Abstract
In urethane–chloralose anaesthetized, neuromuscularly blocked, artificially ventilated rats, we demonstrated that activation of carotid chemoreceptors inhibits the elevated levels of brown adipose tissue (BAT) sympathetic nerve activity (SNA) evoked by hypothermia, by microinjection of prostaglandin E2 into the medial preoptic area or by disinhibition of neurones in the raphe pallidus area (RPa). Peripheral chemoreceptor stimulation with systemic administration of NaCN (50 μg in 0.1 ml) or with hypoxic ventilation (8% O2–92% N2, 30 s) completely inhibited BAT SNA. Arterial chemoreceptor-evoked inhibition of BAT SNA was eliminated by prior bilateral transections of the carotid sinus nerves or by prior inhibition of neurones within the commissural nucleus tractus solitarii (commNTS) with glycine (40 nmol/80 nl) or with the GABAA receptor agonist muscimol (160 pmol/80 nl; 77 ± 10% attenuation), or by prior blockade of ionotropic excitatory amino acid receptors in the commNTS with kynurenate (8 nmol/80 nl; 82 ± 10% attenuation). Furthermore, activation of commNTS neurones following local microinjection of bicuculline (30 pmol/60 nl) completely inhibited the elevated level of BAT SNA resulting from disinhibition of neurones in the RPa. These results demonstrate that hypoxic stimulation of arterial chemoreceptor afferents leads to an inhibition of BAT SNA and BAT thermogenesis through an EAA-mediated activation of second-order, arterial chemoreceptor neurones in the commNTS. Peripheral chemoreceptor-evoked inhibition of BAT SNA could directly contribute to (or be permissive for) the hypoxia-evoked reductions in body temperature and oxygen consumption that serve as an adaptive response to decreased oxygen availability.
Hypoxia evokes a centrally mediated decrease in mammalian body temperature which reduces oxygen consumption and increases the affinity of haemoglobin for oxygen, both of which contribute to the compensation for decreased oxygen availability. This adaptive response, often referred to as anapyrexia (although see Romanovsky, 2004), improves survival rates to severe hypoxia (Wood & Stabenau, 1998) and helps to prevent neuronal injury under conditions of oxygen deprivation (Yager et al. 1993; Coimbra & Wieloch, 1994; Edwards et al. 1995).
The mechanisms underlying the thermoregulatory response to hypoxia have been extensively studied (Janig et al. 1983; Gautier & Bonora, 1992; Gautier et al. 1993; Matsuoka et al. 1994; Steiner & Branco, 2002; Gargaglioni et al. 2003) and these studies suggest that the hypoxia-evoked decrease in body temperature could be mediated by inhibition of cutaneous vasoconstriction and/or inhibition of non-shivering thermogenesis. Brown adipose tissue (BAT) is the principal organ for non-shivering thermogenesis in small mammals and infant primates. Metabolism and heat production in BAT are primarily controlled by the sympathetic outflow to BAT (Cannon & Nedergaard, 2004; Morrison, 2004) and during non-shivering thermogenesis, more than half of all oxygen taken up is consumed by brown adipose (Cannon & Nedergaard, 2004). While direct evidence is lacking for active brown adipose tissue thermogenesis under conditions of normothermia, it is well established that a decrease in hypothalamic temperature (which would likely accompany hypoxia-evoked hypothermia) drives thermogenesis in BAT (Imai-Matsumura & Nakayama, 1987). Thus, the present study was undertaken to test the hypothesis that hypoxia inhibits sympathetically mediated thermogenesis in BAT.
Hypoxia activates arterial chemoreceptors located in the carotid body whose afferent fibres in the carotid sinus nerve excite neurones in the caudal portion of the medial and the commissural subnuclei of the nucleus tractus solitarii (commNTS) (Finley & Katz, 1992). Activation of the arterial chemoreceptor afferents during brief hypoxic episodes increases vasoconstrictor sympathetic outflow and arterial pressure (Guyenet, 2000) and may contribute to the hypertension seen in sleep apnoea (Cutler et al. 2004). Arterial chemoreceptor afferent stimulation also elicits tachycardia in awake animals and bradycardia in anaesthetized, ventilated animals and increases the rate and depth of respiration as monitored by phrenic nerve activity (PHR). In addition to the arterial chemoreceptor reflex, hypoxia may directly excite central neurones controlling sympathetic outflow (Sun & Reis, 1995). The present study specifically tested the effect of arterial chemoreceptor activation on BAT SNA and BAT thermogenesis and the role played by neurones in the commNTS in mediating these effects.
Experimental procedures
All procedures conform to the regulations detailed in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of the Oregon Health and Science University. Male Sprague-Dawley rats (Charles River, Indianapolis, IN, USA, n = 33) weighing 300–450 g were given ad libitum access to standard rat chow and water in a colony room maintained at 22–23°C and kept on a 12: 12-h light–dark cycle. Rats were anaesthetized with isoflurane (2–3% in oxygen) and implanted with femoral arterial and venous catheters and transitioned to intravenous (i.v.) urethane and chloralose anaesthesia (750 mg kg−1 and 60 mg kg−1, respectively) over a 10-min period. Arterial pressure was recorded from the arterial catheter attached to a pressure transducer and heart rate (HR) was derived from the arterial pressure signal. The trachea was cannulated, and the animals were neuromuscularly blocked with d-tubocurarine (0.5 mg i.v., supplemented with 0.1 mg every hour) and ventilated (tidal volume: 1 ml per 100 g body weight, 60 cycles min−1) with 100% oxygen. Adequacy of anaesthesia was assessed prior to neuromuscular blockade by absence of withdrawal reflex, and pressor response to foot pinch as well as absence of eye blink response to gentle probing of the eyeball. In addition, every hour prior to supplementation of neuromuscular blockade, adequacy of anaesthesia was re-assessed and anaesthetic was supplemented (5–10% of initial dose) as necessary. Mixed-expired CO2 was monitored using a capnometer (Traverse medical monitors, model 2200). In rats in which PHR was recorded, bilateral cervical vagus nerve transection was performed to eliminate possible PHR entrainment to the ventilator-driven lung inflation. Colonic (core) temperature was monitored using a copper-constantan thermocouple inserted 6 cm into the rectum and was maintained between 36.5 and 37.5°C with a heating plate and a heat lamp. Animals were placed in a stereotaxic instrument and the coordinates for injections into the RPa (with the incisor bar positioned −4 mm below the interaural line) were 3.0 mm caudal to lambda, 0.0 mm lateral to lambda and 9.6–9.8 mm ventral to dura, or with the incisor bar positioned −11 mm below the interaural line: 2.9 mm rostral to the caudal tip of area postrema, on the midline, and 3.0–3.2 mm ventral to the dorsal surface at the caudal tip of area postrema. The coordinates for the commNTS were (with the incisor bar at −11 mm): 0.4 mm caudal to the caudal tip of the area postrema, on the midline and 0.4 mm ventral to the dorsal surface of the brainstem. The coordinates for microinjections into the medial preoptic area (MPA) were (with the incisor bar at −4 mm): 0.8 mm lateral to bregma, and 8.0 mm ventral to dura at the rostrocaudal level of bregma. Glass micropipettes (outer tip diameter, 20–30 μm) were used for all microinjections which were given over a 10–20 s period using a pressure injection system. All drugs were obtained from Sigma (St Louis, MO, USA) except isofluorane, which was obtained from Abbott Laboratories (North Chicago, IL, USA).
The BAT temperature was monitored with a copper-constantan thermocouple (Physitemp, Clifton, NJ, USA) inserted beneath the intact, left interscapular fat pad. Postganglionic BAT sympathetic nerve activity (SNA) was recorded under mineral oil with a bipolar hook electrode from the central cut end of a nerve bundle isolated from the ventral surface of the right interscapular fat pad after dividing it along the midline and reflecting it laterally. Nerve activity was filtered (1–300 Hz) and amplified (20 000–50 000×) with a Cyberamp 380 (Axon Instruments, Union City, CA, USA). Spike2 software (CED) was used to obtain a continuous measure (4-s bins) of BAT SNA amplitude by calculating the root mean square (r.m.s.) amplitude of the BAT SNA (square root of the total power in the 0–20 Hz band) from the autospectra of sequential 4-s segments of BAT SNA. Control values of BAT SNA were the averages of the BAT SNA amplitudes during the 32-s periods immediately prior to treatments. Response values of BAT SNA were the averages of the BAT SNA values during the 32-s periods of maximal change in BAT SNA evoked by a treatment. Noise levels of BAT SNA (judged from signals recorded following ganglionic blockade in a few initial experiments) were consistently less than 50% of the low levels recorded under our control conditions and consequently were not included in determinations of BAT SNA responses.
In some animals, PHR was recorded (1–3000 Hz, 10 000–20 000×) from the central cut end of the left phrenic nerve with a bipolar hook electrode under mineral oil and integrated PHR was obtained from a real time moving averager (time constant 100 ms; CWE Inc, Ardmore, PA, USA). All physiological variables were digitized (Micro 1401 MKII, CED) and recorded onto a computer hard drive.
At the conclusion of each experiment, the MPA, RPa and commNTS microinjection sites were marked by retracting the microinjection pipettes vertically, filling them with 2% fast green dye, repositioning them to the appropriate dorso-ventral coordinates of the microinjection sites and electrophoretically (15 μA anodal direct current for 10 min) depositing the dye. Rats were perfused transcardially with a 10% paraformaldehyde solution. The brains were removed, postfixed for 12–24 h and sectioned on a vibratome (60 μm sections). Brain sections containing fast green dye deposits were mounted on slides and digital photomicrographs were downloaded to a PC and assembled using Adobe Photoshop software. The locations of microinjection sites were plotted on atlas drawings (Paxinos & Watson, 1986).
All statistics were performed using Systat software (version 10, SPSS Inc.). Data are expressed as means ± s.e.m. Statistical significance was assessed using an ANOVA with repeated measures. Following a significant F-value, post hoc testing was performed using layered Bonferroni analysis. The significance level was P < 0.05.
Experimental protocols
In one group of rats (n = 4) the skin of the abdomen and hindquarters was shaved and the rat was wrapped in a water-perfused blanket. In order to activate BAT thermogenesis, the skin temperature (as monitored by a thermocouple taped to the abdomen of the rat) and colonic temperature were decreased by changing the temperature of the water used to perfuse the blanket. Five to 15 min after the start of cooling, rats were ventilated for 30 s with a hypoxic (8% O2–92% N2) gas mixture.
In another group of rats (n = 4) prostaglandin E2 (PGE2; 170 pmol/60 nl) was injected into the MPA in order to activate BAT thermogenesis (Madden & Morrison, 2004). Five to 10 min after the microinjection of PGE2, rats were ventilated for 30 s with a hypoxic (8% O2–92% N2) gas mixture.
A third group of rats (n = 8) received a microinjection of bicuculline (30 pmol/60 nl) into the RPa and 5–10 min later, the transient responses to activation of peripheral chemoreceptor afferents (Biscoe & Duchen, 1989; Vidruk et al. 2001) with i.v. administration of NaCN (50 μg in 0.1 ml) were recorded. At least 1 h later, half of the rats (n = 4) received an injection of the GABAA agonist muscimol (160 pmol/80 nl) into the commNTS followed 5–10 min later by a second microinjection of bicuculline (30 pmol/60 nl) into the RPa and a subsequent i.v. bolus of NaCN (50 μg in 0.1 ml); the remaining four rats received an injection of saline (60 nl) into the commNTS, followed 5–10 min later by a second microinjection of bicuculline (30 pmol/60 nl) into the RPa and a subsequent i.v. bolus of NaCN (50 μg in 0.1 ml).
In a forth group of rats (n = 10), following bilateral section of the cervical vagus nerves, bicuculline (30 pmol/60 nl) was microinjected into the RPa, followed 5–10 min later by an episode of hypoxic ventilation (8% O2–92% N2; 30 s). Following recovery of BAT SNA to elevated levels, a subsequent microinjection of one of the following was made into the commNTS: saline vehicle (80 nl; n = 5); glycine (40 nmol/80 nl; n = 4); the GABAA agonist muscimol (160 pmol/80 nl; n = 4); or the non-selective ionotropic excitatory amino acid (EAA) receptor antagonist, kynurenic acid (8 nmol/80 nl; n = 4). Within 5 min of a microinjection of one of these agents into the commNTS, rats underwent a second, 30 s episode of hypoxic ventilation with 8% O2. Some rats underwent multiple trials with different drug administrations into the commNTS; in these cases, sufficient time (at least 30 min following glycine or saline, and at least one hour following kynurenic acid) was allowed for recovery from the first drug treatment before a subsequent trial.
In a fifth group of rats (n = 3), at the time of the tracheostomy the carotid sinus nerves were located, isolated and transected bilaterally. Rats with arterial chemoreceptor deafferentations received (a) a microinjection of bicuculline (30 pmol/60 nl) into the RPa, (b) a systemic injection of NaCN (50 μg in 0.1 ml, i.v.) 5–10 min following the bicuculline microinjection into RPa, and (c) a subsequent episode of hypoxic ventilation (8% O2–92% N2; 30 s) following recovery from the NaCN injection. Note that control rats in the second and third groups above underwent similar tracheostomy surgeries but the carotid sinus nerves were not isolated or cut therefore data from these control rats serve as sham surgery comparisons for this experiment.
In a sixth group of rats (n = 4), baseline levels of BAT SNA were increased with a microinjection of bicuculline (30 pmol/60 nl) into the RPa. During the period of sustained elevation in BAT SNA, a microinjection of bicuculline (30 pmol/60 nl) was made into the commNTS.
Results
Effect of hypoxia on thermogenic and cardiovascular responses evoked by hypothermia
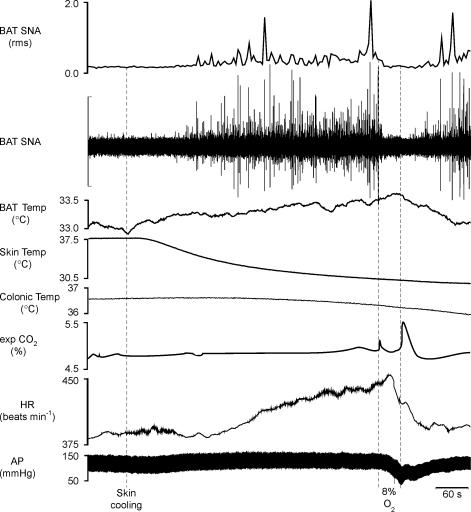
To test the hypothesis that hypothermia-evoked BAT thermogenesis is inhibited by hypoxia, we determined the effect of a 30 s ventilation with a hypoxic gas mixture (8% O2–92% N2) on the elevated level of BAT SNA elicited during skin/whole body cooling. Figure 1 illustrates that in untreated, anaesthetized rats whose core temperature was maintained between 36.5 and 37.5°C, BAT SNA was low, with only a few, small-amplitude bursts occurring every few minutes. Skin/whole body cooling increased BAT SNA and MAP (Fig. 1, Table 1). Hypoxic ventilation for 30 s during the peak of the BAT thermogenic stimulation elicited by hypothermia completely reversed the increase in BAT SNA (Fig. 1; Table 1). Hypoxic ventilation during whole body cooling also decreased MAP (Fig. 1).
Figure 1. Skin cooling and the resulting hypothermia evokes thermogenic and cardiovascular responses that are reversed by ventilation with a hypoxic (8% O2) gas mixture.
Skin/whole body cooling increased brown adipose tissue (BAT) sympathetic nerve activity (SNA; peak: +342% of control), mean arterial pressure (AP, peak: +11 mmHg) and heart rate (HR, peak: +61 beats min−1). Ventilation for 30 s with 8% O2 completely inhibited BAT SNA (nadir: 97% of precooling control), and markedly decreased mean arterial pressure (−49 mmHg) and heart rate (−56 beats min−1). Vertical scale bar in BAT SNA tracing represents 40 μV.
Table 1.
Thermogenic, metabolic and cardiovascular variables in the control condition, during skin/whole body cooling, and during ventilation with 8% O2
| Control | Hypothermia | 8% O2 | |
|---|---|---|---|
| BAT SNA (% control) | 100 | 659 ± 184* | 95 ± 14† |
| BAT temp. (°C) | 33.9 ± 0.4 | 33.9 ± 0.3 | 33.6 ± 0.4 |
| Skin temp. (°C) | 37.5 ± 0.2 | 32.4 ± 0.7* | 32.3 ± 0.7*† |
| Colonic temp. (°C) | 37.2 ± 0.4 | 36.3 ± 0.1* | 36.1 ± 0.1*† |
| Expired CO2 (%) | 4.0 ± 0.4 | 4.1 ± 0.5 | 3.6 ± 0.5 |
| HR (beats min−1) | 413 ± 8 | 444 ± 5 | 378 ± 25 |
| MAP (mmHg) | 96 ± 9 | 106 ± 7* | 48 ± 11† |
Values are means ± s.e.m. (n = 4) in the control condition, at the peak response within 15 min of the start of skin/whole body cooling, and at the minimum level during ventilation with 8% O2.
P < 0.05, compared to the control condition.
P < 0.05, compared to the hypothermia-evoked level.
Effect of hypoxia on thermogenic and cardiovascular responses evoked by prostaglandin in the MPA
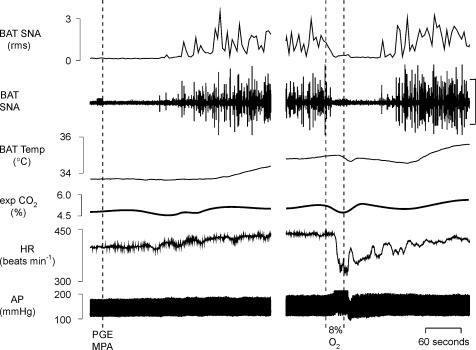
To test the hypothesis that hypoxia reduces BAT thermogenesis through a centrally mediated inhibition of BAT SNA, we determined the effect of a 30 s ventilation with a hypoxic gas mixture (8% O2–92% N2) on the elevated level of BAT thermogenesis and BAT SNA elicited during the febrile response to microinjection of prostaglandin E2 (PGE2) into the MPA (Madden & Morrison, 2003). Figure 2 illustrates that in untreated, anaesthetized rats whose core temperature was maintained between 36.5 and 37.5°C, BAT SNA was low, with only a few, small-amplitude bursts occurring every few minutes. Microinjection of PGE2 into the MPA increased BAT SNA, BAT temperature, expired CO2 and HR (Fig. 2, Table 2). Hypoxic ventilation for 30 s during the peak of the BAT thermogenic stimulation elicited by PGE2 completely, but temporarily, reversed the increase in BAT SNA, leading to a pause in the rise in BAT temperature (Fig. 2; Table 2). Hypoxic ventilation also evoked a short-lived bradycardia and a stimulus-bound pressor response (Fig. 2).
Figure 2. Microinjection of prostaglandin E2 (PGE2) into the medial preoptic area (MPA) evokes thermogenic and cardiovascular responses that are reversed by ventilation with a hypoxic (8% O2) gas mixture.
Microinjection of PGE2 into the MPA increased brown adipose tissue (BAT) sympathetic nerve activity (SNA; peak: +698% of control), BAT temperature (peak: +1.1°C), expired CO2 (peak: +0.5%), and heart rate (HR, peak: +37 beats min−1). Ventilation for 30 s with 8% O2 completely inhibited BAT SNA (nadir: 109% of prePGE2 control), markedly decreased heart rate (−104 beats min−1) and increased mean arterial pressure (peak: +14 mmHg). Vertical scale bar in BAT SNA tracing represents 80 μV.
Table 2.
Thermogenic, metabolic and cardiovascular variables in the control condition, following microinjection of PGE2 into the MPA, and during ventilation with 8% O2
| Control | PGE2 MPA | 8% O2 | |
|---|---|---|---|
| BAT SNA (% control) | 100 | 1282 ± 322* | 115 ± 5† |
| BAT temp. (°C) | 33.3 ± 0.6 | 34.9 ± 0.3* | 34.7 ± 0.4* |
| Expired CO2 (%) | 4.5 ± 0.3 | 5.0 ± 0.3* | 4.7 ± 0.3 |
| HR (beats min−1) | 359 ± 26 | 412 ± 27* | 389 ± 32 |
| MAP (mmHg) | 102 ± 11 | 113 ± 9 | 104 ± 18 |
Values are means ± s.e.m. (n = 4) in the control condition, at the peak response within 10 min of the microinjection of PGE2 into the MPA, and at the minimum level during ventilation with 8% O2.
P < 0.05, compared to the control condition.
P < 0.05, compared to the PGE2 evoked level.
Effect of intravenous NaCN on thermogenic and cardiovascular responses before and after muscimol-evoked inhibition of the commNTS
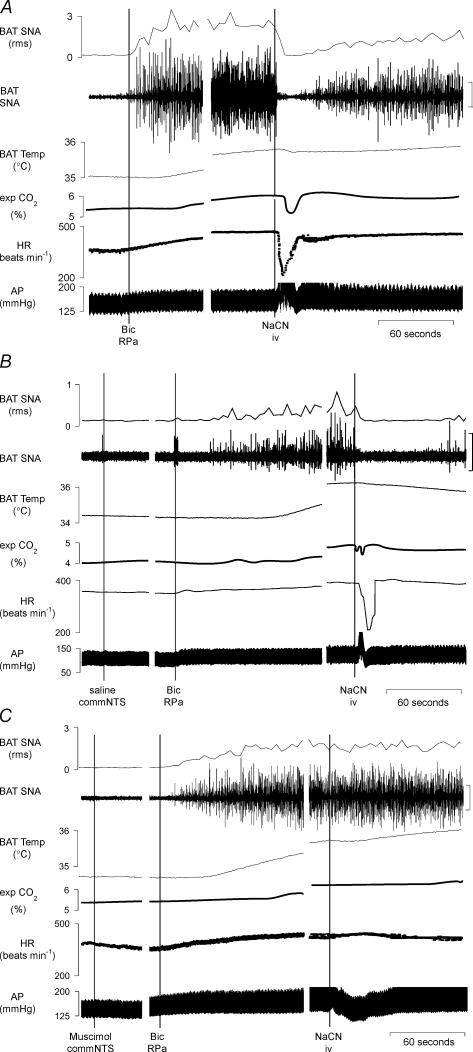
To test the hypothesis that arterial chemoreceptor reflex activation inhibits BAT SNA, a bolus of NaCN (50 μg in 0.1 ml, i.v.) was administered to rats whose BAT SNA had been artificially increased from low resting levels by disinhibiting neurones in RPa (Morrison et al. 1999). As illustrated in Fig. 3A, bicuculline microinjection into RPa increased BAT SNA from the low levels exhibiting only a few, small-amplitude bursts every few minutes in rats whose core temperature was maintained between 36.5 and 37.5°C, to elevated levels characterized by nearly continuous bursting occurring at 4–10 Hz seen in situations of near-maximal BAT activation. Microinjection of bicuculline into the RPa increased BAT SNA (+1547 ± 298% of control, n = 8, Fig. 3), BAT temperature (+1.7 ± 0.2°C), expired CO2 (+0.7 ± 0.1%) and HR (+60 ± 15 beats min−1). Subsequent i.v. injection of NaCN produced a brief (lasting for approximately 15–30 s), and nearly complete (96 ± 2%) inhibition of the bicuculline-evoked increase in BAT SNA (Fig. 3A), leading to a pause in the rise of BAT temperature and contributing to a brief fall in the level of expired CO2. Stimulation of the arterial chemoreceptor reflex with NaCN also decreased HR (−92 ± 19 beats min−1) and increased AP, paralleling the cardiovascular responses previously seen with bolus administration of cyanide in vagus-intact, awake rats (Franchini & Krieger, 1992; Haibara et al. 1995; Callera et al. 1999; Haibara et al. 1999).
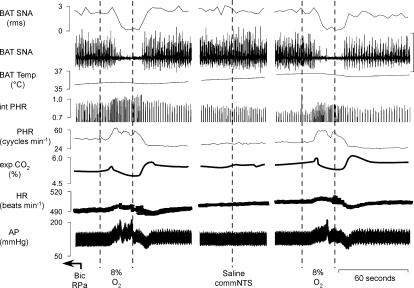
Figure 3. The inhibition of BAT SNA and the bradycardia evoked by intravenous administration of NaCN is prevented by microinjection of the GABAA receptor agonist muscimol into the commissural nucleus tractus solitarii (commNTS).
A, microinjection of bicuculline (Bic) into the raphe pallidus area (RPa) increased brown adipose tissue sympathetic nerve activity (BAT SNA) by +2157% of control, BAT temperature by +0.8°C, expired CO2 by +0.7%, and heart rate (HR) by +109 beats min−1. Intravenous (i.v.) injection of NaCN reversed the increase in BAT SNA (nadir: 100% of control prior to bicuculline in RPa), briefly halted the rise in BAT temperature, decreased expired CO2 (minimum:0.6%), decreased the HR (minimum: −180 beats min−1) and increased mean arterial pressure (AP; peak: +15 mmHg). Panels separated by 1.5 min.
B, microinjections of saline into the commNTS did not alter the Bic- or NaCN-evoked responses compared to the trials in which the commNTS was not injected. Microinjection of Bic into the RPa increased BAT SNA by +537% of control, BAT temperature by +2.2°C, expired CO2 by +0.8%, and HR by +23 beats min−1. Intravenous (i.v.) injection of NaCN reversed the increase in BAT SNA (nadir: 100% of control prior to bicuculline in RPa), halted the rise in BAT temperature, decreased expired CO2 (minimum:0.2%), decreased the HR (minimum: −196 beats min−1) and increased mean AP (peak: +30 mmHg). Panels separated by 4 and 2 min. C, microinjection of muscimol into the commNTS markedly attenuated the NaCN-evoked inhibition of BAT SNA (BAT SNA: 1665% of control before NaCN versus 1470% of control after NaCN), prevented the decrease in expired CO2 (exp. CO2: 6.1% before NaCN versus 6.1% after NaCN), prevented the bradycardia (HR: 436 beats min−1 before NaCN versus 451 beats min−1 after NaCN) and converted the pressor response (panel A) into a depressor response (−26 mmHg). Panels separated by 4 and 1.5 min. Vertical scale bars in BAT SNA tracing represent 80 μV.
To determine if neurones in the commNTS, the site of termination of arterial chemoreceptor afferents, are involved in mediating the inhibition of BAT SNA evoked by arterial chemoreceptor reflex activation, we compared the ability of NaCN to inhibit BAT SNA after microinjection of saline vehicle or muscimol into the commNTS in separate experiments. In each animal, microinjection into the NTS was preceded by an NTS-untreated trial in which NaCN was injected i.v. following microinjection of bicuculline into RPa (Fig. 3A). Neither the BAT sympathoexcitatory responses to microinjection of bicuculline into RPa nor the magnitude of the sympathoinhibitory responses to the subsequent i.v. injection of NaCN differed between the NTS-untreated trials of the groups receiving a microinjection of saline or muscimol into the commNTS. Microinjection of either saline or muscimol into the commNTS had no effect on the amplitudes of the evoked increases in BAT SNA or BAT temperature evoked by the subsequent microinjection of bicuculline into the RPa (Fig. 3B and C, Table 3). As illustrated in Fig. 3B, microinjection of saline into the commNTS did not alter the NaCN-evoked responses compared to the NTS-untreated trial (Fig. 3A). In contrast, as illustrated in Fig. 3C, microinjection of muscimol into the commNTS eliminated the NaCN-evoked inhibition of BAT SNA (muscimol in commNTS: 26% inhibition versus saline in commNTS: 98% inhibition; Table 3), and prevented the NaCN-evoked decrease in HR and increase in AP (Fig. 3C, Table 3).
Table 3.
Effects on cardiovascular, metabolic and thermogenic variables of bicuculline (Bic) injected into the raphe pallidus area (RPa) and of subsequent intravenous injection of NaCN following microinjection of either saline or muscimol into the commissural nucleus tractus solitarii (commNTS)
| Saline commNTS (n = 4) | Muscimol commNTS (n = 4) | ||||
|---|---|---|---|---|---|
| Control(n=8) | Bic RPa | NaCN | Bic RPa | NaCN | |
| BAT SNA (% control) | 100 | 1279 ± 329* | 148 ± 30† | 1935 ± 405* | 1599 ± 396*†‡ |
| BAT Temp. (°C) | 33.8 ± 0.3 | 35.3 ± 0.3* | 34.9 ± 0.2 | 35.7 ± 0.3* | 35.7 ± 0.3 |
| Expired CO2 (%) | 4.7 ± 0.3 | 5.6 ± 0.6* | 5.4 ± 0.6† | 5.4 ± 0.3* | 5.2 ± 0.3† |
| HR (beats min−1) | 364 ± 11 | 426 ± 38* | 351 ± 20† | 436 ± 20* | 444 ± 18*‡ |
| MAP (mmHg) | 101 ± 7 | 96 ± 9 | 119 ± 10† | 152 ± 4*‡ | 134 ± 4† |
Values are means ± s.e.m. for physiological variables in the control condition, during the peak responses to microinjection of bicuculline into the RPa (Bic RPa) subsequent to microinjection of saline or muscimol into the commNTS and at the minimum levels within 5 min of intravenous injections of NaCN delivered at the peak of the responses evoked by Bic in RPa.
P < 0.05, increase compared to the control condition.
P < 0.05, compared to immediately preceding Bic in RPa response.
P < 0.05, compared to the corresponding saline in commNTS response.
Effect of modulating commNTS neuronal excitability on the thermogenic, cardiovascular and respiratory responses to hypoxia
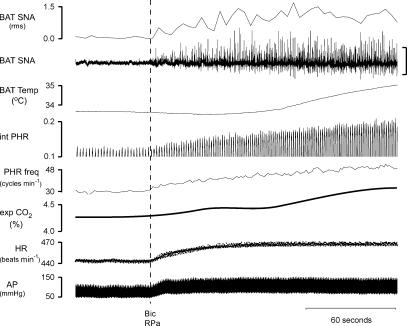
In the three data sets to be described, the amplitude of BAT SNA was low in rats under control conditions with core temperature maintained at 37.0 ± 0.5°C. Microinjection of bicuculline into the RPa produced significant (P < 0.05) increases in BAT SNA (+639 ± 109% of control, n = 10), BAT temperature (+0.6 ± 0.1°C from 34.2 ± 0.2°C), expired CO2 (+0.5 ± 0.1% from 5.0 ± 0.1%), HR (+28 ± 6 beats min−1 from 457 ± 8 beats min−1), MAP (+9 ± 4 mmHg from 108 ± 8 mmHg) and PHR amplitude (+14 ± 5% of control) and frequency (+9 ± 2 cycles min−1 from 39 ± 2 cycles min−1) (Fig. 4). The thermogenic and cardiovascular responses to disinhibition of RPa neurones in these vagotomized rats paralleled those previously described in vagus-intact rats (Morrison et al. 1999, 2000; Madden & Morrison, 2003). The increase in PHR amplitude and frequency evoked by microinjection of bicuculline into RPa began within seconds of the microinjection and reached peak levels within several minutes.
Figure 4. Microinjection of bicuculline (Bic) into the raphe pallidus (RPa) evokes thermogenic, respiratory and cardiovascular responses.
Microinjection of Bic into the RPa increased brown adipose tissue (BAT) sympathetic nerve activity (SNA; peak: +514% of control), BAT temperature (peak: +1.2°C), phrenic nerve activity (PHR) amplitude (peak: +38% of control) and frequency (peak: +20 cycles min−1), expired CO2 (peak: +0.4%), heart rate (HR, peak: +25 beats min−1), and mean arterial pressure (AP, peak: +22 mmHg). Vertical scale bar in BAT SNA tracing represents 80 μV.
Brief hypoxic ventilation (8% O2, 30 s) in these vagotomized rats produced a nearly complete inhibition of the increased level of BAT SNA evoked by microinjection of bicuculline into RPa (Figs 5, 6 and 7; Tables 4, 5 and 6), leading to a pause in the rise of BAT temperature and a brief fall in expired CO2. In addition, hypoxic ventilation increased MAP, HR and PHR amplitude and frequency (Figs 5, 6 and 7; Tables 4, 5 and 6). These responses paralleled those elicited by brief hypoxia during the BAT sympathoexcitatory response to microinjection of PGE2 into the MPA (Fig. 2).
Figure 5. Hypoxic ventilation-evoked thermogenic, respiratory and cardiovascular responses are not affected by microinjection of saline vehicle into the commNTS.
Bicuculline (Bic) was microinjected into the raphe pallidus (RPa) 3 min prior to the data in the first panel. Ventilation for 30 s with 8% O2 completely inhibited BAT SNA, increased mean arterial pressure (AP; peak: +20 mmHg) and increased PHR amplitude (peak: +12% of control above the Bic-evoked level) and frequency (peak: +11 cycles min−1) with a post-hypoxic frequency decline of −12 cycles min−1. Following microinjection of saline into the commNTS, hypoxic ventilation completely inhibited BAT SNA, and increased mean AP (peak: +13 mmHg), PHR amplitude (peak: +13%), and PHR frequency (peak: +12 cycles min−1) with a post-hypoxia frequency decline of −12 cycles min−1. Panels separated by 1 and 2 min. Vertical scale bar in BAT SNA tracing represents 80 μV.
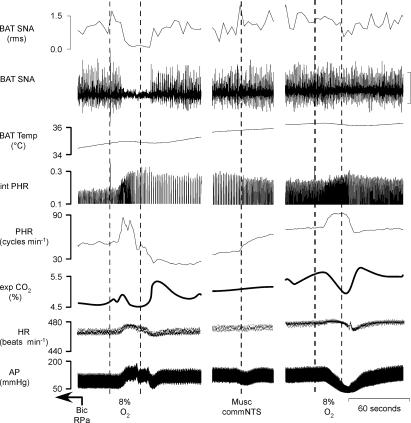
Figure 6. Hypoxic ventilation-evoked thermogenic, respiratory and cardiovascular responses are markedly reduced by microinjection of muscimol into the commNTS.
Bicuculline (Bic) was microinjected into the raphe pallidus (RPa) 2.5 min prior to the data in the first panel. Ventilation for 30 s with 8% O2 completely inhibited BAT SNA, increased mean arterial pressure (AP; peak: +24 mmHg) and increased PHR amplitude (peak: +110% of control above the Bic-evoked level) and frequency (peak: +8 cycles min−1) followed by post-hypoxic frequency decline (PHFD) of −20 cycles min−1. Microinjection of muscimol into the commNTS eliminated the hypoxic ventilation-evoked inhibition of BAT SNA (100% inhibition before muscimol compared to 1% inhibition after muscimol), converted the control hypoxic ventilation-evoked pressor response into a depressor response (nadir: −38 mmHg), attenuated the hypoxic-ventilation-evoked increases in PHR amplitude (peak: +25% of control above the Bic-evoked level) and attenuated the PHFD (−4 cycles min−1). Following microinjection of muscimol into the commNTS the hypoxic ventilation-evoked increase in PHR frequency was +10 cycles min−1. Panels separated by 0.5 and 1 min. Vertical scale bar in BAT SNA tracing represents 80 μV.
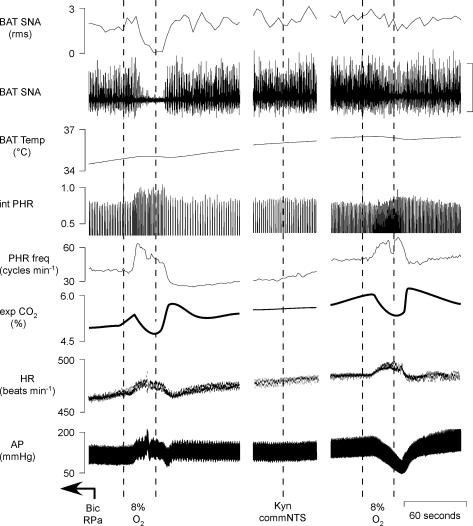
Figure 7. Hypoxic ventilation-evoked thermogenic, respiratory and cardiovascular responses are markedly reduced by microinjection of kynurenate into the commNTS.
Bicuculline (Bic) was microinjected into the raphe pallidus (RPa) 2.5 min prior to the data in the first panel. Ventilation for 30 s with 8% O2 completely inhibited BAT SNA, increased mean arterial pressure (AP; peak: +27 mmHg) and increased PHR amplitude (peak: +25% of control above the Bic-evoked level) and frequency (peak: +11 cycles min−1) followed by a post-hypoxic frequency decline (PHFD) of −14 cycles min−1. Microinjection of kynurenate into the commNTS attenuated the hypoxic ventilation-evoked inhibition of BAT SNA (100% inhibition before kynurenate compared to 11% inhibition after kynurenate), converted the control hypoxic ventilation-evoked pressor response into a depressor response (nadir: −32 mmHg), attenuated the hypoxic ventilation-evoked increase PHR amplitude (peak: +5% of control above the Bic-evoked level) and frequency (peak: +6 cycles min−1) and nearly eliminated the post-hypoxic frequency decline (−1 cycles min−1). Panels separated by 1 and 1 min. Vertical scale bar in BAT SNA tracing represents 80 μV.
Table 4.
Effects of microinjection of saline into the commissural nucleus tractus solitarii (commNTS) on thermogenic, metabolic, cardiovascular, and respiratory responses to ventilation with 8% O2
| Untreated commNTS | Saline commNTS | |||
|---|---|---|---|---|
| Bic RPa | 8% O2 | Bic RPa | 8% O2 | |
| BAT SNA (% control) | 871 ± 203 | 319 ± 110* | 878 ± 417 | 262 ± 89* |
| BAT temp. (°C) | 35.2 ± 0.3 | 35.3 ± 0.3 | 35.5 ± 0.4 | 35.5 ± 0.3 |
| Expired CO2 (%) | 5.6 ± 0.3 | 5.4 ± 0.3 | 5.7 ± 0.4 | 5.6 ± 0.4 |
| HR (beats min−1) | 494 ± 13 | 501 ± 14 | 499 ± 10 | 520 ± 9 |
| MAP (mmHg) | 123 ± 12 | 142 ± 13* | 133 ± 9 | 150 ± 12* |
| PHR amp. (% control) | 114 ± 6 | 132 ± 9* | 117 ± 11 | 131 ± 13* |
| PHR freq. (cycles min−1) | 52 ± 4 | 62 ± 4* | 51 ± 4 | 62 ± 4* |
Values are means ± s.e.m. (n = 5) for physiological variables at the peak within 5 min of the microinjection of bicuculline (Bic) into the RPa, the value during a subsequent 30 s ventilation with 8% O2, at the peak after microinjection of Bic into the RPa following a saline microinjection into the commNTS, and the value during a second 30 s episode of ventilation with 8% O2. Note the Bic responses after saline injection into the commNTS did not differ from those in the untreated trial. In addition, the hypoxia-evoked responses did not differ between the untreated trial and the subsequent commNTS injected trial.
P < 0.05, compared to the immediately preceding Bic-evoked response.
Table 5.
Effects of microinjection of muscimol or glycine into the commissural nucleus tractus solitarii (commNTS) on thermogenic, metabolic, cardiovascular, and respiratory responses to ventilation with 8% O2
| Untreated commNTS | Inhibition of commNTS | |||
|---|---|---|---|---|
| Bic RPa | 8% O2 | Bic RPa | 8% O2 | |
| BAT SNA (% control) | 755 ± 134 | 286 ± 72* | 838 ± 259 | 749 ± 202† |
| BAT temp. (°C) | 34.7 ± 0.1 | 34.8 ± 0.1 | 35.0 ± 0.3 | 35.0 ± 0.3 |
| Expired CO2 (%) | 5.4 ± 0.2 | 5.3 ± 0.2 | 5.6 ± 0.2 | 5.4 ± 0.2 |
| HR (beats min−1) | 479 ± 6 | 487 ± 6 | 482 ± 5 | 467 ± 10 |
| MAP (mmHg) | 118 ± 9 | 144 ± 8* | 123 ± 13 | 97 ± 12*† |
| PHR amp. (% control) | 115 ± 7 | 146 ± 18* | 112 ± 13 | 122 ± 15* |
| PHR freq.(cycles min−1) | 45 ± 2 | 60 ± 3* | 60 ± 4* | 67 ± 4*† |
Values are means ± s.e.m. (n = 8) for physiological variables at the peak value within 5 min of the microinjection of bicuculline (Bic) into the RPa, the values during subsequent ventilation with 8% O2, the peak values after microinjection of Bic into the RPa plus muscimol or glycine microinjection into the commNTS, and the values during a subsequent episode of ventilation with 8% O2.
P < 0.05 compared to the preceding Bic-evoked response.
P < 0.05 for the hypoxia-evoked change compared to that in the NTS-untreated response.
Table 6.
Effects of microinjection of kynurenate into the commissural nucleus tractus solitarii (commNTS) on thermogenic, metabolic, cardiovascular and respiratory responses to ventilation with 8% O2
| Untreated commNTS | Kynurenate commNTS | |||
|---|---|---|---|---|
| Bic RPa | 8% O2 | Bic RPa | 8% O2 | |
| BAT SNA (% control) | 962 ± 237 | 376 ± 108* | 952 ± 451 | 870 ± 397† |
| BAT temp. (°C) | 35.3 ± 0.3 | 35.4 ± 0.2 | 35.6 ± 0.3 | 35.6 ± 0.3 |
| Expired CO2 (%) | 5.4 ± 0.2 | 5.2 ± 0.3 | 5.6 ± 0.3 | 5.3 ± 0.3 |
| HR (beats min−1) | 503 ± 13 | 514 ± 12 | 496 ± 4 | 502 ± 4 |
| MAP (mmHg) | 117 ± 9 | 144 ± 8* | 137 ± 6 | 116 ± 12*† |
| PHR amp. (% control) | 119 ± 7 | 139 ± 7* | 103 ± 8* | 112 ± 8*† |
| PHR freq.(cycles min−1) | 49 ± 6 | 61 ± 5* | 56 ± 5 | 71 ± 8* |
Values are means ± s.e.m. (n = 4) for physiological variables at the peak values within 5 min of the microinjection of bicuculline (Bic) into the RPa, the values during subsequent ventilation with 8% O2, the peak values after microinjection of Bic into the RPa plus kynurenate microinjection into the commNTS, and the values during a subsequent episode of ventilation with 8% O2.
P < 0.05 compared to the immediately preceding Bic-evoked response.
P < 0.05 for the hypoxia-evoked change compared to that in the NTS-untreated response.
Microinjection of saline vehicle into the commNTS had no effect on the amplitudes of the ongoing sympathoexcitatory responses or respiratory stimulation evoked by microinjection of bicuculline into RPa (Fig. 5). Similarly, in comparison to the NTS-untreated condition, saline vehicle microinjection into commNTS did not alter the changes in the thermogenic, metabolic and cardiovascular variables evoked by hypoxic ventilation (Fig. 5, Table 4). In contrast, microinjection of glycine or muscimol (Fig. 6, Table 5), or kynurenate (Fig. 7, Table 6) into the commNTS, while it did not affect the sympathoexcitation following microinjection of bicuculline into the RPa, did markedly attenuate the hypoxic ventilation-evoked inhibition of BAT SNA (attenuated by 77 ± 10%, n = 8; and 82 ± 10%, n = 4, respectively, when compared to the NTS-untreated hypoxia responses). In addition, microinjection of glycine or muscimol (Fig. 6, Table 5), or kynurenate (Fig. 7, Table 6) into the commNTS converted the hypoxia-evoked pressor responses into depressor responses and reduced the hypoxia-evoked increase in respiratory frequency (microinjection of glycine or muscimol, Table 5) and the increase in PHR amplitude (microinjection of kynurenate, Table 6).
Following the respiratory stimulation produced by ventilation with 8% O2 for 30 s, PHR frequency was decreased (Figs 5, 6 and 7), a phenomenon described as post-hypoxic frequency decline (PHFD) (Coles & Dick, 1996). Microinjection of saline into the commNTS did not alter PHFD (saline in commNTS: −13 ± 1 cycles min−1versus NTS-untreated trial: −17 ± 2 cycles min−1); however, PHFD was prevented by microinjection of muscimol into the commNTS (muscimol in commNTS: −3 ± 1 cycles min−1versus untreated trial: −22 ± 8 cycles min−1), or microinjection of kynurenate into the commNTS (kynurenate in commNTS: 1 ± 3 cycles min−1versus untreated trial: −20 ± 9 cycles min−1).
Effect of chemoreceptor afferent denervation on the thermogenic and cardiovascular responses to NaCN and to hypoxic ventilation
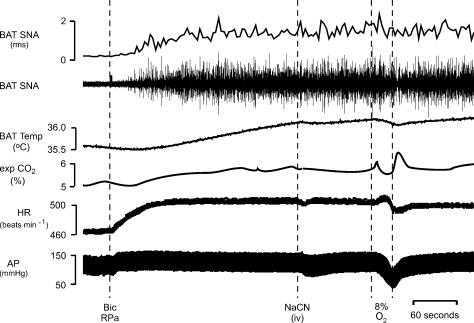
To establish that the responses to i.v. NaCN and to 30-s hypoxic ventilation were due to activation of the arterial chemoreceptor reflex, the effects of these stimuli were determined in three rats following elimination of their chemoreceptor afferents with bilateral transection of the carotid sinus nerves. As shown in the example in Fig. 8, similar to rats with intact carotid sinus nerves, BAT SNA was low in rats following chemoreceptor afferent denervation when core temperature was maintained between 36.5 and 37.5°C and microinjection of bicuculline into the RPa produced significant increases in BAT SNA, BAT temperature, expired CO2, HR and MAP. In rats with sectioned carotid sinus nerves, systemic administration of NaCN failed to decrease BAT SNA, expired CO2, or HR (Fig. 8, Table 7) as it had in intact rats (Fig. 3A). In contrast to the NaCN-evoked pressor response in intact rats (Fig. 3A), MAP decreased (−23 ± 5 mmHg) in the absence of arterial chemoreceptor afferents (Fig. 8, Table 7). Similarly, ventilation with 8% O2 for 30 s failed to alter any of the measured variables (Fig. 8, Table 7) with the exception of a decrease in MAP (−44 ± 7 mmHg).
Figure 8. NaCN-evoked and hypoxic ventilation-evoked responses arise from arterial chemoreceptors.
Following section of chemoreceptor afferent nerves, microinjection of bicuculline (Bic) into the raphe pallidus (RPa) increased brown adipose tissue (BAT) sympathetic nerve activity (SNA; peak: +535% of control), BAT temperature (peak: +0.6°C), expired CO2 (peak: +0.6%), and heart rate (HR, peak: +41 beats min−1). After section of the chemoreceptor afferent nerves, the BAT SNA amplitude was unchanged by intravenous administration of NaCN (618% of control prior to NaCN versus 623% of control after NaCN) or by hypoxic ventilation (635% of control before ventilation with 8% O2versus 798% of control during ventilation with 8% O2). Mean arterial pressure (AP) was reduced by hypoxic ventilation (nadir: −31 mmHg). Vertical scale bar in BAT SNA tracing represents 80 μV.
Table 7.
Effect of chemoreceptor deafferentations on the thermogenic, metabolic and cardiovascular responses to systemic administration of NaCN and to ventilation with 8% O2
| Control | Bic RPa | NaCN | 8% O2 | |
|---|---|---|---|---|
| BAT SNA (% control) | 100 | 923 ± 193* | 1102 ± 339* | 819 ± 14* |
| BAT temp. (°C) | 34.7 ± 0.6 | 35.4 ± 0.5* | 35.3 ± 0.6* | 35.4 ± 0.5* |
| Expired CO2 (%) | 5.1 ± 0.3 | 5.7 ± 0.2* | 5.7 ± 0.2* | 5.6 ± 0.1* |
| HR (beats min−1) | 434 ± 13 | 476 ± 14* | 476 ± 13* | 455 ± 35 |
| MAP (mmHg) | 114 ± 6 | 125 ± 6 | 106 ± 3 | 82 ± 1*† |
Physiological variables in rats in which the carotid sinus nerves have been transected bilaterally. Values are means ± s.e.m. (n = 3) in the control condition, at the peak response within 10 min of the microinjection of bicuculline (Bic) into the RPa, at the minimum level within five minutes of subsequent intravenous injection of NaCN, and at the minimum level during ventilation with 8% O2.
P < 0.05 compared to the control condition.
P < 0.05 compared to the Bic RPa condition.
Effect of disinhibition of commNTS neurones on thermogenic and cardiovascular responses
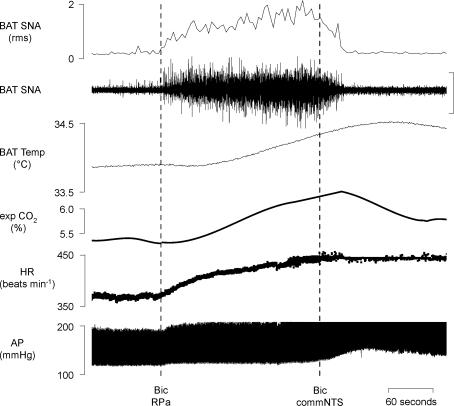
To determine if an increase in the discharge of neurones in commNTS, the site of termination of arterial chemoreceptor afferents, leads to inhibition of BAT SNA, bicuculline was microinjected into commNTS to disinhibit local neurones, including those receiving arterial chemoreceptor afferent input. Microinjection of bicuculline into the RPa (Fig. 9) increased BAT SNA (peak: +616 ± 113% of control, P < 0.05, n = 4), BAT temperature (peak: +1.3 ± 0.5°C, P < 0.05, n = 4), expired CO2 (peak: +1.2 ± 0.2%, P < 0.05, n = 4) and HR (peak: +67 ± 13 beats min−1, P < 0.05, n = 4). Microinjection of bicuculline into the commNTS completely reversed the evoked increases in BAT SNA, BAT temperature, and expired CO2 (Table 8).
Figure 9. Disinhibition of neurones within the commissural nucleus tractus solitarii (commNTS) inhibits brown adipose tissue (BAT) thermogenesis.
Microinjection of bicuculline (Bic) into the raphe pallidus (RPa) increased BAT sympathetic nerve activity (SNA; peak: +577% of control), BAT temperature (peak: +0.3°C), expired CO2 (peak: +0.9%), and heart rate (HR, peak: +70 beats min−1). Microinjection of Bic into the commNTS inhibited BAT SNA (nadir: 100% of control), decreased BAT temperature, and decreased expired CO2 (nadir: −0.9%). Vertical scale bar in BAT SNA tracing represents 40 μV.
Table 8.
Effects on thermogenic, metabolic and cardiovascular variables of disinhibition of neurones within the raphe pallidus area (RPa), and subsequent disinhibition of neurones within the commNTS
| Control | Bic RPa | Bic commNTS | |
|---|---|---|---|
| BAT SNA (% control) | 100 | 716 ± 113* | 91 ± 9† |
| BAT temp. (°C) | 34.8 ± 0.6 | 36.1 ± 0.5* | 34.7 ± 0.3† |
| Expired CO2 (%) | 5.0 ± 0.3 | 6.2 ± 0.2* | 4.9 ± 0.2† |
| HR (beats min−1) | 386 ± 26 | 453 ± 17* | 404 ± 10† |
| MAP (mmHg) | 109 ± 12 | 121 ± 9 | 136 ± 14 |
Values are means ± s.e.m. (n = 4) for physiological variables in the control condition, at the peak response within 10 min of the microinjection of bicuculline (Bic) into the RPa, and at the minimum level within five minutes (except for BAT temperature and core temperature, within 10 min) of subsequent microinjection of Bic into the commNTS.
P < 0.05, compared to the control condition.
P < 0.05, compared to the immediately preceding response evoked by microinjection of Bic into RPa.
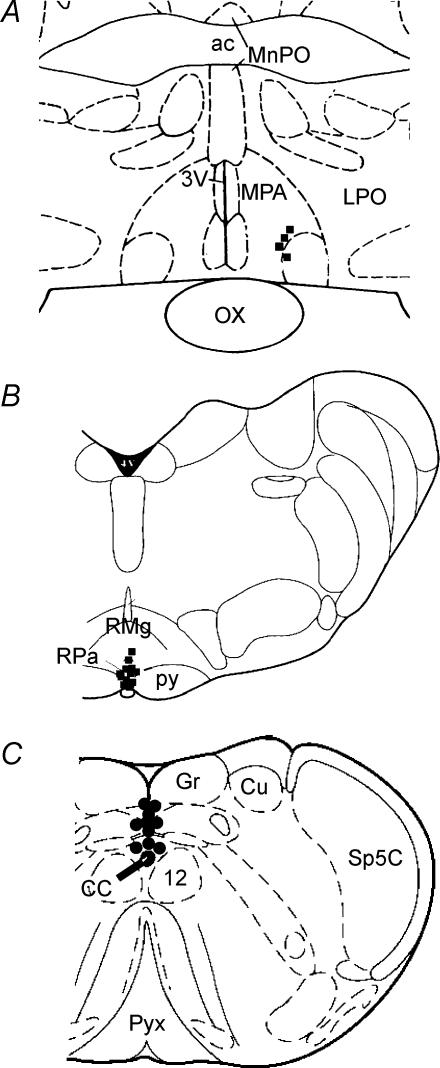
Histological localization of microinjection sites
The locations of microinjection sites in the MPA, the RPa and the commNTS are shown in Fig. 10. The approximate centres of the injection sites targeting the MPA were located primarily dorsal and medial to the anteroventral preoptic nucleus at the rostrocaudal level of the decussation of the anterior commissure (Fig. 10A). The approximate centres of the injection sites targeting the RPa (Fig. 10B) were located at the level of the caudal half of the facial nucleus within the RPa, or the midline caudal raphe magnus. The approximate centres of the injection sites targeting the midline commNTS (Fig. 10C) were located on the midline just caudal to the area postrema and dorsal to the central canal.
Figure 10. Histological localization of microinjection sites.
Histological localization of microinjection sites targeting the medial preoptic area (MPA) (A), the raphe pallidus area (RPa) (B), and the commissural nucleus tractus solitarii (commNTS) (C) plotted on atlas drawings of the rat medulla (+8.74, −2.3 and −5.3 mm from interaural, respectively) (Paxinos & Watson, 1986). 3V: third ventricle; 4V: forth ventricle; 7n: facial nerve; 12: hypoglossal nucleus; ac anterior commissure; cc: central canal; Gr: gracilis nucleus; Cu: cuneate nucleus; LPO: lateral preoptic; LSO: lateral superior olive; MnPO: median preoptic; ox: optic chiasm; py: pyramidal tract; pyx; pyramidal decussation; RMg: raphe magnus.
Discussion
The present study is the first to demonstrate that hypoxic activation of arterial chemoreceptor afferents or increased activity of their second-order target neurones in the commNTS leads to inhibition of BAT SNA and BAT thermogenesis. Although prolonged hypoxia can reduce metabolism and oxygen consumption in animals whose arterial chemoreceptor afferents have been cut (Gautier & Bonora, 1992; Gautier et al. 1993; Matsuoka et al. 1994), the present study directly demonstrates the significant inhibitory effect of the arterial chemoreceptor reflex in reducing BAT SNA and BAT thermogenesis during an acute hypoxic challenge. In determining the basis for the reductions in body temperature observed during prolonged hypoxia, the interrelationship among (a) the hypometabolic responses elicited by peripheral chemoreceptor afferents, (b) those arising from central neurones responding directly to oxygen deficit, and (c) heat loss responses such as reduced cutaneous vasoconstriction remains to be examined.
Chemoreceptor stimulation inhibits the sympathetic outflow to the cutaneous vasculature (Janig et al. 1983; Rowell & Blackmon, 1987), consistent with an important role for heat loss in the initiation and maintenance of hypoxic hypothermia. Similar to the hypothermia elicited by hypoxic exposure, inhibition of neuronal discharge in the RPa of conscious rats causes a precipitous fall in body temperature (Zaretsky et al. 2003). Since cutaneous vasoconstriction (Blessing, 2003; Ootsuka et al. 2004) and non-shivering thermogenesis (Morrison et al. 1999; Morrison, 2004) are dependent on the activity of populations of putative sympathetic premotor neurones in RPa, a reduction in either mechanism could contribute to the fall in body temperature seen with inhibition of RPa activity. Although no direct measurements of non-shivering thermogenesis at various ambient temperatures are available, at normal room temperature, BAT thermogenesis is thought to make little contribution to the maintenance of body temperature under normal conditions. Thus, in hypoxia, an increase in cutaneous heat loss may be the principal factor initiating hypothermia. However, if non-shivering thermogenesis were not simultaneously inhibited, such a fall in core temperature would stimulate a compensatory reflex stimulation of BAT thermogenesis, resulting in a large energy and oxygen utilization to counteract the effects of increased heat loss. Thus, hypoxic inhibition of non-shivering thermogenesis is an important aspect of the coordinated pattern of responses comprising the hypothermic component of the homeostatic mechanism to restrict oxygen consumption during the reduced cellular oxygen availability that may arise in hypoxic environments such as high altitude or in respiratory or circulatory diseases that compromise oxygen transport.
We have previously demonstrated that disinhibition of cells in the RPa results in activation of BAT SNA and BAT thermogenesis (Morrison et al. 1999; Madden & Morrison, 2003), suggesting that a potent inhibitory input impinges upon putative sympathetic premotor neurones within the RPa. In the current study both chemical and hypoxic activation of arterial chemoreceptors, as well as disinhibition of neurones within the midline commNTS inhibited the increase in BAT SNA and BAT thermogenesis evoked by disinhibition of neurones within the RPa. Since the increase in BAT thermogenesis evoked by microinjection of bicuculline into the RPa was completely reversed by chemoreceptor activation, it is unlikely that the mechanism of this chemoreceptor reflex-evoked inhibition of BAT SNA involves the activation of a GABAergic input directly onto putative sympathetic premotor neurones in the RPa. In this regard, hypoxia could drive an inhibitory input (a) to sympathetic premotor neurones of the RPa that is not mediated by GABA, (b) to the source of the synaptic excitation of the BAT sympathetic premotor neurones in the RPa, or (c) to the spinal preganglionic neurones controlling BAT thermogenesis. To elucidate the neural circuit involved in the hypoxia-evoked inhibition of BAT SNA will require further experimentation to define the brainstem pathway between second-order arterial chemoreceptor neurones in commNTS and BAT sympathetic preganglionic neurones.
Our results demonstrate that in urethane–chloralose anaesthetized, artificially ventilated rats, peripheral chemoreceptor-evoked, vagally mediated bradycardia can be blocked by inhibition of neurones within the commNTS. Consistent with our results, a similar study using awake freely breathing rats demonstrated that peripheral chemoreceptor-evoked bradycardia was prevented by activation of GABAA receptors within the commNTS (Callera et al. 1999). In contrast, the peripheral chemoreceptor-evoked pressor response was not blocked by inhibition of neurones in the commNTS (Callera et al. 1999), nor was it prevented by blockade of excitatory amino acid (EAA) receptors within the commNTS in awake rats (Haibara et al. 1999). However, in the present study, inhibition of neurones within the commNTS, blockade of EAA input to neurones within the commNTS or transection of the carotid sinus nerves converted the chemoreceptor-evoked pressor response into a depressor response. Consistent with our results, studies in anaesthetized rats have reported that blockade of ionotropic excitatory amino acid receptors within the commNTS (Vardhan et al. 1993; Zhang & Mifflin, 1993) or carotid sinus denervation (Ilyinsky et al. 2003) prevents the peripheral chemoreceptor-evoked pressor response. The question of whether the mechanisms mediating chemoreceptor-evoked pressor responses differ between anaesthetized and awake rats could be addressed if sequential chemoreceptor stimulation and NTS blockade experiments were performed in the same rats that were initially awake and then given anaesthesia.
We observed a significant increase in PHR amplitude and frequency following microinjection of bicuculline into the rostral RPa. Previous studies have suggested that serotonergic neurones located in the ventral medullary raphe are central chemoreceptors and that activation of these cells plays a role in hypercapnia-evoked respiratory stimulation (Richerson et al. 2001; Nattie et al. 2004). Thus, a potential, albeit untested, mechanism underlying the increase in respiratory drive observed in the current study following disinhibition of neurones in the rostral RPa is that removal of a tonic GABAergic input to serotonergic, chemosensitive neurones of the RPa stimulates a central chemoreceptor-like activation of phrenic nerve activity.
Our results agree with previous demonstrations that hypoxia increases PHR amplitude and frequency (Dick & Coles, 2000; Coles et al. 2002; Ilyinsky et al. 2003). In addition, our findings that inhibition of commNTS neurones or blockade of EAA receptors within the midline commNTS attenuated the hypoxia-evoked increases in PHR frequency and amplitude are consistent with the demonstration that removal of peripheral chemoreceptors completely prevents the hypoxia-evoked increases in the PHR amplitude and frequency (Ilyinsky et al. 2003) or in respiratory frequency (Cardenas & Zapata, 1983; Coles et al. 2002). The difference in the magnitude of the effect between the present study and those involving removal of peripheral chemoreceptors could be explained by the fact that transection of the carotid sinus nerves would be expected to remove all carotid chemoreceptor input, while our microinjections only targeted neurones in the midline commNTS (which mediate inhibition of BAT SNA) and likely spared potential chemoreceptor inputs to the lateral commNTS (which may partially mediate respiratory stimulation).
In the present study, inhibition of neurones within the midline commNTS, or blockade of EAA receptors within the midline commNTS prevented PHFD. This decrease in respiratory frequency (Coles et al. 2002) or in PHR burst frequency (Ilyinsky et al. 2003) seen after acute hypoxia occurs in the absence of carotid chemoreceptor afferents and requires activation of neurones in the ventrolateral pons (Coles & Dick, 1996). PHFD is not elicited by stimulation of peripheral chemoreceptor afferents with NaCN (Cardenas & Zapata, 1983). Taken together these data suggest that PHFD requires an EAA-evoked activation of neurones within the midline commNTS and that this activation may be independent of carotid chemoreceptor inputs.
In conclusion, the findings of the present study demonstrate that hypoxic stimulation of arterial chemoreceptor afferents leads to an inhibition of BAT SNA and BAT thermogenesis through an EAA-mediated activation of second-order chemoreceptor neurones in the commNTS. Peripheral chemoreceptor-evoked inhibition of BAT SNA could contribute to hypoxia-evoked reductions in body temperature and oxygen consumption and serve as an adaptive response to decreased oxygen availability, at least under conditions associated with non-shivering thermogenesis.
Acknowledgments
This work was supported by NIH grants NS40987 (S.F.M.) and DK065401 (C.J.M.). We are grateful to Brad Sugden for histological assistance.
References
- Biscoe TJ, Duchen MR. Electrophysiological responses of dissociated type I cells of the rabbit carotid body to cyanide. J Physiol. 1989;413:447–468. doi: 10.1113/jphysiol.1989.sp017663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing WW. Lower brainstem pathways regulating sympathetically mediated changes in cutaneous blood flow. Cell Mol Neurobiol. 2003;23:527–538. doi: 10.1023/A:1025020029037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callera JC, Bonagamba LG, Nosjean A, Laguzzi R, Machado BH. Activation of GABAA but not GABAB receptors in the NTS blocked bradycardia of chemoreflex in awake rats. Am J Physiol. 1999;276:H1902–H1910. doi: 10.1152/ajpheart.1999.276.6.H1902. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Cardenas H, Zapata P. Ventilatory reflexes originated from carotid and extracarotid chemoreceptors in rats. Am J Physiol. 1983;244:R119–R125. doi: 10.1152/ajpregu.1983.244.1.R119. [DOI] [PubMed] [Google Scholar]
- Coimbra C, Wieloch T. Moderate hypothermia mitigates neuronal damage in the rat brain when initiated several hours following transient cerebral ischemia. Acta Neuropathologica. 1994;87:325–331. doi: 10.1007/BF00313599. [DOI] [PubMed] [Google Scholar]
- Coles SK, Dick TE. Neurones in the ventrolateral pons are required for post-hypoxic frequency decline in rats. J Physiol. 1996;497:79–94. doi: 10.1113/jphysiol.1996.sp021751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles SK, Miller R, Huela J, Wolken P, Schlenker E. Frequency responses to hypoxia and hypercapnia in carotid body-denervated conscious rats. Respiratory Physiol Neurobiol. 2002;130:113–120. doi: 10.1016/s0034-5687(02)00005-1. [DOI] [PubMed] [Google Scholar]
- Cutler MJ, Swift NM, Keller DM, Wasmund WL, Burk JR, Smith ML. Periods of intermittent hypoxic apnea can alter chemoreflex control of sympathetic nerve activity in humans. Am J Physiol Heart Circ Physiol. 2004;287:H2054–H2060. doi: 10.1152/ajpheart.00377.2004. [DOI] [PubMed] [Google Scholar]
- Dick TE, Coles SK. Ventrolateral pons mediates short-term depression of respiratory frequency after brief hypoxia. Respiration Physiol. 2000;121:87–100. doi: 10.1016/s0034-5687(00)00121-3. [DOI] [PubMed] [Google Scholar]
- Edwards AD, Yue X, Squier MV, Thoresen M, Cady EB, Penrice J, Cooper CE, Wyatt JS, Reynolds EO, Mehmet H. Specific inhibition of apoptosis after cerebral hypoxia-ischaemia by moderate post-insult hypothermia. Biochem Biophys Res Comms. 1995;217:1193–1199. doi: 10.1006/bbrc.1995.2895. [DOI] [PubMed] [Google Scholar]
- Finley JC, Katz DM. The central organization of carotid body afferent projections to the brainstem of the rat. Brain Res. 1992;572:108–116. doi: 10.1016/0006-8993(92)90458-l. [DOI] [PubMed] [Google Scholar]
- Franchini KG, Krieger EM. Carotid chemoreceptors influence arterial pressure in intact and aortic-denervated rats. Am J Physiol. 1992;262:R677–R683. doi: 10.1152/ajpregu.1992.262.4.R677. [DOI] [PubMed] [Google Scholar]
- Gargaglioni LH, Coimbra NC, Branco LG. The nucleus raphe magnus modulates hypoxia-induced hyperventilation but not anapyrexia in rats. Neuroscience Lett. 2003;347:121–125. doi: 10.1016/s0304-3940(03)00671-2. [DOI] [PubMed] [Google Scholar]
- Gautier H, Bonora M. Ventilatory and metabolic responses to cold and hypoxia in intact and carotid body-denervated rats. J Appl Physiol. 1992;73:847–854. doi: 10.1152/jappl.1992.73.3.847. [DOI] [PubMed] [Google Scholar]
- Gautier H, Bonora M, Trinh HC. Ventilatory and metabolic responses to cold and CO2 in intact and carotid body-denervated awake rats. J Appl Physiol. 1993;75:2570–2579. doi: 10.1152/jappl.1993.75.6.2570. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. Neural structures that mediate sympathoexcitation during hypoxia. Respiration Physiol. 2000;121:147–162. doi: 10.1016/s0034-5687(00)00125-0. [DOI] [PubMed] [Google Scholar]
- Haibara AS, Bonagamba LG, Machado BH. Sympathoexcitatory neurotransmission of the chemoreflex in the NTS of awake rats. Am J Physiol. 1999;276:R69–R80. doi: 10.1152/ajpregu.1999.276.1.R69. [DOI] [PubMed] [Google Scholar]
- Haibara AS, Colombari E, Chianca DA, Jr, Bonagamba LG, Machado BH. NMDA receptors in NTS are involved in bradycardic but not in pressor response of chemoreflex. Am J Physiol. 1995;269:H1421–H1427. doi: 10.1152/ajpheart.1995.269.4.H1421. [DOI] [PubMed] [Google Scholar]
- Ilyinsky O, Tolstykh G, Mifflin S. Chronic hypoxia abolishes posthypoxia frequency decline in the anesthetized rat. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1322–R1330. doi: 10.1152/ajpregu.00033.2003. [DOI] [PubMed] [Google Scholar]
- Imai-Matsumura K, Nakayama T. The central efferent mechanism of brown adipose tissue thermogenesis induced by preoptic cooling. Can J Physiol Pharmacol. 1987;65:1299–1303. doi: 10.1139/y87-206. [DOI] [PubMed] [Google Scholar]
- Janig W, Krauspe R, Wiedersatz G. Reflex activation of postganglionic vasoconstrictor neurones supplying skeletal muscle by stimulation of arterial chemoreceptors via non-nicotinic synaptic mechanisms in sympathetic ganglia. Pflugers Arch. 1983;396:95–100. doi: 10.1007/BF00615511. [DOI] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Excitatory amino acid receptor activation in the raphe pallidus area mediates prostaglandin-evoked thermogenesis. Neuroscience. 2003;122:5–15. doi: 10.1016/s0306-4522(03)00527-x. [DOI] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Excitatory amino acid receptors in the dorsomedial hypothalamus mediate prostaglandin-evoked thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2004;286:R320–R325. doi: 10.1152/ajpregu.00515.2003. [DOI] [PubMed] [Google Scholar]
- Matsuoka T, Dotta A, Mortola JP. Metabolic response to ambient temperature and hypoxia in sinoaortic-denervated rats. Am J Physiol. 1994;266:R387–R391. doi: 10.1152/ajpregu.1994.266.2.R387. [DOI] [PubMed] [Google Scholar]
- Morrison SF. Central pathways controlling brown adipose tissue thermogenesis. News Physiol Sci. 2004;19:67–74. doi: 10.1152/nips.01502.2003. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Ramamurthy S, Young JB. Reduced rearing temperature augments responses in sympathetic outflow to brown adipose tissue. J Neurosci. 2000;20:9264–9271. doi: 10.1523/JNEUROSCI.20-24-09264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF, Sved AF, Passerin AM. GABA-mediated inhibition of raphe pallidus neurons regulates sympathetic outflow to brown adipose tissue. Am J Physiol. 1999;276:R290–R297. doi: 10.1152/ajpregu.1999.276.2.R290. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A, Richerson G, Lappi DA. Medullary serotonergic neurones and adjacent neurones that express neurokinin-1 receptors are both involved in chemoreception in vivo. J Physiol. 2004;556:235–253. doi: 10.1113/jphysiol.2003.059766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ootsuka Y, Blessing WW, McAllen RM. Inhibition of rostral medullary raphe neurons prevents cold-induced activity in sympathetic nerves to rat tail and rabbit ear arteries. Neurosci Lett. 2004;357:58–62. doi: 10.1016/j.neulet.2003.11.067. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Orlando, FL, USA: Academic Press; 1986. [Google Scholar]
- Richerson GB, Wang W, Tiwari J, Bradley SR. Chemosensitivity of serotonergic neurons in the rostral ventral medulla. Respir Physiol. 2001;129:175–189. doi: 10.1016/s0034-5687(01)00289-4. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA. Do fever and anapyrexia exist? Analysis of set point-based definitions. Am J Physiol Regul Integr Comp Physiol. 2004;287:R992–R995. doi: 10.1152/ajpregu.00068.2004. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Blackmon JR. Human cardiovascular adjustments to acute hypoxaemia. Clin Physiol. 1987;7:349–376. doi: 10.1111/j.1475-097x.1987.tb00179.x. [DOI] [PubMed] [Google Scholar]
- Steiner AA, Branco LG. Hypoxia-induced anapyrexia: implications and putative mediators. Annu Rev Physiol. 2002;64:263–288. doi: 10.1146/annurev.physiol.64.081501.155856. [DOI] [PubMed] [Google Scholar]
- Sun MK, Reis DJ. NMDA receptor-mediated sympathetic chemoreflex excitation of RVL-spinal vasomotor neurones in rats. J Physiol. 1995;482:53–68. doi: 10.1113/jphysiol.1995.sp020499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardhan A, Kachroo A, Sapru HN. Excitatory amino acid receptors in commissural nucleus of the NTS mediate carotid chemoreceptor responses. Am J Physiol. 1993;264:R41–R50. doi: 10.1152/ajpregu.1993.264.1.R41. [DOI] [PubMed] [Google Scholar]
- Vidruk EH, Olson EB, Jr, Ling L, Mitchell GS. Responses of single-unit carotid body chemoreceptors in adult rats. J Physiol. 2001;531:165–170. doi: 10.1111/j.1469-7793.2001.0165j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SC, Stabenau EK. Effect of gender on thermoregulation and survival of hypoxic rats. Clin Exp Pharmacol Physiol. 1998;25:155–158. doi: 10.1111/j.1440-1681.1998.tb02196.x. [DOI] [PubMed] [Google Scholar]
- Yager J, Towfighi J, Vannucci RC. Influence of mild hypothermia on hypoxic-ischemic brain damage in the immature rat. Pediatric Res. 1993;34:525–529. doi: 10.1203/00006450-199310000-00029. [DOI] [PubMed] [Google Scholar]
- Zaretsky DV, Zaretskaia MV, DiMicco JA. Stimulation and blockade of GABAA receptors in the raphe pallidus: effects on body temperature, heart rate, and blood pressure in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2003;285:R110–R116. doi: 10.1152/ajpregu.00016.2003. [DOI] [PubMed] [Google Scholar]
- Zhang W, Mifflin SW. Excitatory amino acid receptors within NTS mediate arterial chemoreceptor reflexes in rats. Am J Physiol. 1993;265:H770–H773. doi: 10.1152/ajpheart.1993.265.2.H770. [DOI] [PubMed] [Google Scholar]