Abstract
Serotonergic neurones in the mammalian medullary raphe region (MRR) have been implicated in central chemoreception and the modulation of the ventilatory response to hypercapnia, and may also be involved in the ventilatory response to hypoxia. In this study, we ask whether ventilatory responses across arousal states are affected when the 5-hydroxytryptamine 1A receptor (5-HT1A) agonist (R)-(+)-8-hydroxy-2(di-n-propylamino)tetralin (DPAT) is microdialysed into the MRR of the unanaesthetized adult rat. Microdialysis of 1, 10 and 30 mm DPAT into the MRR significantly decreased absolute ventilation values  during 7% CO2 breathing by 21%, 19% and 30%, respectively, in wakefulness compared to artificial cerebrospinal fluid (aCSF) microdialysis, due to decreases in tidal volume (VT) and not in frequency (f), similar to what occurred during non-rapid eye movement (NREM) sleep. The concentration-dependence of the hypercapnic ventilatory effect might be due to differences in tissue distribution of DPAT. DPAT (30 mm) changed room air breathing pattern by increasing f and decreasing VT. As evidenced by a sham control group, repeated experimentation and microdialysis of aCSF alone had no effect on the ventilatory response to 7% CO2 during wakefulness or sleep. Unlike during hypercapnia, microdialysis of 30 mm DPAT into the MRR did not change the ventilatory response to 10% O2. Additionally, 10 and 30 mm DPAT MRR microdialysis decreased body temperature, and 30 mm DPAT increased the percentage of experimental time in wakefulness. We conclude that serotonergic activity in the MRR plays a role in the ventilatory response to hypercapnia, but not to hypoxia, and that MRR 5-HT1A receptors are also involved in thermoregulation and arousal.
during 7% CO2 breathing by 21%, 19% and 30%, respectively, in wakefulness compared to artificial cerebrospinal fluid (aCSF) microdialysis, due to decreases in tidal volume (VT) and not in frequency (f), similar to what occurred during non-rapid eye movement (NREM) sleep. The concentration-dependence of the hypercapnic ventilatory effect might be due to differences in tissue distribution of DPAT. DPAT (30 mm) changed room air breathing pattern by increasing f and decreasing VT. As evidenced by a sham control group, repeated experimentation and microdialysis of aCSF alone had no effect on the ventilatory response to 7% CO2 during wakefulness or sleep. Unlike during hypercapnia, microdialysis of 30 mm DPAT into the MRR did not change the ventilatory response to 10% O2. Additionally, 10 and 30 mm DPAT MRR microdialysis decreased body temperature, and 30 mm DPAT increased the percentage of experimental time in wakefulness. We conclude that serotonergic activity in the MRR plays a role in the ventilatory response to hypercapnia, but not to hypoxia, and that MRR 5-HT1A receptors are also involved in thermoregulation and arousal.
Serotonin-containing neurones located in the medullary raphe region (which includes those in the raphe magnus, pallidus and obscurus, parapyramidally, and in the juxta-facial paragigantocellularis lateralis), or the MRR, modulate several physiological processes. With regard to respiration, neurones from the MRR share synaptic connections with the dorsal and ventral respiratory groups (Connelly et al. 1989; Voss et al. 1990), and send afferents to both the hypoglossal and phrenic motor nuclei (Lalley, 1986; Jelev et al. 2001), thereby affecting respiratory motoneurone output. The MRR is also recognized to be among several brainstem regions that are chemosensitive; that is, they are able to specifically detect increases in tissue PCO2 and decreases in pH, leading to increased ventilation in the conscious animal (for review, see Nattie, 2000). In unanaesthetized rats and goats, focal acidification via microdialysis of aCSF containing an elevated fraction of CO2 in the MRR increases ventilation (Nattie & Li, 2001; Hodges et al. 2004).
Electrophysiological studies of rat MRR neurones in primary tissue culture show that many cells exhibit an increased firing rate in response to acidosis and increased PCO2 (Wang et al. 1998). Subsequently, immunohistochemical analysis identified the majority of these CO2-stimulated neurones as serotonergic (Wang et al. 2001). However, a recent study suggested that glutamatergic neurones on the ventral medullary surface in the retrotrapezoid nucleus (RTN) region are the central CO2 chemoreceptors in vivo, and that serotonergic neurones are not involved (Mulkey et al. 2004). Nonetheless, selectively lesioning neurones expressing serotonin transporter (SERT) in the MRR with microinjection of a SERT antibody–saporin conjugate decreased the ventilatory response to CO2 during both wakefulness and non-rapid eye movement (NREM) sleep states in the conscious rat (Nattie et al. 2004), arguing that serotonergic neurones in this region may be involved with central chemosensitivity. Studies that perturbed medullary serotonergic function using microdialysis of pharmacological compounds into the MRR also suggest these neurones are involved with modulating the CO2 response. Daily microdialysis into the MRR of the selective serotonin re-uptake inhibitor (SSRI) fluoxetine over a 2-week period increased the ventilatory response to 7% CO2 during wakefulness and sleep after 7 and 15 days (Taylor et al. 2004). In contrast, inhibition of serotonergic neurones in the MRR via microdialysis of the 5-HT1A receptor agonist (R)-(+)-8-hydroxy-2(di-n-propylamino)tetralin (DPAT) decreased the ventilatory response to CO2 in conscious 10- to 16-day-old piglets, but had the opposite effect in younger animals, indicative of an age-dependent effect (Messier et al. 2004).
The potential role of MRR neurones in the ventilatory response to hypoxia is not yet understood. Serotonin-containing neurones originating in the MRR project to the nucleus of the solitary tract (NTS) (Thor & Helke, 1987), the primary termination point of carotid sinus nerve (CSN) afferents from oxygen chemoreceptors in the carotid body (Housley & Sinclair, 1988). As neurones in the NTS have direct efferent connections with respiratory motoneurones affecting ventilation (Dobbins & Feldman, 1994), it is logical to suppose that serotonergic neurones from the MRR may play a role in the ventilatory response to hypoxia. A recent study performed in conscious rats determined that non-specific lesioning of MRR neurones using microinjection of ibotenic acid leads to an increased ventilatory response to 7% O2 compared to control animals (Gargaglioni et al. 2003). Therefore, unlike in hypercapnic conditions, where MRR neurones are chemosensitive and can potentiate the ventilatory response, during hypoxia they may have an inhibitory role. However, the phenotypes of MRR neurones responsible for inhibiting the ventilatory response to hypoxia have not been characterized.
We had two primary objectives in the current study: (1) to verify whether reversible pharmacological inhibition of serotonergic neurones (as opposed to saporin-induced lesions that occur over a period of days) in the MRR of conscious adult rats would decrease the ventilatory response to CO2; and (2) to determine whether serotonergic neurones in the MRR modulate the ventilatory response to hypoxia. To acutely inhibit serotonergic activity in the MRR, we have used focal microdialysis of the 5-HT1A agonist DPAT. The MRR contains neurones that express somatodendritic 5-HT1A receptors (Thor et al. 1990; Azmitia et al. 1996), and DPAT specifically acts upon these receptors, leading to inhibition of serotonergic neurones (Hjorth & Magnusson, 1988). As serotonergic deficiencies within the MRR have been found in infants succumbing to the sudden infant death syndrome (SIDS) (Kinney et al. 2001), it is important to understand the influence of medullary serotonergic neurones over responses to ventilatory stress.
Methods
Animal preparation
Experiments were performed in 21 adult male Sprague-Dawley rats weighing between 250 and 350 g, and all protocols were approved by the Institutional Animal Care and Use Committee at Dartmouth College Animal Resource Center. Animals received food and water ad libitum, and were housed on a 12 h light−12 h dark cycle beginning at midnight in a temperature-controlled environment. Ventilation experiments were completed in the morning or early afternoon of the working day (with the light period ending at noon) to allow ample periods of quiet wakefulness and NREM sleep ventilation data to be recorded.
Anaesthesia consisted of an intramuscular injection of 100 mg kg−1 ketamine and an intraperitoneal injection of 20 mg kg−1 xylazine. Anaesthetic depth was evaluated by firm hind-paw pinching, and if necessary, additional one-quarter doses of ketamine and xylazine were administered to maintain adequate anaesthesia. The neck, head and abdomen were thoroughly shaved and sterilized with betadine solution and 70% ethanol. A sterile telemetric temperature probe (TA-F20, Data Sciences International, St Paul, MN, USA) was placed within the abdominal cavity via an incision through the linea alba. The head of the rat was then centred on a Kopf stereotaxic apparatus, and a midline incision was made from the frontal bone to the base of the skull, exposing the skull surface. Three electroencephalogram (EEG) electrode screws were fastened to the skull: one 2 mm rostral to bregma and 2 mm lateral to the midline, one 2 mm rostral to lambda and 2 mm lateral to the midline, and a ground laterally between the two. Two wire electromyogram (EMG) electrodes were sutured within the neck skeletal muscle, and all electrode wires were connected to a 6-prong plastic pedestal. A microdialysis probe cannula and dummy were then inserted into the medulla approximately 11.0–13.0 mm caudal to bregma and 0 mm from the midline, and 10.6–10.8 mm deep into the dorsal surface of the cerebellum. These coordinates assured placement into the medullary raphe nuclei at the rostral–caudal level of the facial nucleus in accordance with the stereotaxic rat brain atlas of Paxinos & Watson (1982). The EEG–EMG pedestal and microdialysis probe cannula were secured to the skull with cranioplastic cement and the wound was closed. Animals were allowed to recover for at least 4 days before beginning the experimental protocol, and any animal showing signs of illness was immediately killed by an overdose (> 200 mg kg−1) of sodium pentobarbital injected intraperitoneally, and not used in the experiments. Animals in each group used in this study were healthy, active and gained weight similarly over the course of the experiments.
Reverse microdialysis
The microdialysis probe used had an 11-mm stainless steel shaft with 0.38 mm o.d., and a 1-mm cuprophane tip that allowed for diffusion of molecules up to 6000 Da (CMA/11, CMA microdialysis, Solna, Sweden). In all experiments, flow through the microdialysis probe was kept constant at 0.05 ml h−1. aCSF was used in sham experiments and as the drug vehicle and consisted of (mm): sodium 152.0, chloride 131.0, potassium 3.0, bicarbonate 26.0, magnesium 2.1, and calcium 2.2. Before addition of calcium, the aCSF was allowed to equilibrate with 5% CO2. (R)-(+)-8-OH-DPAT HBr (Sigma, St Louis, MO, USA), was prepared in aCSF in 1, 10 and 30 mm concentrations. These concentrations were chosen based upon previous studies, which used 30 mm DPAT microdialysis into the medullary raphe of an unanaesthetized piglet (Messier et al. 2004). Due to obvious brainstem size differences and potential drug spread beyond the medullary raphe, we also wanted to determine whether smaller concentrations of DPAT would be effective at altering ventilatory responses in an unanaesthetized rat. We initially began with 100, 250 and 500 μm concentrations in addition to 1, 10 and 30 mm concentrations in two preliminary rat experiments. However, as there were no noticeable changes in ventilation until at least 1 mm DPAT was used in these two animals, we decided to use 1, 10 and 30 mm concentrations only within the remainder of the group.
Data acquisition
To record breathing in a conscious rat, we used a whole body plethysmograph chamber as previously described (Nattie & Li 2001, 2002a,b; Nattie et al. 2004). The analog signal from the pressure transducer was sampled at 150 Hz and digitally converted by a computer using the DATAPAC 2000 system (RUN Technologies, Laguna Hills, CA, USA). Inflow and outflow rates in the plethysmograph were balanced to one another such that flow through was ≥ 1.4 l min−1 (flow meter model 601E, Matheson Tri-gas, Montgomeryville, PA, USA), and the plethysmograph was kept at atmospheric pressure. CO2 and O2 levels were sampled in the outflow line at ∼100 ml min−1 by a combined CO2 and O2 analyser (Gemini Respiratory Gas Analyser, CWE Inc., Ardmore PA, USA). The plethysmograph was calibrated with five 0.3-ml air injections before the beginning of each experiment. EEG and EMG signals from skull and skeletal muscle electrodes were sampled at 150 Hz, filtered at 0.3–50 and 0.1–100 Hz, respectively, and also directly recorded using the DATAPAC 2000 system.
By applying the Fick principle, we were able to calculate O2 consumption  by using the difference in O2 content between inflow air and outflow air:
by using the difference in O2 content between inflow air and outflow air:
where  and
and 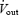 are inflow and outflow rates, and FIO2 and FOO2 are the fraction of inflow and outflow O2. In the plethysmograph, a gas mixture (7% CO2 or 10% O2) once added to the inflow (at a flow rate of 1.4 l min−1), takes approximately 10–15 min to equilibrate to appropriate concentrations in the outflow. Therefore,
are inflow and outflow rates, and FIO2 and FOO2 are the fraction of inflow and outflow O2. In the plethysmograph, a gas mixture (7% CO2 or 10% O2) once added to the inflow (at a flow rate of 1.4 l min−1), takes approximately 10–15 min to equilibrate to appropriate concentrations in the outflow. Therefore,  was calculated from oxygen content data collected after 10-min intervals during the ventilation experiments, and analysed as an average value during room air, 7% CO2, or 10% O2 conditions. Rat body temperature was measured continuously using the analog signal from the telemetric temperature probe within the abdomen, and chamber temperature was measured using a thermometer located inside the plethysmograph.
was calculated from oxygen content data collected after 10-min intervals during the ventilation experiments, and analysed as an average value during room air, 7% CO2, or 10% O2 conditions. Rat body temperature was measured continuously using the analog signal from the telemetric temperature probe within the abdomen, and chamber temperature was measured using a thermometer located inside the plethysmograph.
Experimental protocol: hypercapnic response
There were two groups of animals used in these experiments: one group (n = 7) underwent DPAT microdialysis, and another (n = 6) that served as a sham control group and received aCSF microdialysis alone. On the first day of the experiments, the EEG–EMG was connected, the microdialysis probe was inserted, and the rat was placed into the plethysmograph. After the rat had acclimated and appeared calm, aCSF was microdialysed for 30 min. Ventilation and sleep data in room air conditions were recorded for 30 min. The flow into the plethysmograph was changed to a mixture of room air and 7% CO2, and once the chamber reached 7% CO2 as indicated by the gas analyser, data were recorded for a further 30 min. Microdialysis was continued throughout the entire ventilation experiment. Identical ventilation experiments were then performed with 1, 10 and 30 mm DPAT microdialysis, and at least 48 h was allowed in between the ventilation experiments, as shown in Fig. 1A. This allowed each animal to serve as its own aCSF control during data analysis in a repeated measures design. The sham control animals received aCSF microdialysis and underwent ventilation experiments on four separate occasions at least 48 h apart, identical to the rats receiving DPAT. In this way, it could be determined whether repeated exposure to 7% CO2 or repeated microdialysis alone could alter ventilation. Again, in the sham control group, each subsequent aCSF microdialysis ventilation experiment was compared to the first in a repeated measures design.
Figure 1. Ventilation experiment protocols for (A) the 7% CO2 experiments (n = 7) and (B) the 10% O2 experiments (n = 8).
The sham control group for the 7% CO2 experiments (n = 6) were identical to (A), except rats received aCSF microdialysis on all four experimental days.
Experimental protocol: hypoxic response
After completing the hypercapnic response experiments, we used a different group of rats (n = 8) to determine whether DPAT microdialysis into the medullary raphe would alter the ventilatory response to hypoxia. On the first day of the experiments, the animals were placed into the plethysmograph and allowed to acclimate as described as above. aCSF was microdialysed for 30 min, at the end of which 30 min of room air ventilation data were recorded. Microdialysis was always allowed to continue throughout the ventilation experiment, similar to the hypercapnia experiments. The inflow to the box was changed to a mixture of 10% O2 balanced with nitrogen, and once the gas analyser read 10% O2, 1 h of ventilation was recorded. A longer period of hypoxic breathing was used in these experiments in an attempt to acquire some sleep during the hypoxic challenge, as animals in general are less likely to sleep during hypoxia as compared to hypercapnia (Pappenheimer, 1977). After 1 h of hypoxia, a further 30 min of post hypoxic room air ventilation was recorded. At least 48 h after the first experiment, a second ventilation experiment was performed with 30 mm DPAT microdialysis. We chose to only use the 30 mm concentration of DPAT in the hypoxic response experiments because this was the concentration that we found to be most effective in altering ventilatory responses during the hypercapnia experiments. A second aCSF microdialysis control ventilation experiment was performed at least 48 h after the DPAT experiment for an additional control. The experimental protocol for the hypoxic experiments is shown in Fig. 1B. This experimental design allowed the ventilatory response to hypoxia during aCSF microdialysis to be compared to the response during DPAT microdialysis within the same group of animals.
Anatomy
At the conclusion of the ventilation experiments, rats were killed with an overdose (> 200 mg kg−1) of sodium pentobarbital, injected intraperitoneally. The brainstem was immediately dissected out of the animal and quickly frozen on dry ice. Tissue was stored at −15°C until required for cuting into consecutive 50-μm sections on a cryostat (Leica model CM1850) and mounted upon gelatine-coated slides. Following fixation in 4% paraformaldehyde solution, this tissue was stained with cresyl violet so that the location of the microdialysis probe could be clearly seen. To estimate the distance between bregma and the probe tip, the distance between the probe tip and the caudal aspect of the facial nerve (approximately −11.0 mm from bregma in accordance with the rat brain atlas of Paxinos & Watson (1982) was calculated. Any animals that had probes falling outside of the MRR were excluded from data analysis.
Data analysis
For sleep analysis, a fast Fourier transform was performed on the EEG–EMG signal at 3.6 s-long epochs, in addition to observing the raw record. Frequency bands delta, theta and sigma were distinguished as 0.3–5, 6–9 and 10–17 Hz, respectively. Experiments were performed either between 0900 h and 12.00 h (right before the light period ended) or 12.00 h and 15.00 h. (directly after the dark period began) such that both wakefulness and sleep states were observed. Arousal states were defined in the following way as previously described (Li et al. 1999; Nattie & Li 2001, 2002a): (1) wakefulness, raw EEG low, EMG present, delta power low; (2) NREM sleep, raw EEG high, EMG low, delta power high, the product of sigma and theta power high; and (3) rapid eye movement (REM) sleep, raw EEG low, EMG absent, delta power low, theta to delta power ratio high. REM periods were brief and did not occur in every experiment, and there were also certain periods during the experiments for which we could not determine sleep state. Therefore, breath events occurring during REM or when sleep state was indeterminate were excluded from the ventilation analysis.
Individual breath events were selected with the DATAPAC 2000 software. Any breaths occurring during activity (sniffing, grooming, moving) were eliminated. Breaths were grouped into bins of 50–500 events depending upon arousal state as determined by sleep analysis.  , f and VT were calculated for each breath with rat body temperature, plethysmograph chamber temperature and the barometric pressure of the day the experiment was performed; and
, f and VT were calculated for each breath with rat body temperature, plethysmograph chamber temperature and the barometric pressure of the day the experiment was performed; and  and VT were normalized (per 100 g rat body weight). Ventilatory parameters are expressed as mean values for quiet wakefulness and NREM sleep during room air, 7% CO2 or 10% O2 conditions. Statistics performed on the data included one-way repeated measures (RM) ANOVA, two-way RM ANOVA, Student t tests and Friedman RM ANOVA on ranks as appropriate (Sigma Stat 3.0, systat Software Inc, Point Richmond, CA, USA). When significant differences were found between groups, post hoc analysis was used as indicated.
and VT were normalized (per 100 g rat body weight). Ventilatory parameters are expressed as mean values for quiet wakefulness and NREM sleep during room air, 7% CO2 or 10% O2 conditions. Statistics performed on the data included one-way repeated measures (RM) ANOVA, two-way RM ANOVA, Student t tests and Friedman RM ANOVA on ranks as appropriate (Sigma Stat 3.0, systat Software Inc, Point Richmond, CA, USA). When significant differences were found between groups, post hoc analysis was used as indicated.
Results
Microdialysis probe placement
Anatomical locations of the microdialysis probe tips are shown schematically in Fig. 2. The mean distances (in mm, ± s.e.m.) from bregma for the three treatment groups in this study were as follows: DPAT hypercapnia group, 11.5 ± 0.2; hypercapnia sham control group, 12.3 ± 0.2; and DPAT hypoxia group 12.1 ± 0.2. Probe tips in the DPAT hypercapnia group were located further rostral compared to the hypercapnia sham control and DPAT hypoxia groups; however, all probe tips fell into regions containing the raphe magnus, pallidus or obscurus. Tissue damage and gliosis were observed surrounding the location of the probe tip, but never extended more than a 0.4-mm radius from the tip centre. This damage did not appear to alter ventilatory responses, as repeated microdialysis of aCSF in the hypercapnia sham control group did not alter the ventilatory response to CO2 over the course of repeated experimentation.
Figure 2. Locations of microdialysis probes in the rats included in data analysis.
Probe tips fell between −11.0 to −12.8 mm from bregma in reference to the stereotaxic rat brain atlas of Paxinos & Watson (1982). Top diagrams indicate probes falling between −11.0 to −11.6 mm, and bottom diagrams indicate those between −11.6 to −12.8 mm from bregma. A, DPAT hypercapnia experiments (•, n = 7); B, hypercapnia sham control group (▪, n = 6); C, DPAT hypoxia experiments (▴n = 8). VII nuc, facial nucleus; Rmg, raphe magnus; Rpa, raphe pallidus; Rob, raphe obscurus.
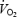 , ventilatory responses and body temperature: DPAT hypercapnia group
, ventilatory responses and body temperature: DPAT hypercapnia group
There was no significant change in  in room air conditions when any of the three concentrations of DPAT were microdialysed compared to aCSF (one-way RM ANOVA). Absolute ventilatory values during wakefulness in room air and 7% CO2 conditions in the DPAT hypercapnia group are illustrated in Fig. 3. During room air breathing, there was a slight but significant decrease in
in room air conditions when any of the three concentrations of DPAT were microdialysed compared to aCSF (one-way RM ANOVA). Absolute ventilatory values during wakefulness in room air and 7% CO2 conditions in the DPAT hypercapnia group are illustrated in Fig. 3. During room air breathing, there was a slight but significant decrease in  seen when animals were microdialysed with 1 mm DPAT compared to aCSF (*P < 0.05, one-way RM AVOVA, Dunnett post hoc comparison to aCSF). This decrease in
seen when animals were microdialysed with 1 mm DPAT compared to aCSF (*P < 0.05, one-way RM AVOVA, Dunnett post hoc comparison to aCSF). This decrease in  in room air was not present during microdialysis of the 10 or 30 mm concentrations, therefore this result should be interpreted with caution. During 7% CO2 exposure, 1, 10 and 30 mm DPAT microdialysis all decreased
in room air was not present during microdialysis of the 10 or 30 mm concentrations, therefore this result should be interpreted with caution. During 7% CO2 exposure, 1, 10 and 30 mm DPAT microdialysis all decreased  compared to aCSF (+P < 0.05, one-way RM ANOVA, Dunnett post hoc comparison to aCSF). The decrease in
compared to aCSF (+P < 0.05, one-way RM ANOVA, Dunnett post hoc comparison to aCSF). The decrease in  during 7% CO2 conditions in wakefulness when all three DPAT concentrations were given was not due to a decrease in f but in VT (+P < 0.05, one-way RM ANOVA, Dunnett post hoc comparison to aCSF). Additionally, microdialysis of 30 mm DPAT appeared to alter breathing pattern in room air conditions. There was a significant decrease in VT and a striking yet insignificant increase in f when 30 mm DPAT was microdialysed, and this breathing pattern effect in normocapnia was not observed during 1 or 10 mm DPAT treatments.
during 7% CO2 conditions in wakefulness when all three DPAT concentrations were given was not due to a decrease in f but in VT (+P < 0.05, one-way RM ANOVA, Dunnett post hoc comparison to aCSF). Additionally, microdialysis of 30 mm DPAT appeared to alter breathing pattern in room air conditions. There was a significant decrease in VT and a striking yet insignificant increase in f when 30 mm DPAT was microdialysed, and this breathing pattern effect in normocapnia was not observed during 1 or 10 mm DPAT treatments.
Figure 3. Mean  , f and VT absolute values in the DPAT hypercapnia group during quiet wakefulness (n = 7).
, f and VT absolute values in the DPAT hypercapnia group during quiet wakefulness (n = 7).
During 30 min of room air breathing, 1 mm DPAT microdialysis significantly decreased  , and 30 mm DPAT microdialysis significantly decreased VT compared to aCSF microdialysis (*P < 0.05, one-way repeated measures ANOVA, Dunnett post hoc comparison to aCSF). During 30 mm DPAT microdialysis, f in room air conditions appears elevated compared to aCSF; however, it was not found to be significant. During 30 min of 7% CO2 breathing,
, and 30 mm DPAT microdialysis significantly decreased VT compared to aCSF microdialysis (*P < 0.05, one-way repeated measures ANOVA, Dunnett post hoc comparison to aCSF). During 30 mm DPAT microdialysis, f in room air conditions appears elevated compared to aCSF; however, it was not found to be significant. During 30 min of 7% CO2 breathing,  was significantly decreased during 1, 10 and 30 mm DPAT microdialysis compared to aCSF, due to significant decreases in VT but not in f (+P < 0.05, one-way repeated measures ANOVA, Dunnett post hoc comparison to aCSF).
was significantly decreased during 1, 10 and 30 mm DPAT microdialysis compared to aCSF, due to significant decreases in VT but not in f (+P < 0.05, one-way repeated measures ANOVA, Dunnett post hoc comparison to aCSF).
All seven animals in the DPAT hypercapnia group had adequate NREM sleep data during aCSF and 1 mm DPAT microdialysis, but when 10 mm was given only five of seven animals slept, and 30 mm microdialysis led to an absence of NREM sleep in every animal. Microdialysis of 10 and 30 mm DPAT led to a visible increase in animal activity within the plethysmograph, and many animals appeared highly alert and maintained an upright posture throughout the experiment. Due to lack of NREM sleep ventilation data, and to keep analysis consistent, Fig. 4 illustrates only absolute ventilatory values in the five animals that slept during 10 mm DPAT microdialysis. As in wakefulness, 1 and 10 mm DPAT treatment led to a significant decrease in  during 7% CO2 breathing, and no change was seen in room air breathing (+P < 0.05 one-way RM ANOVA, Dunnett post hoc comparison to aCSF). Again, this decrease in
during 7% CO2 breathing, and no change was seen in room air breathing (+P < 0.05 one-way RM ANOVA, Dunnett post hoc comparison to aCSF). Again, this decrease in  during hypercapnia appeared to be caused by a decrease in VT and not f, but the change in VT was not significant.
during hypercapnia appeared to be caused by a decrease in VT and not f, but the change in VT was not significant.
Figure 4. Mean  , f and VT absolute values in the DPAT hypercapnia group during NREM sleep (n = 5).
, f and VT absolute values in the DPAT hypercapnia group during NREM sleep (n = 5).
There were no significant changes found in  , f or VT during 30 min of room air breathing. During 30 min of 7% CO2 exposure,
, f or VT during 30 min of room air breathing. During 30 min of 7% CO2 exposure,  was significantly decreased during 1 and 10 mm DPAT microdialysis compared to when aCSF was microdialysed (+P < 0.05, one-way repeated measures ANOVA, Dunnett post hoc comparison to aCSF). As found in wakefulness, these significant decreases in
was significantly decreased during 1 and 10 mm DPAT microdialysis compared to when aCSF was microdialysed (+P < 0.05, one-way repeated measures ANOVA, Dunnett post hoc comparison to aCSF). As found in wakefulness, these significant decreases in  in hypercapnic conditions were probably due to decreased VT during 1 and 10 mm DPAT microdialysis; however, no statistical differences were detected.
in hypercapnic conditions were probably due to decreased VT during 1 and 10 mm DPAT microdialysis; however, no statistical differences were detected.
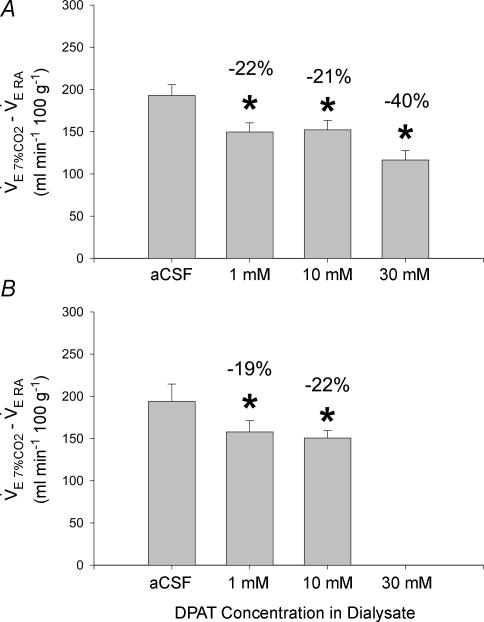
To quantify the DPAT-induced changes in the ventilatory response to 7% CO2, the mean  during 30 min of room air breathing was subtracted from mean
during 30 min of room air breathing was subtracted from mean  during 30 min of 7% CO2 breathing in each animal. These results are displayed in Fig. 5. During quiet wakefulness (Fig. 5A), 1, 10 and 30 mm DPAT led to significant decreases in the 7% CO2 ventilatory response (22%, 21% and 40%, respectively; *P < 0.05, one-way RM ANOVA, Dunnett post hoc comparison to aCSF). In the five animals that had sufficient NREM sleep data, there were significant decreases in the 7% CO2 ventilatory response during 1 and 10 mm DPAT microdialysis (19% and 22%, respectively; *P < 0.05, one-way RM ANOVA, Dunnett post hoc comparison to aCSF). Percentage decreases in the 7% CO2 ventilatory response were similar during NREM sleep to those in wakefulness.
during 30 min of 7% CO2 breathing in each animal. These results are displayed in Fig. 5. During quiet wakefulness (Fig. 5A), 1, 10 and 30 mm DPAT led to significant decreases in the 7% CO2 ventilatory response (22%, 21% and 40%, respectively; *P < 0.05, one-way RM ANOVA, Dunnett post hoc comparison to aCSF). In the five animals that had sufficient NREM sleep data, there were significant decreases in the 7% CO2 ventilatory response during 1 and 10 mm DPAT microdialysis (19% and 22%, respectively; *P < 0.05, one-way RM ANOVA, Dunnett post hoc comparison to aCSF). Percentage decreases in the 7% CO2 ventilatory response were similar during NREM sleep to those in wakefulness.
Figure 5. Mean differences in  between 7% CO2 and room air conditions in the DPAT hypercapnia group during (A) quiet wakefulness (n = 7) and (B) NREM sleep (n = 5).
between 7% CO2 and room air conditions in the DPAT hypercapnia group during (A) quiet wakefulness (n = 7) and (B) NREM sleep (n = 5).
Microdialysis of 1, 10 and 30 mm DPAT in quiet wakefulness and 1 and 10 mm DPAT microdialysis in NREM sleep significantly decreased the ventilatory response to 7% CO2 compared to that seen during aCSF microdialysis (*P < 0.05, one-way RM ANOVA, Dunnett post hoc comparison to aCSF).
Figure 6 shows average core body temperatures of rats in the DPAT hypercapnia group during the aCSF, 1, 10 and 30 mm microdialysis experiments. Temperatures were recorded every 10 min during room air and 7% CO2 conditions. There was a significant decrease in average body temperature during the 10 and 30 mm DPAT experiments compared to when aCSF was microdialysed (*P < 0.05, two-way RM ANOVA with treatment and time as factors, Dunnett post hoc comparison to aCSF). In this analysis, a significant interaction (P= 0.029) was also detected between treatment and time (t). Temperature in all treatment groups at t = 40, 50 and 60 min was decreased compared to t = 10 min (P < 0.05, Bonferroni post hoc pair-wise comparison to t = 10 min), indicating that 7% CO2 exposure itself caused a significant decrease in body temperature. The average temperature of the plethysmograph in this group of animals (± s.e.m.) during the ventilation experiments was 26.2 ± 0.2°C.
Figure 6. Mean core body temperatures during the 6 min ventilation experiments in the DPAT hypercapnia group (n = 7).
Microdialysis of 10 and 30 mm DPAT elicited significant decreases in body temperature compared to aCSF microdialysis (*P < 0.05, two-way repeated measures ANOVA with time and treatment as factors, Dunnett post hoc comparison to aCSF). There was a significant interaction found between treatment group and time (P= 0.029); however, further analysis found that DPAT microdialysis did not exacerbate the normal body temperature decrease found during 30 min of 7% CO2 breathing.
Initial body temperature analysis indicated that there was a significant interaction between treatment and time factors, therefore it was necessary to assess whether DPAT microdialysis exacerbated the decrease in body temperature observed during 7% CO2 conditions. To do this, mean body temperatures at each time point during 1, 10 and 30 mm DPAT microdialysis were subtracted from body temperature values during aCSF microdialysis in each animal (data not shown). A significant change in core body temperature differences across the three DPAT treatment groups was detected (P < 0.001, two-way RM ANOVA, post hoc Bonferroni pair-wise comparison of treatment groups). There was also a significant interaction found between treatment and time in this analysis (P= 0.020), however, post hoc pair-wise comparisons revealed that there were no significant effects in body temperature differences at each time point compared to t = 10 min. Although it appears that DPAT microdialysis increases the drop in body temperature during 7% CO2 breathing, this effect could not be statistically validated.
 , ventilatory responses and body temperature: hypercapnia sham control group
, ventilatory responses and body temperature: hypercapnia sham control group
As seen in the DPAT hypercapnia group, there were no differences found in normocapnic 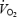 values in this group across the four aCSF microdialysis trials (one-way RM ANOVA). In the six animals serving as hypercapnia sham controls, repeated aCSF microdialysis did not lead to changes in wakefulness or NREM sleep ventilation during 30 min of room air breathing nor 30 min of 7% CO2 (one-way RM ANOVA). Absolute ventilatory values during wakefulness are shown in Fig. 7, and during NREM sleep in Fig. 8. Values for f, VT and
values in this group across the four aCSF microdialysis trials (one-way RM ANOVA). In the six animals serving as hypercapnia sham controls, repeated aCSF microdialysis did not lead to changes in wakefulness or NREM sleep ventilation during 30 min of room air breathing nor 30 min of 7% CO2 (one-way RM ANOVA). Absolute ventilatory values during wakefulness are shown in Fig. 7, and during NREM sleep in Fig. 8. Values for f, VT and  for room air and 7% CO2 in each aCSF microdialysis experiment were similar to those observed during aCSF microdialysis in the DPAT hypercapnia group. Additionally, the differences between
for room air and 7% CO2 in each aCSF microdialysis experiment were similar to those observed during aCSF microdialysis in the DPAT hypercapnia group. Additionally, the differences between  in 7% CO2 and in room air (the ventilatory response to 7% CO2) during wakefulness or NREM sleep were unchanged (data not shown).
in 7% CO2 and in room air (the ventilatory response to 7% CO2) during wakefulness or NREM sleep were unchanged (data not shown).
Figure 7. Mean  , f and VT absolute values in the hypercapnia sham control group during wakefulness (n = 6).
, f and VT absolute values in the hypercapnia sham control group during wakefulness (n = 6).
Repeated aCSF microdialysis trials did not significantly affect ventilatory parameters during room air or 7% CO2 breathing (one-way RM ANOVA).
Figure 8. Mean  , f and VT absolute values in the hypercapnia sham control group during NREM sleep (n = 6).
, f and VT absolute values in the hypercapnia sham control group during NREM sleep (n = 6).
Repeated aCSF microdialysis trials did not significantly affect ventilatory parameters during room air or 7% CO2 breathing (one-way RM ANOVA).
Repeated aCSF microdialysis experiments did not result in any changes in core body temperature (Table 1). Breathing 7% CO2 did cause a slight but significant decrease in body temperature at t = 40, 50 and 60 min, and this occurred in every aCSF trial within the group (*P < 0.05, two-way RM ANOVA, Dunnett post hoc comparison to t = 10 min). The slight decrease in body temperature during 7% CO2 was similar to that seen during aCSF microdialysis in the DPAT hypercapnia group. The average temperature of the plethysmograph (± s.e.m.) during the ventilatory experiments in this group of animals was 26.0 ± 0.2°C.
Table 1.
Core body temperatures in the sham control group (n = 6)
| Time in room air (min) | Time in 7% CO2 (min) | ||||||
|---|---|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | 50 | 60 | ||
| Tb | aCSF 1 | 37.75 ± 0.06 | 37.78 ± 0.05 | 37.72 ± 0.05 | 37.63 ± 0.04* | 37.6 ± 0.06* | 37.57 ± 0.06* |
| aCSF 2 | 37.77 ± 0.05 | 37.7 ± 0.05 | 37.67 ± 0.04 | 37.62 ± 0.05* | 37.57 ± 0.04* | 37.55 ± 0.05* | |
| aCSF 3 | 37.70 ± 0.05 | 37.65 ± 0.04 | 37.65 ± 0.04 | 37.52 ± 0.05* | 37.5 ± 0.06* | 37.48 ± 0.05* | |
| aCSF 4 | 37.73 ± 0.06 | 37.72 ± 0.08 | 37.67 ± 0.08 | 37.65 ± 0.09* | 37.63 ± 0.08* | 37.62 ± 0.07* | |
Mean values are shown (± s.e.m.). 7% CO2 breathing caused a slight but significant decrease in body temperature compared to that at t = 0(*P < 0.05, two-way RM ANOVA with aCSF trial and time as factors, Dunnett post hoc comparison to t = 10 min).
 , ventilatory responses and body temperature: DPAT hypoxia group
, ventilatory responses and body temperature: DPAT hypoxia group
 was calculated in the DPAT hypoxia group before (prehypoxia), during and after (posthypoxia) the 1-h exposure to 10% O2. There was no significant effect of 30 mm DPAT microdialysis found on either pre-, during or posthypoxia
was calculated in the DPAT hypoxia group before (prehypoxia), during and after (posthypoxia) the 1-h exposure to 10% O2. There was no significant effect of 30 mm DPAT microdialysis found on either pre-, during or posthypoxia  values compared to when aCSF was microdialysed (one-way RM ANOVA). We were unable to detect any effects of hypoxia on
values compared to when aCSF was microdialysed (one-way RM ANOVA). We were unable to detect any effects of hypoxia on 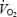 during our experimental conditions during the first aCSF trial (aCSF1), 30 mm DPAT microdialysis or the second aCSF trial (aCSF2) when comparing prehypoxia
during our experimental conditions during the first aCSF trial (aCSF1), 30 mm DPAT microdialysis or the second aCSF trial (aCSF2) when comparing prehypoxia  to
to  during 10% O2 breathing (one-way RM ANOVA). Compared to prehypoxia values,
during 10% O2 breathing (one-way RM ANOVA). Compared to prehypoxia values,  was significantly increased in the 30 mm DPAT and aCSF2 trials during the posthypoxia period (P < 0.05, one-way RM ANOVA, Dunnett post hoc comparison to prehypoxia), and the aCSF1 trial approached significance.
was significantly increased in the 30 mm DPAT and aCSF2 trials during the posthypoxia period (P < 0.05, one-way RM ANOVA, Dunnett post hoc comparison to prehypoxia), and the aCSF1 trial approached significance.
Absolute ventilatory values at 10-min intervals for the 30-min prehypoxic room air recording, 1-h 10% O2 recording and 30-min posthypoxic room air recording in wakefulness are shown in Fig. 9. Even with 1 h of recording during 10% O2 conditions, we did not obtain adequate NREM sleep ventilation for statistical analysis, so the data are not shown. As there were no significant differences found between  , f or VT values during the aCSF1 and aCSF2 trials (two-way RM ANOVA with treatment and time as factors), the mean of the two values was compared to the values during the 30 mm DPAT microdialysis experiment for simplicity. Microdialysis of 30 mm DPAT into the MRR did not have any effect on
, f or VT values during the aCSF1 and aCSF2 trials (two-way RM ANOVA with treatment and time as factors), the mean of the two values was compared to the values during the 30 mm DPAT microdialysis experiment for simplicity. Microdialysis of 30 mm DPAT into the MRR did not have any effect on 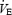 during room air or 10% O2 breathing compared with aCSF microdialysis (two-way RM ANOVA with treatment and time as factors). Posthypoxia
during room air or 10% O2 breathing compared with aCSF microdialysis (two-way RM ANOVA with treatment and time as factors). Posthypoxia  during aCSF and 30 mm DPAT microdialysis appears slightly higher than prehypoxia
during aCSF and 30 mm DPAT microdialysis appears slightly higher than prehypoxia  , but there was no significant effect, as determined by comparing mean prehypoxic values to mean posthypoxic values (one-way RM ANOVA). Treatment with 30 mm DPAT did lead to a significant increase in f and decrease in VT throughout the experiment (#P < 0.05 treatment effect, two-way RM ANOVA with treatment and time as factors, Dunnett post hoc comparison to aCSF1 and aCSF2 average). The alteration in breathing pattern (increase in f, decrease in VT) observed in room air and in hypoxia in the DPAT hypoxia group was similar to the changes in breathing pattern found during the original DPAT hypercapnia experiments when 30 mm DPAT was microdialysed. Unlike the DPAT hypercapnia experiments, however, there was no additional change in the ventilatory response to 10% O2 brought about by 30 mm DPAT microdialysis. When the differences between ventilatory parameters during prehypoxia room air and 10% O2 were calculated, there were no significant effects detected as a result of 30 mm DPAT treatment (two-way RM ANOVA with treatment and time as factors, data not shown).
, but there was no significant effect, as determined by comparing mean prehypoxic values to mean posthypoxic values (one-way RM ANOVA). Treatment with 30 mm DPAT did lead to a significant increase in f and decrease in VT throughout the experiment (#P < 0.05 treatment effect, two-way RM ANOVA with treatment and time as factors, Dunnett post hoc comparison to aCSF1 and aCSF2 average). The alteration in breathing pattern (increase in f, decrease in VT) observed in room air and in hypoxia in the DPAT hypoxia group was similar to the changes in breathing pattern found during the original DPAT hypercapnia experiments when 30 mm DPAT was microdialysed. Unlike the DPAT hypercapnia experiments, however, there was no additional change in the ventilatory response to 10% O2 brought about by 30 mm DPAT microdialysis. When the differences between ventilatory parameters during prehypoxia room air and 10% O2 were calculated, there were no significant effects detected as a result of 30 mm DPAT treatment (two-way RM ANOVA with treatment and time as factors, data not shown).
Figure 9. Mean  , f and VT absolute values in the hypoxia DPAT group during quiet wakefulness (n = 8).
, f and VT absolute values in the hypoxia DPAT group during quiet wakefulness (n = 8).
As there were no significant differences found between ventilatory values during the first (aCSF1) and second aCSF (aCFS2) trials in this group, the average is presented for clarity. Microdialysis of 30 mm DPAT had no effect on 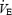 during room air breathing or hypoxia; however, it significantly increased f and decreased VT during both room air and 10% O2 compared to aCSF microdialysis (#P < 0.05, two-way RM ANOVA with treatment and time as factors, Dunnett post hoc comparison to aCSF).
during room air breathing or hypoxia; however, it significantly increased f and decreased VT during both room air and 10% O2 compared to aCSF microdialysis (#P < 0.05, two-way RM ANOVA with treatment and time as factors, Dunnett post hoc comparison to aCSF).
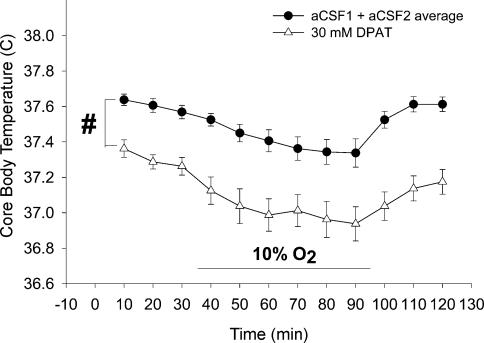
Rat body temperatures in the DPAT hypoxia group are shown in Fig. 10. As body temperature at each 10-min interval in the aCSF1 and aCSF2 trials were not significantly different (two-way RM ANOVA with treatment and time as factors), the mean of the two were again used for clarity. During the aCSF microdialysis trials, 10% O2 exposure resulted in a significant decrease in body temperature from t = 50 to t = 90 min when compared to t = 10 min (P < 0.05, two-way RM ANOVA with treatment and time as factors, post hoc Bonferroni pair-wise comparison). Hypoxia-induced anapyrexia was also observed with 30 mm DPAT microdialysis, as there was a significant decrease in temperature seen from t = 40 to t = 90 min compared to t = 10 min (P < 0.05, two-way RM ANOVA with treatment and time as factors, post hoc Bonferroni pair-wise comparison). Microdialysis of 30 mm DPAT into the MRR significantly decreased body temperature throughout the ventilation experiment compared to aCSF microdialysis, similar to the body temperature effect seen during 30 mm DPAT microdialysis in the DPAT hypercapnia group (#P < 0.05, two-way RM ANOVA with treatment and time as factors, Dunnett post hoc comparison to aCSF1 and aCSF2 average). To determine whether 30 mm DPAT microdialysis in the MRR exaggerated the hypoxia-induced decrease in body temperature, we subtracted the average body temperature prehypoxia from body temperatures at each time point during 10% O2 breathing for both the aCSF microdialysis experiments and the 30 mm DPAT microdialysis experiments. There was no change detected in the body temperature response to 10% O2 when 30 mm DPAT was microdialysed compared to aCSF (two-way RM ANOVA with treatment and time as factors). The average temperature within the plethysmograph during the ventilatory experiments in this group of animals was 26.2 ± 0.1°C.
Figure 10. Mean core body temperature of rats in the hypoxia DPAT group.
There was no significant body temperature difference found between the aCSF1 and aCSF2 trials so they are presented as an average value. As in the hypercapnia experiments, 30 mm DPAT microdialysis significantly decreased body temperature compared to aCSF microdialysis (P < 0.05 two-way RM ANOVA with treatment and time as factors, Dunnett post hoc comparison to aCSF). Further analysis revealed DPAT treatment did not change the body temperature decrease observed during hypoxia.
Sleep states
Table 2 shows the percentage experimental time in wakefulness and NREM sleep in all three groups of animals in this study. Percentage time in REM sleep or when sleep state was indeterminate never amounted to more than 5% of the experimental time during any experiment in any group (data not shown). In the DPAT hypercapnia group, microdialysis of 30 mm DPAT caused a significant increase in percentage time awake and decrease in percentage time in NREM sleep compared to the aCSF trial during 7% CO2 breathing but not in room air conditions (*P < 0.05, one-way RM ANOVA, Dunnett post hoc comparison to aCSF). Repeated aCSF microdialysis experiments did not result in any significant changes in percentage time in wakefulness or NREM sleep during room air or 7% CO2 conditions in the hypercapnia sham control group (one-way RM ANOVA). Microdialysis of 30 mm DPAT into the MRR in the DPAT hypoxia group led to significant increases in percentage time spent in wakefulness and decreases in time in NREM sleep during prehypoxia room air, 10% O2 and posthypoxia room air conditions (*P < 0.05, one-way RM ANOVA, Dunnett post hoc comparison to aCSF). Overall, 30 mm DPAT microdialysis in both the DPAT hypercapnia and DPAT hypoxia group increased time spent in wakefulness during the ventilation experiments.
Table 2.
Percentage experimental time spent in quiet wakefulness (QW) and non-rapid eye movement (NREM) sleep
| Room Air 1 | 7% CO2 or 10% O2 | Room Air 2 (hypoxia group only) | ||||
|---|---|---|---|---|---|---|
| QW | NREM | QW | NREM | QW | NREM | |
| 7% CO2 DPAT group (n = 7) | ||||||
| aCSF | 81.8 ± 6.6 | 17.7 ± 6.5 | 70.3 ± 11.3 | 28.0 ± 11.0 | — | — |
| 1 mm | 68.2 ± 11.4 | 29.3 ± 11.3 | 85.2 ± 7.4 | 14.5 ± 7.3 | — | — |
| 10 mm | 85.8 ± 5.8 | 13.3 ± 5.4 | 91.3 ± 2.4 | 7.8 ± 2.2 | — | — |
| 30 mm | 92.7 ± 4.3 | 7.3 ± 4.3 | 98.5 ± 1.1* | 1.5 ± 1.1* | — | — |
| 7% CO2 Sham group (n = 6) | ||||||
| aCSF 1 | 71.9 ± 4.9 | 28.1 ± 4.8 | 72.2 ± 7.5 | 26.0 ± 6.8 | — | — |
| aCSF 2 | 62.3 ± 10.2 | 35.8 ± 9.6 | 67.7 ± 8.6 | 33.3 ± 8.8 | — | — |
| aCSF 3 | 66.5 ± 4.4 | 32.0 ± 4.3 | 71.0 ± 7.8 | 28.0 ± 7.9 | — | — |
| aCSF 4 | 69.7 ± 5.4 | 29.0 ± 5.2 | 63.2 ± 10.9 | 35.8 ± 10.8 | — | — |
| 10% O2 DPAT group (n = 8) | ||||||
| aCSF | 62.5 ± 5.1 | 32.4 ± 6.2 | 91.3 ± 2.8 | 8.8 ± 2.8 | 59.9 ± 4.9 | 40.5 ± 3.4 |
| 30 mm | 90.3 ± 6.5* | 9.0 ± 5.9* | 99.0 ± 1.0* | 1.0 ± 0.5* | 78.3 ± 8.5* | 21.4 ± 8.3* |
P < 0.05, one-way repeated measures ANOVA, Dunnett post hoc analysis comparison to aCSF trial within the group.
Discussion
Major findings
Microdialysis of the 5-HT1A receptor agonist DPAT into the MRR in adult unanaesthetized rats (1) led to significant decreases in the ventilatory response to 7% CO2 compared to aCSF microdialysis alone, (2) decreased core body temperature, (3) increased the time spent in wakefulness during the experiments and (4) had no effect on the ventilatory response to hypoxia. Microdialysis of 30 mm DPAT produced the largest decrease in the ventilatory response to 7% CO2 (40%), and this concentration also increased f and decreased VT in room air conditions, evidence of a baseline effect on ventilatory pattern.
Ventilation effects of MRR DPAT microdialysis: hypercapnia and hypoxia
This study provides a new piece of evidence that acutely and reversibly activating the 5-HT1A receptor (thereby decreasing serotonergic activity) within the MRR of an unanaesthetized adult rat decreases the ventilatory response to hypercapnia. The inhibitory effects of DPAT on the ventilatory response to hypercapnia did not appear to vary between wakefulness and NREM sleep during microdialysis of either 1 or 10 mm concentrations, which is similar to what was found in the SERT antibody–saporin study (Nattie et al. 2004). These data reinforce the hypothesis that MRR serotonergic activity is involved in central chemosensitivity and in modulating the ventilatory response to hypercapnia in vivo (Messier et al. 2004; Nattie et al. 2004; Taylor et al. 2004; Richerson et al. 2005). Though the decrease in the ventilatory response to 7% CO2 observed when DPAT was microdialysed into the rat MRR does not directly prove that serotonergic neurones serve as central chemoreceptors, it shows that they are deeply involved in regulating the ventilatory response to hypercapnia. A recent study by Mulkey et al. (2004) suggested that glutamatergic, and not serotonergic neurones in the nearby RTN served as the central ‘CO2 sensors’ on the ventral medullary surface. However, in vivo studies suggest that multiple brainstem regions (including the RTN, MRR and NTS) are involved in central chemoreception (Li et al. 1999; Nattie & Li 2001, 2002a). Given the evidence that MRR chemosensitive neurones are serotonergic (Wang et al. 2001; Nattie et al. 2004; Richerson et al. 2005), and that RTN chemosensitive neurones are glutamatergic (Mulkey et al. 2004; Guyenet et al. 2005), it is likely that more than one neuronal phenotype in the brainstem is capable of responding to increases in tissue PCO2.
In agreement with the present finding, microdialysis of 30 mm DPAT into a conscious piglet decreased the ventilatory response to 5% CO2, but only in piglets within their second postnatal week (Messier et al. 2004). In consideration of possible age-related differences in MRR chemosensitivity, a recent anatomical study established that expression of serotonin receptors in several nuclei of the neonatal piglet brainstem, including the MRR, varies over the first 30 days of life (Niblock et al. 2004). These physiological and anatomical results in piglets, together with our current data in the adult rat, indicate that the serotonergic system may undergo developmental changes in the early postnatal period prior to playing a role in the hypercapnic ventilatory response or central chemosensitivity.
During systemic hypoxia, peripheral chemoreceptors present in the carotid body detect decreases in arterial PCO2, and synapse via the carotid sinus nerve directly to caudal regions of the NTS including the ventrolateral and commissural subnuclei (Housley & Sinclair, 1988). In the NTS, cardiovascular and respiratory information is probably integrated and modulated secondarily by a variety of neurotransmitters (Kalia et al. 1984; Richter et al. 1999). Although serotonergic terminals originating in the MRR project to the caudal NTS (Thor & Helke, 1987), and electrical or chemical stimulation of neurones in the MRR inhibit NTS activity during peripheral chemoreceptor activation (Perez & Ruiz, 1995), our data in this study suggest that 5-HT1A-expressing neurones within the MRR are not involved in the ventilatory response to hypoxia. It is still logical to propose that non-serotonergic inputs to the NTS from the MRR are involved in modulating the ventilatory response to hypoxia. Erickson & Millhorn (1994) observed c-fos staining in neurones within the MRR after exposing rats to hypoxia or carotid sinus nerve stimulation; however, only ∼30% of c-fos-staining neurones were identified by immunohistochemistry as serotonergic. Non-serotonergic inputs to the NTS from the MRR that might be involved in modulating the ventilatory response to hypoxia could possibly include but are not limited to substance P (Thor & Helke, 1987), l-glutamate (Howe, 1985), thyrotropin-releasing hormone (Palkovits et al. 1986) and GABA (Gao et al. 1993).
Body temperature effects of MRR DPAT microdialysis
In both the DPAT hypercapnia and DPAT hypoxia group, microdialysis of 30 mm DPAT into the MRR significantly lowered core body temperature by approximately 0.4°C compared to aCSF. Smaller concentrations of DPAT also decreased body temperature, in a seemingly concentration-dependent fashion (Fig. 6). Previous studies that applied DPAT directly to the MRR region in rats indicated a role for MRR serotonergic neurones in thermoregulation. Berner et al. (1999) found that microinjecting DPAT in an amount equivalent to 12.5 mm in conscious rats blunted elevations in oxygen consumption and increases in electromyographic activity (i.e. shivering) caused by cooling the preoptic/anterior hypothalamus (POAH), suggesting that MRR serotonergic activity modulates communication between the POAH and thermoregulatory effector responses. Morrison (2004) also linked 5-HT1A activity in the MRR to thermoregulation in the anaesthetized rat. He found that 10 mm DPAT microinjection into the raphe pallidus inhibited leptin-evoked increases in brown adipose tissue (BAT) sympathetic nerve activity, BAT temperature, expired CO2, heart rate and mean arterial pressure. Furthermore, MRR serotonergic neurones have been reported to project to the sympathetic innervations of the tail artery (Smith et al. 1998), and inhibition of the MRR with muscimol microdialysis results in a decrease in the cold-induced activity in sympathetic cutaneous vasomotor nerves in the rat tail (Ootsuka et al. 2004). This is physiologically relevant, as ∼20% of heat loss in the rat occurs through anastomoses in the skin of the tail (Young & Dawson, 1982). Though the present experiments were not designed to study the effects of MRR DPAT on thermoregulation, data from others suggest that MRR serotonergic activity plays a role in modulating effector responses. Because we did not observe any changes in oxygen consumption as a result of MRR DPAT microdialysis in our study, it is possible that the decrease in body temperature was the result of inhibiting sympathetic vasomotor nerves serving the rat tail vasculature.
Relationships between respiratory responses and body temperature
Is the decrease in the ventilatory response to 7% CO2 the result of the decrease in body temperature found during MRR DPAT microdialysis? A previous study directly investigating body temperature effects on hypoxia and hypercapnia responses in conscious rats allows us to estimate that approximately 3% of the decrease in the ventilatory response to 7% CO2 could be attributed to the 0.5°C drop in body temperature caused by 30 mm MRR DPAT microdialysis (Maskrey, 1990). As 30 mm DPAT microdialysis caused a 40% decrease in the ventilatory response to 7% CO2, body temperature most probably had a very minimal contribution to this effect.
Arousal effects of MRR DPAT microdialysis
Microdialysis of 30 mm DPAT into the MRR compared to aCSF increased the percentage experimental time spent in wakefulness during both the hypercapnia and hypoxia experiments, such that NREM sleep data could not be completely analysed. This effect echoes a previous study performed in unanaesthetized piglets, where 30 mm DPAT microdialysis into the MRR significantly increased time spent in wakefulness (Messier et al. 2004). In piglets, unlike in rats, MRR DPAT microdialysis did not affect core body temperature. Decreases in body temperature in rats might be expected to promote wakefulness, as sleep decreases directly with ambient temperature because of increased thermoregulatory activity (Schmidek et al. 1972). However, increased arousal observed in the piglet independent of a change in body temperature argues that these two effects in the rat may be unrelated during MRR DPAT microdialysis.
The disruption of sleep cycling found when DPAT is microdialysed into the MRR is difficult to interpret, as the MRR has never been directly implicated in sleep modulation. DPAT microinjection in the dorsal raphe nuclei (DR) promotes arousal and increases hippocampal theta activity (Nitz & McNaughton, 1999), and DR 5-HT1A neurones directly affect activity in the REM-generating laterodorsal and the pedunculopontine tegmental nuclei of the midbrain (Monti & Monti, 2000). In contrast, serotonergic neurones in the MRR primarily project to sensory and motor neurones in the spinal cord, and their activity has been found to relate highly to tonic and repetitive motor activity in a freely behaving cat (Jacobs et al. 2002). Serotonergic neurones in the MRR have very few projections to REM centres, especially compared to the number of serotonergic inputs from the DR (Semba, 1993). Additionally, activity of serotonin-containing raphe pallidus neurones is highest during wakefulness, decreases with sleep stage, and is nearly absent during REM sleep; though serotonergic MRR activity is not correlated with cortical EEG activity as is serotonergic DR activity (Heym et al. 1982). It is possible that the increased arousal observed during MRR DPAT microdialysis is a result of an alteration of sensory and motor activity in the brainstem and spinal cord, and not due to the negligible effects that the treatment may have upon sleep control regions of the midbrain.
Non-specific effects of MRR DPAT microdialysis
Other studies that have used the reverse microdialysis technique to administer pharmacological compounds to the mammalian brainstem have co-dialysed dyes of similar molecular weight to estimate the tissue radius affected by treatment (Cream et al. 1999; Messier et al. 2004). Dyes provide some proof that reverse microdialysis is an effective method of delivering compounds, but can never replicate the actual tissue boundaries of diffusion when the drug itself is microdialysed. Because microdialysis of 30 mm DPAT into the MRR significantly changed the breathing pattern during room air conditions in this study, and 1 and 10 mm concentrations did not have this effect, it is possible that the high concentration affected a great enough number of 5-HT1A receptor-expressing MRR neurones to the point of altering baseline respiration. We also speculate that the 40% decrease in the hypercapnic ventilatory response seen after 30 mm DPAT treatment, double to that seen with 1 or 10 mm, is attributable to a larger spread of DPAT in the ventral medulla.
DPAT has been widely used since its discovery as a highly selective 5-HT1A agonist (Arvidsson et al. 1981). It binds preferentially to 5-HT1A receptors subcellularly located on the soma and dendrites of neurones that synthesize serotonin; leading to hyperpolarization, decreased serotonin synthesis, and decreased serotonin release at synaptic terminals (Hjorth & Magnusson, 1988). Nonetheless, DPAT has been reported to act upon postsynaptic 5-HT1A receptors located upon non-serotonergic neurones (5-HT1A heteroreceptors) and lead to non-specific hyperpolarization (Kirby et al. 2003). As 5-HT1A has been detected on non-serotonergic neurones in the ventral medulla (Helke et al. 1997), and perhaps even glia (Kia et al. 1996), non-serotonergic effects of DPAT must be included as one of the limiting factors during interpretation of this study. DPAT has also been discovered to be an agonist of the 5-HT7 class of serotonin receptor, but the presence of 5-HT7 has not yet been described in the rat MRR, and its biological function in the CNS is still unclear (Vanhoenacker et al. 2000). Despite its limitations, we still believe that DPAT is a powerful tool to inhibit serotonergic neurones in the MRR by activating 5-HT1A autoreceptors.
Conclusions
From our results, we conclude that MRR serotonergic neurones mediate the CO2 response in unanaesthetized rats, affect body temperature regulation and influence arousal. While it is currently debated whether medullary serotonergic neurones are indeed central CO2 chemoreceptors (Guyenet et al. 2005; Richerson et al. 2005), our data support their acute role in the hypercapnic ventilatory response. As these neurones do not affect the hypoxic ventilatory response, further studies will hopefully determine which neuronal phenotypes in the MRR could possibly be involved.
Acknowledgments
This work was supported by funding from the National Institutes of Health HL 28066, National Institute of Child Health and Human Development 36379 and the Albert J. Ryan Foundation.
References
- Arvidsson LE, Hacksell U, Nilsson JL, Hjorth S, Carlsson A, Lindberg P, et al. 8-Hydroxy-2-(di-n-propylamino) tetralin, a new centrally acting 5-hydroxytryptamine receptor agonist. J Med Chem. 1981;24:921–923. doi: 10.1021/jm00140a002. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Gannon PJ, Kheck NM, Whitaker-Azmitia PM. Cellular localization of the 5-HT1A receptor in primate brain neurons and glial cells. Neuropsychopharmacology. 1996;14:35–46. doi: 10.1016/S0893-133X(96)80057-1. [DOI] [PubMed] [Google Scholar]
- Berner NJ, Grahn DA, Heller HC. 8-OH-DPAT-sensitive neurons in the nucleus raphe magnus modulate thermoregulatory output in rats. Brain Res. 1999;831:155–164. doi: 10.1016/s0006-8993(99)01426-2. [DOI] [PubMed] [Google Scholar]
- Connelly CA, Ellenberger HH, Feldman JL. Are there serotonergic projections from raphe and retrotrapezoid nuclei to the ventral respiratory group in the rat? Neurosci Lett. 1989;105:34–40. doi: 10.1016/0304-3940(89)90007-4. [DOI] [PubMed] [Google Scholar]
- Cream C, Nattie E, Li A. TRH microdialysis into the RTN of the conscious rat increases breathing, metabolism, and temperature. J Appl Physiol. 1999;87:673–682. doi: 10.1152/jappl.1999.87.2.673. [DOI] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol. 1994;347:64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Millhorn DE. Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotoninergic neurons of the rat brainstem. J Comp Neurol. 1994;348:161–182. doi: 10.1002/cne.903480202. [DOI] [PubMed] [Google Scholar]
- Gao B, Fritschy JM, Benke D, Mohler H. Neuron specific expression of GABAA-receptor subtypes: differential association of the [alpha]1- and [alpha]3- subunits with serotonergic and gabaergic neurons. Neuroscience. 1993;54:881–892. doi: 10.1016/0306-4522(93)90582-z. [DOI] [PubMed] [Google Scholar]
- Gargaglioni LH, Coimbra NC, Branco LG. The nucleus raphe magnus modulates hypoxia-induced hyperventilation but not anapyrexia in rats. Neurosci Lett. 2003;347:121–125. doi: 10.1016/s0304-3940(03)00671-2. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA, Mulkey DK. Retrotrapezoid nucleus: a litmus test for the identification of central chemoreceptors. Exp Physiol. 2005;90:247–253. doi: 10.1113/expphysiol.2004.029637. [DOI] [PubMed] [Google Scholar]
- Helke CJ, Capuano S, Tran N, Zhuo H. Immunocytochemical studies of the 5-HT(1A) receptor in ventral medullary neurons that project to the intermediolateral cell column and contain serotonin or tyrosine hydroxylase immunoreactivity. J Comp Neurol. 1997;379:261–270. [PubMed] [Google Scholar]
- Heym J, Steinfels GF, Jacobs BL. Activity of serotonin-containing neurons in the nucleus raphe pallidus of freely moving cats. Brain Res. 1982;251:259–276. doi: 10.1016/0006-8993(82)90743-0. [DOI] [PubMed] [Google Scholar]
- Hjorth S, Magnusson T. The 5-HT 1A receptor agonist, 8-OH-DPAT, preferentially activates cell body 5-HT autoreceptors in rat brain in vivo. Naunyn Schmiedebergs Arch Pharmacol. 1988;338:463–471. doi: 10.1007/BF00179315. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Klum L, Leekley T, Brozoski DT, Bastasic J, Davis S, et al. Effects on breathing in awake and sleeping goats of focal acidosis in the medullary raphe. J Appl Physiol. 2004;96:1815–1824. doi: 10.1152/japplphysiol.00992.2003. [DOI] [PubMed] [Google Scholar]
- Housley GD, Sinclair JD. Localization by kainic acid lesions of neurones transmitting the carotid chemoreceptor stimulus for respiration in rat. J Physiol. 1988;406:99–114. doi: 10.1113/jphysiol.1988.sp017371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe PR. Blood pressure control by neurotransmitters in the medulla oblongata and spinal cord. J Auton Nerv Syst. 1985;12:95–115. doi: 10.1016/0165-1838(85)90054-2. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Martin-Cora FJ, Fornal CA. Activity of medullary serotonergic neurons in freely moving animals. Brain Res Brain Res Rev. 2002;40:45–52. doi: 10.1016/s0165-0173(02)00187-x. [DOI] [PubMed] [Google Scholar]
- Jelev A, Sood S, Liu H, Nolan P, Horner RL. Microdialysis perfusion of 5-HT into hypoglossal motor nucleus differentially modulates genioglossus activity across natural sleep-wake states in rats. J Physiol. 2001;532:467–481. doi: 10.1111/j.1469-7793.2001.0467f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia M, Fuxe K, Hokfelt T, Johansson O, Lang R, Ganten D, et al. Distribution of neuropeptide immunoreactive nerve terminals within the subnuclei of the nucleus of the tractus solitarius of the rat. J Comp Neurol. 1984;222:409–444. doi: 10.1002/cne.902220308. [DOI] [PubMed] [Google Scholar]
- Kia HK, Brisorgueil MJ, Hamon M, Calas A, Verge D. Ultrastructural localization of 5-hydroxytryptamine1A receptors in the rat brain. J Neurosci Res. 1996;46:697–708. doi: 10.1002/(SICI)1097-4547(19961215)46:6<697::AID-JNR7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Filiano JJ, White WF. Medullary serotonergic network deficiency in the sudden infant death syndrome: review of a 15-year study of a single dataset. J Neuropathol Exp Neurol. 2001;60:228–247. doi: 10.1093/jnen/60.3.228. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Pernar L, Valentino RJ, Beck SG. Distinguishing characteristics of serotonin and non-serotonin-containing cells in the dorsal raphe nucleus: electrophysiological and immunohistochemical studies. Neuroscience. 2003;116:669–683. doi: 10.1016/s0306-4522(02)00584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalley PM. Serotoninergic and non-serotoninergic responses of phrenic motoneurones to raphe stimulation in the cat. J Physiol. 1986;380:373–385. doi: 10.1113/jphysiol.1986.sp016291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Randall M, Nattie EE. CO2 microdialysis in retrotrapezoid nucleus of the rat increases breathing in wakefulness but not in sleep. J Appl Physiol. 1999;87:910–919. doi: 10.1152/jappl.1999.87.3.910. [DOI] [PubMed] [Google Scholar]
- Maskrey M. Body temperature effects on hypoxic and hypercapnic responses in awake rats. Am J Physiol. 1990;259:R492–R498. doi: 10.1152/ajpregu.1990.259.3.R492. [DOI] [PubMed] [Google Scholar]
- Messier ML, Li A, Nattie EE. Inhibition of medullary raphe serotonergic neurons has age-dependent effects on the CO2 response in newborn piglets. J Appl Physiol. 2004;96:1909–1919. doi: 10.1152/japplphysiol.00805.2003. [DOI] [PubMed] [Google Scholar]
- Monti JM, Monti D. Role of dorsal raphe nucleus serotonin 5-HT1A receptor in the regulation of REM sleep. Life Sci. 2000;66:1999–2012. doi: 10.1016/s0024-3205(99)00649-9. [DOI] [PubMed] [Google Scholar]
- Morrison SF. Central pathways controlling brown adipose tissue thermogenesis. News Physiol Sci. 2004;19:67–74. doi: 10.1152/nips.01502.2003. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, et al. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Nattie E. Multiple sites for central chemoreception: their roles in response sensitivity and in sleep and wakefulness. Respir Physiol. 2000;122:223–235. doi: 10.1016/s0034-5687(00)00161-4. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. CO2 dialysis in the medullary raphe of the rat increases ventilation in sleep. J Appl Physiol. 2001;90:1247–1257. doi: 10.1152/jappl.2001.90.4.1247. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. CO2 dialysis in nucleus tractus solitarius region of rat increases ventilation in sleep and wakefulness. J Appl Physiol. 2002a;92:2119–2130. doi: 10.1152/japplphysiol.01128.2001. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. Substance P-saporin lesion of neurons with NK1 receptors in one chemoreceptor site in rats decreases ventilation and chemosensitivity. J Physiol. 2002b;544:603–616. doi: 10.1113/jphysiol.2002.020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie EE, Li A, Richerson G, Lappi DA. Medullary serotonergic neurones and adjacent neurones that express neurokinin-1 receptors are both involved in chemoreception in vivo. J Physiol. 2004;556:235–253. doi: 10.1113/jphysiol.2003.059766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niblock MM, Kinney HC, Luce CJ, Belliveau RA, Filiano JJ. The development of the medullary serotonergic system in the piglet. Auton Neurosci. 2004;110:65–80. doi: 10.1016/j.autneu.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Nitz DA, McNaughton BL. Hippocampal EEG and unit activity responses to modulation of serotonergic median raphe neurons in the freely behaving rat. Learn Mem. 1999;6:153–167. [PMC free article] [PubMed] [Google Scholar]
- Ootsuka Y, Blessing WW, McAllen RM. Inhibition of rostral medullary raphe neurons prevents cold-induced activity in sympathetic nerves to rat tail and rabbit ear arteries. Neurosci Lett. 2004;357:58–62. doi: 10.1016/j.neulet.2003.11.067. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Mezey E, Eskay RL, Brownstein MJ. Innervation of the nucleus of the solitary tract and the dorsal vagal nucleus by thyrotropin-releasing hormone-containing raphe neurons. Brain Res. 1986;373:246–251. doi: 10.1016/0006-8993(86)90338-0. [DOI] [PubMed] [Google Scholar]
- Pappenheimer JR. Sleep and respiration of rats during hypoxia. J Physiol. 1977;266:191–207. doi: 10.1113/jphysiol.1977.sp011763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Sterotaxic Coordinates. New York: Academic Press; 1982. [Google Scholar]
- Perez H, Ruiz S. Medullary responses to chemoreceptor activation are inhibited by locus coeruleus and nucleus raphe magnus. Neuroreport. 1995;6:1373–1376. doi: 10.1097/00001756-199507100-00003. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Wang W, Hodges MR, Dohle CI, Diez-Sampedro A. Homing in on the specific phenotype (s) of central respiratory chemoreceptors. Exp Physiol. 2005;90:259–266. doi: 10.1113/expphysiol.2005.029843. [DOI] [PubMed] [Google Scholar]
- Richter DW, Schmidt-Garcon P, Pierrefiche O, Bischoff AM, Lalley PM. Neurotransmitters and neuromodulators controlling the hypoxic respiratory response in anaesthetized cats. J Physiol. 1999;514:567–578. doi: 10.1111/j.1469-7793.1999.567ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidek WR, Hoshino K, Schmidek M, Timo-Iaria C. Influence of environmental temperature on the sleep-wakefulness cycle in the rat. Physiol Behav. 1972;8:363–371. doi: 10.1016/0031-9384(72)90384-8. [DOI] [PubMed] [Google Scholar]
- Semba K. Aminergic and cholinergic afferents to REM sleep induction regions of the pontine reticular formation in the rat. J Comp Neurol. 1993;330:543–556. doi: 10.1002/cne.903300410. [DOI] [PubMed] [Google Scholar]
- Smith JE, Jansen AS, Gilbey MP, Loewy AD. CNS cell groups projecting to sympathetic outflow of tail artery: neural circuits involved in heat loss in the rat. Brain Res. 1998;786:153–164. doi: 10.1016/s0006-8993(97)01437-6. [DOI] [PubMed] [Google Scholar]
- Taylor NC, Li A, Green A, Kinney HC, Nattie EE. Chronic fluoxetine microdialysis into the medullary raphe nuclei of the rat, but not systemic administration, increases the ventilatory response to CO2. J Appl Physiol. 2004;97:1763–1773. doi: 10.1152/japplphysiol.00496.2004. [DOI] [PubMed] [Google Scholar]
- Thor KB, Blitz-Siebert A, Helke CJ. Discrete localization of high-density 5-HT1A binding sites in the midline raphe and parapyramidal region of the ventral medulla oblongata of the rat. Neurosci Lett. 1990;108:249–254. doi: 10.1016/0304-3940(90)90649-t. [DOI] [PubMed] [Google Scholar]
- Thor KB, Helke CJ. Serotonin- and substance P-containing projections to the nucleus tractus solitarii of the rat. J Comp Neurol. 1987;265:275–293. doi: 10.1002/cne.902650210. [DOI] [PubMed] [Google Scholar]
- Vanhoenacker P, Haegeman G, Leysen JE. 5-HT7 receptors: current knowledge and future prospects. Trends Pharmacol Sci. 2000;21:70–77. doi: 10.1016/s0165-6147(99)01432-7. [DOI] [PubMed] [Google Scholar]
- Voss MD, De Castro D, Lipski J, Pilowsky PM, Jiang C. Serotonin immunoreactive boutons form close appositions with respiratory neurons of the dorsal respiratory group in the cat. J Comp Neurol. 1990;295:208–218. doi: 10.1002/cne.902950205. [DOI] [PubMed] [Google Scholar]
- Wang W, Pizzonia JH, Richerson GB. Chemosensitivity of rat medullary raphe neurones in primary tissue culture. J Physiol. 1998;511:433–450. doi: 10.1111/j.1469-7793.1998.433bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Tiwari JK, Bradley SR, Zaykin RV, Richerson GB. Acidosis-stimulated neurons of the medullary raphe are serotonergic. J Neurophysiol. 2001;85:2224–2235. doi: 10.1152/jn.2001.85.5.2224. [DOI] [PubMed] [Google Scholar]
- Young AA, Dawson NJ. Evidence for on-off control of heat dissipation from the tail of the rat. Can J Physiol Pharmacol. 1982;60:392–398. doi: 10.1139/y82-057. [DOI] [PubMed] [Google Scholar]