in hypoxic chemotransduction. Proc Natl Acad Sci U S A. 1995;92:295–299. doi: 10.1073/pnas.92.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngson C, Nurse C, Yeger H, Cutz E. Oxygen sensing in airway chemoreceptors. Nature. 1993;365:153–155. doi: 10.1038/365153a0. [DOI] [PubMed] [Google Scholar]