Abstract
Nitric oxide (NO) is an intercellular messenger regulating neuronal functions. To visualize NO signalling in the brain, we generated a novel fluorescent NO indicator, which consists of the heme-binding region (HBR) of soluble guanylyl cyclase and the green fluorescent protein. The indicator (HBR–GFP) was expressed in the Purkinje cells of the mouse cerebellum and we imaged NO signals in acute cerebellar slices upon parallel fibre (PF) activation with a train of burst stimulations (BS, each BS consisting of five pulses at 50 Hz). Our results showed that the intensity of synaptic NO signal decays steeply with the distance from the synaptic input near PF–Purkinje cell synapses and generates synapse-specific long-term potentiation (LTP). Furthermore, the NO release level has a bell-shaped dependence on the frequency of PF activity. At an optimal frequency (1 Hz), but not at a low frequency (0.25 Hz) of a train of 60 BS, NO release as well as LTP was induced. However, both NO release and LTP were significantly reduced at higher frequencies (2–4 Hz) of BS train due to cannabinoid receptor-mediated retrograde inhibition of NO generation at the PF terminals. These results suggest that synaptic NO signalling decodes the frequency of neuronal activity to mediate synaptic plasticity at the PF–Purkinje cell synapse.
Nitric oxide (NO) is generated from arginine as catalysed by NO synthase, and NO as an intercellular messenger plays important roles in various biological systems (Ignarro & Kadowitz, 1985; Haley, 1998). In the central nervous system, neuronal NO synthase (nNOS) is expressed in certain types of neurones and glial cells, and its activity is regulated by intracellular Ca2+ concentration. Thus, NO is considered to be produced by firing neurones and to spread over synaptic clefts to reach neighbouring cells, contributing to the regulation of various neuronal functions (Snyder, 1992; Bredt & Snyder, 1994; Garthwaite & Boulton, 1995; Prast & Philippu, 2001). NO is an important candidate for a retrograde messenger in the induction of long-term potentiation (LTP) in the hippocampus (Schuman & Madison, 1991) and an anterograde messenger in long-term depression (LTD) and LTP in the cerebellum (Shibuki & Okada, 1991; Lev-Ram et al. 1995, 2002). However, it has not been fully understood how NO signalling is regulated and how far it propagates at synapses. NO as a neurotransmitter has unique properties. NO is generated on demand, while conventional neurotransmitters are stored in exocytotic vesicles. Since it is highly diffusible and passes rapidly through cell membranes, NO has been considered to have a wide range of effects exceeding 100 μm in all directions (Lancaster, 1994; Wood & Garthwaite, 1994; Daniel et al. 1998; Schweighofer & Ferriol, 2000; Prast & Philippu, 2001). Indeed, NO-dependent heterosynaptic LTP has been reported in the cerebellum (Jacoby et al. 2001). Therefore, the elucidation of NO dynamics within the brain during nerve activities is expected to facilitate the understanding of the biological actions of this messenger.
Although various methods have been used to estimate NO dynamics in biological samples (Shibuki, 1990; Kojima et al. 1998), the direct measurement of NO dynamics at synapses has not yet been carried out. For direct and selective NO detection at subcellular levels, we developed a new fluorescent NO indicator, which contains the heme-binding region of soluble guanylyl cyclase (sGC) for the molecular recognition of NO. Although all haemoproteins have high reactivities with NO, the nitrosyl complex of haemoproteins is usually unstable in the presence of oxygen because NO bound to heme is easily oxidized, and oxygen itself binds to heme. However, the nitrosyl complex of sGC does not react with oxygen (Traylor & Sharma, 1992; Stone & Marletta, 1994). This unique property of sGC suggests that the heme-binding region of sGC can be used for the selective molecular recognition of NO. Therefore, we constructed candidate indicator proteins containing the heme-binding region of sGC as a NO sensor moiety, and variants of the green fluorescent protein (GFP). We showed that one of the thus designed molecules can be used as a fluorescent NO indicator.
The parallel fibre (PF)–Purkinje cell synapse in the cerebellum is one of the most extensively studied synapses, and several patterns of synaptic plasticity have been reported (Lev-Ram et al. 2002). Using the new NO indicator, we succeeded in imaging NO dynamics within Purkinje cells upon electric stimulation of PFs in cerebellar slices. Our study showed that the intensity of synaptically generated NO signal sharply decreases with the distance from the activated synapses and depends biphasically on the frequency of burst-like PF activity due to retrograde inhibition of NO synthesis at higher frequencies via the endocannabinoid receptor. Furthermore, this NO signalling is the key factor for the generation of activity-dependent LTP at the PF–Purkinje cell synapse. Thus, these results extend the understanding of the roles of NO signalling in synaptic mechanisms.
Methods
HBR–GFP construction
The cDNA encoding sGC β1 (1–260) was PCR-amplified from pBluescript SK (−)-β1 (1–385) (Namiki et al. 2001) using the forward primer 5′-GGAATTCCATATGTACGGTTTTGTGAACTA-3′ and the reverse primer 5′-GGAATTCATGAGGGCGGACCAGAGA-3′, and was subcloned into the EcoRI site of pBluescript SK(−). The cDNA fragment encoding the enhanced green fluorescent protein (EGFP) was PCR-amplified from pEGFP-1 (Clontech) using the forward primer 5′-CCCAAGCTTGAATTCGTGAGCAAGGGCGAGGA-3′ and the reverse primer 5′-CCGCTCGAGCTTGTACAGCTCGTCCATGC-3′, and was subcloned into the HindIII/XhoI site of pBluescript SK(−). The NdeI–EcoRI fragment of sGC β1 (1–260) and the EcoRI–XhoI fragment of EGFP were subcloned into the NdeI/XhoI site of pET23a. The HBR-GFP protein was expressed in E. coli strain BL21 SI (Gibco BRL) cultured in the 2 × YT medium supplemented with δ-aminolevulinic acid (Sigma) and purified using TALON metal affinity resin (Clontech) and a HiLoad 16/60 Superdex 200 pg column (Amersham Pharmacia). For the construction of the Sindbis virus expression systems, the cDNA encoding HBR–GFP was subcloned into the XbaI/ApaI site of the pSinRep5 vector (Invitrogen), yielding pSinRep5–HBR–GFP.
In vitro measurements
Absorption and fluorescence spectra were measured in phosphate-buffered saline consisting of 137 mm NaCl, 2.7 mm KCl, 8.1 mm Na2HPO4, and 1.5 mm KH2PO4 at pH 7.4, unless otherwise indicated, using a DU640 spectrophotometer (Beckman) at room temperature (23–25°C) or using an FP6300 spectrof-luorometer (JASCO) at 25°C. The concentration of NO generated from 3-(2-hydroxy-1-methyl-2-nitrosohydrazino)-N-methyl-1-propanamine (NOC7) was determined using a NO-selective electrode (ISO-NO, WPI).
Model
The following differential equation is derived from Scheme (1) described in Results.
| (1) |
where x=[NO·HBR−GFP]/([HBR−GFP]+[NO·HBR−GFP]). Numerical solutions to eqn (1) for time-dependent changes in NO concentration were obtained by Euler's method.
Sindbis virus infection
Infectious Sindbis virus particles carrying the gene encoding HBR–GFP were prepared according to the manufacturer's instructions (Invitrogen). Briefly, pSinRep5-HBR–GFP was used as the template for in vitro transcription using SP6 RNA polymerase (Invitrogen). The RNA transcript and the helper RNA from the DH (26S) cDNA template (Invitrogen) were cotransfected into BHK cells by electroporation. The culture medium was harvested 24 h after transfection.
For the in vivo infection of the virus particles, mice (C57BL/6) on postnatal days 24–30 were anaesthetized with pentobarbital (0.1 mg (g body weight)−1). Experiments were carried out according to the guidelines established by the Animal Welfare Committee of the University of Tokyo. Through an incision in the scalp, a small piece of the occipital bone and dura covering the surface of cerebellar lobule VII were removed (Kakizawa et al. 2000; Kakizawa et al. 2003). Then the solution containing the Sindbis virus particles carrying the gene encoding HBR–GFP or EGFP was injected into the cerebellar lobule VII cortex with a microglass needle attached to a manipulator. Glass needles were pulled and broken to a tip diameter of 20–40 μm. A volume of 0.5–1.0 μl was delivered unilaterally within 5–10 min by air pressure. After the injection, the scalp was sutured, and the mice were allowed to recover from anaesthesia at 37°C.
Slice experiments
Twenty-four hours after the injection of virus vectors, parasagittal cerebellar slices (250 μm thick) were prepared from the infected mice brain, as previously described (Edwards et al. 1989; Kakizawa et al. 2000; McGee et al. 2001; Okubo et al. 2001; Kakizawa et al. 2003). The slices were incubated in a standard bath solution for 1 h before imaging in a recording chamber mounted on the stage of an upright microscope (BX61WI; Olympus) equipped with a confocal scanning unit and an argon laser (FV300, Olympus). The standard bath solution, containing (mm) 125 NaCl, 2.5 KCl, 2 CaCl2, 1 MgSO4, 1.25 NaH2PO4, 26 NaHCO3 and 20 glucose, was bubbled continuously with a mixture of 95% O2 and 5% CO2 in a reservoir, and was used to perfuse the recording chamber at a rate of 40 ml h−1. Drugs added to the bath solution in the reservoir entered the recording chamber within 1 min. Bicuculline (10 μm) was always present in the bath solution to block spontaneous activities of inhibitory interneurones. Purkinje cells expressing HBR–GFP were visually identified under a water-immersion objective (40 ×, NA 0.80) (see Fig. 3). For the focal stimulation of parallel fibres, a glass pipette with a 5- to 10-μm-diameter tip and filled with the standard bath solution was placed 15–50 μm above the distal dendrites of Purkinje cells, and square pulses were applied (duration, 0.1 ms; amplitude, <10 V). The successful stimulation of parallel fibres was confirmed on the basis of the electrophysiological characteristics of postsynaptic currents, namely, paired-pulse facilitation and sensitivity to 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo [f]quinoxaline-7-sulphonamide disodium (NBQX) (Konnerth et al. 1990). Six to 13 sequential confocal images (excitation at 488 nm) obtained at 3–4 μm z-axis intervals were acquired every 0.5 s, and were projected onto a plane to obtain images of dendrites at 15 s intervals. For intracellular Ca2+ measurement, uninfected cerebellar slices were used. Oregon Green BAPTA-1 (100 μm) was introduced into Purkinje cells through a patch pipette, and three to five sequential images were projected onto a plane to obtain Ca2+ signal images of dendrites at 3 s intervals. In the experiments whose results are shown in Fig. 4D and E, to minimize the electrotonic spread of Ca2+ signals, 2.5 μm NBQX was added to the bath solution to block AMPA receptors, while keeping stimulus intensity constant as in other experiments. Experiments were carried out at room temperature (24–26°C).
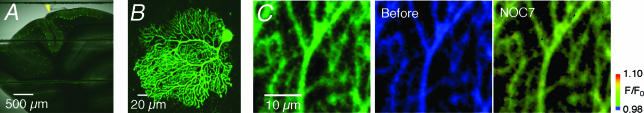
Figure 3. Fluorescence images of a cerebellar slice expressing HBR–GFP after infection with Sindbis virus.
A and B, fluorescence images of Purkinje cells expressing HBR–GFP in a cerebellar slice at two different magnifications. The yellow arrowhead in A indicates the site of penetration of virus injection needle. C, fluorescence image of Purkinje cell dendrites expressing HBR–GFP (left panel). Pseudocolor images showing the fluorescence intensities of HBR–GFP before (middle panel) and after (right panel) NOC7 application to the perfusing solution at 1 mm. Images are presented in the intensity-modulated-display (IMD) mode. Representative result of six experiments.
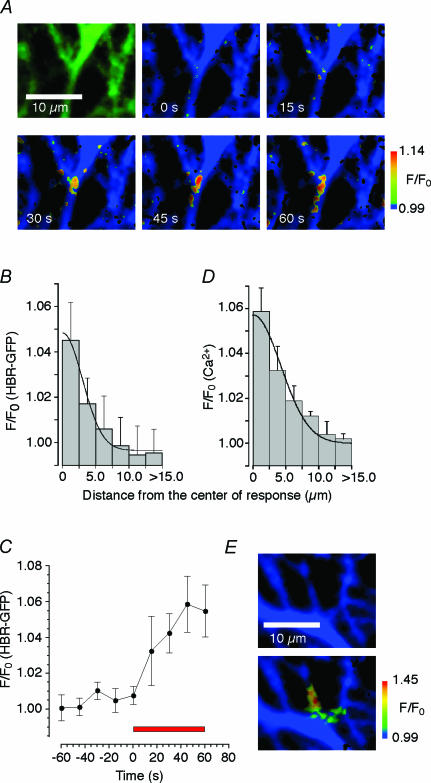
Figure 4. Parallel fibre-induced NO dynamics in Purkinje cell within cerebellar slice.
A, fluorescence image of dendrites of a Purkinje cell expressing HBR–GFP. Pseudocolor images showing the fluorescence intensities of HBR–GFP before and during 60 BSs (each BS = 5 pulses at 50 Hz) repetitively applied to PFs at 1 Hz. Representative result of five experiments. B, distribution of the average increase in HBR–GFP fluorescence intensity after a parallel fibre stimulation (60 BSs) plotted against distance from the centre of response (mean ± s.e.m., n = 5). The continuous curve indicates the best-fit Gaussian curve. C, time course of HBR–GFP fluorescence intensity change at the centre of response during parallel fibre stimulation (60 BSs, red horizontal bar). Mean ± s.e.m. (n = 4). D, range of parallel fibre stimulation. Distribution of average increase in Oregon Green BAPTA-1 fluorescence intensity during parallel fibre stimulation plotted against distance from the centre of response (mean ± s.e.m., n = 4). The continuous curve indicates the best-fit Gaussian curve. E, pseudocolor images of Purkinje cell dendrites showing the fluorescence intensities of Oregon Green BAPTA-1 before and within 3 s after the electric stimulation of parallel fibres.
Electrophysiology
Whole-cell recordings were performed in visually identified Purkinje cells (Edwards et al. 1989). The resistance of patch pipettes was 1.8–3.5 MΩ when filled with an intracellular solution composed of (mm): 130 potassium gluconate, 10 KCl, 10 NaCl, 1 EGTA, 10 Hepes, 4 ATP and 0.4 GTP (pH 7.3, adjusted with KOH). PFs were stimulated as described above. The membrane potential was held constant between −90 and −80 mV for recording the excitatory postsynaptic current (EPSC) of PF synapses, after the compensation of liquid junction potential. Ionic current was recorded using an EPC-9 patch-clamp amplifier (HEKA, Elektronik, Lambrecht/Pfalz, Germany). EPSC signals were filtered at 3 kHz and digitized at 20 kHz. On-line data acquisition and EPSC analysis were performed using the PULSE and PULSE-FIT programs (HEKA), respectively. A train of burst stimulation (BS) was applied under a current-clamp condition. In the experiments on LTP, to monitor the amplitude of PF-EPSC, test pulses were applied to PFs at 0.1 Hz, except for the period of BS. Series resistance (initial value: 6–12 MΩ) was monitored throughout the experiment, and data were discarded when series resistance varied by >10%. In the experiments on the synaptic specificity of LTP (Fig. 5D and E), test pulses were alternately applied using two stimulating electrodes. The intensity of each stimulus was adjusted to evoke PF-EPSCs with an amplitude of 70–120 pA.
Figure 5. NO-dependent LTP at PF–Purkinje cell synapses.
A, LTP was induced by 60 BSs repetitively applied at 1 Hz, but not when the bath solution contained the NOS inhibitor l-NAME (100 μm). Left panel, changes in PF-EPSC amplitude before and after BS (arrow). The amplitude was normalized by the mean value observed for 10 min before BS. Middle panel, sample traces immediately before (1) and 30 min after BS (2). Right panel, average PF-EPSC amplitude during the 21–30 min period after BS. Mean ± s.e.m. (n = 5). B, absence of change in PPR after BS-induced LTP. Left panel, the PPR values 10 min before (○) and 30 min after (•) BS. Ten paired-pulse stimuli were repeatedly applied at 0.2 Hz at each interpulse interval (50, 100 or 200 ms). Mean ± s.e.m. (n = 5). Right panel, typical traces of PF-EPSC evoked by paired-pulse stimulus (100 ms interpulse interval) 10 min before and 30 min after BS. Each trace represents an average of 5–10 sweeps. C, no effect of ODQ on the BS-induced LTP. Left panel, changes in the amplitude of PF-EPSC before and after BS (arrow). The amplitude was normalized by the mean value observed for 10 min before BS. Middle panel, sample traces immediately before (1) and 30 min after (2) BS. Each trace represents average of 5–10 sweeps. Right panel, averaged amplitude of PF-EPSC during the 21–30 min period after BS.Mean ± s.e.m. (n = 5). D, synaptic specificity of LTP induced by 1 Hz BS. Two separate beams of PFs were stimulated using two electrodes (S1 and S2) placed 23–43 μm apart. LTP was observed at S1-stimulated synapses, in which 60 BSs were applied at 1 Hz (arrow), but was absent in S2-stimulated synapses, in which BS was not applied. Mean ± s.e.m. (n = 4). E, the distance between the two electrodes (S1 and S2) was varied, and the extent of LTP at the S2-stimulated synapses was normalized by that at S1 (left panel). The extent of overlap between S1-stimulated and S2-stimulated synapses was estimated by cross facilitation (right panel). The data point at zero distance is the paired-pulse ratio at S1. (Mean ± s.e.m., n = 10). BS was applied only to S1 as in D. Statistical differences were evaluated by student's t-test.
Results
Generation of NO indicator (HBR–GFP)
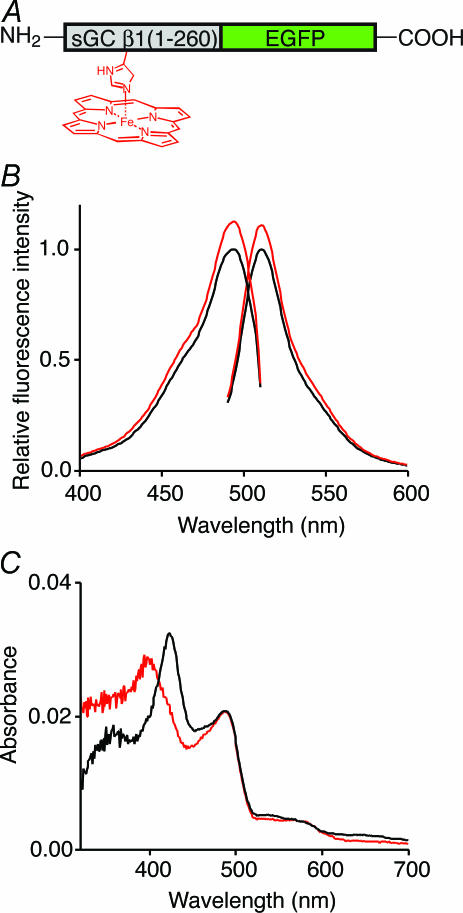
The deletion mutants of the sGC β1 subunit containing residues 80–195 bind heme and NO in the same manner as the native sGC, forming a five-coordinate, high-spin ferrous complex (Namiki et al. 2001). Therefore, we used the heme-binding region of sGC for NO recognition, and generated 39 recombinant proteins in which GFP variants were fused to the N- and/or C- terminus of the heme-binding region of various lengths. Among the NO indicator candidates, the fusion protein between the heme-binding region, which consists of residues 1–260 of the sGC β1 subunit, and EGFP (HBR–GFP, Fig. 1A) showed the most preferable properties.
Figure 1. Spectral properties of HBR–GFP.
A, schematic representation of the structure of HBR–GFP. Heme is bound to histidine 105. B, excitation (emission at 509 nm) and emission (excitation at 480 nm) spectra of HBR–GFP in the absence (black) and presence (red) of 67 μm NOC7. Data were normalized by the peak values of the spectra obtained in the absence of NOC7. C, electronic absorption spectra of HBR–GFP in the absence (black) and presence (red) of 142 μm NOC7. In response to NO application, the heme-derived peak at 423 nm shifted to 399 nm, while there was no effect on the 487 nm absorption peak of EGFP within HBR–GFP.
Both the excitation and emission spectra of HBR–GFP were similar to those of EGFP in the absence of NO, but the fluorescence intensity of HBR–GFP increased upon the addition of a NO donor, NOC7 (Figs 1B and 2A). In response to NO application, the 423 nm heme absorption peak shifted to 399 nm, while the 487 nm absorption peak of EGFP within HBR–GFP did not change (Fig. 1C). This result suggests that intramolecular interaction increases the quantum yield of EGFP fluorescence upon NO binding. The spectrum of HBR–GFP after the addition of NOC7 is characteristic of five-coordinate, high-spin ferrous heme and is the same as those of cytochrome c′ from Alcaligenes (Yoshimura et al. 1986) and native sGC (Stone & Marletta, 1994). Most heme proteins form such a complex only in an anaerobic environment, because oxygen readily binds to the sixth coordination position. However, the heme of HBR–GFP appears to have an unusually low affinity for oxygen in the same manner as that of native sGC (Stone & Marletta, 1994, 1996), indicating that HBR–GFP specifically recognizes NO in the presence of oxygen. Under the same conditions, EGFP showed no changes in fluorescence intensity upon NOC7 addition (Fig. 2A).
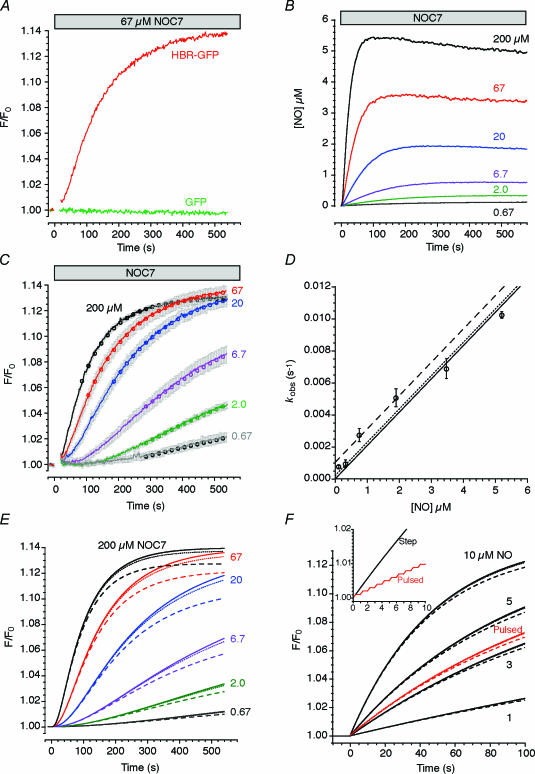
Figure 2. Kinetic properties of HBR–GFP.
A, fluorescence intensity changes of HBR–GFP (red) and EGFP (green) upon NOC7 (67 μm) addition. The measurements were interrupted during NOC7 addition. Representative traces of four and three experiments. B, time courses of NO concentration changes upon addition of NOC7 at various concentrations. Measurement with a NO electrode under the same conditions as in C. C, time courses of fluorescence intensity changes of HBR–GFP at 509 nm with excitation at 480 nm upon addition of NOC7 at various concentrations. Each trace represents mean ± s.e.m. (n = 4). Circles show the best-fit exponential curve for each data segment. D, the rate constants of exponential curves fitted to the data segments of C plotted against plateau NO concentration (mean ± s.d., n = 4). The continuous line shows the best linear fit with a slope of 2.08 × 103m−1s−1. Dotted and dashed lines are parallel to the continuous line, having intercepts of 2 × 10−4 s−1 and 1 × 10−3 s−1, respectively. E, model-based prediction of HBR–GFP fluorescence intensity change in response to NO concentration changes shown in B. koff = 0 or 2 × 10−5 s−1 (continuous lines, which are indistinguishable), 2 × 10−4 s−1 (dotted line) and 1 × 10−3 s−1 (dashed line). F, model-based prediction of HBR–GFP fluorescence intensity change in response to either a stepwise increase (black trace, 1–10 μm) or a train of pulsed increases (red trace, 10 μm, 350 ms pulses at 1 Hz) in NO concentration. koff was varied as in E. Inset, HBR–GFP response to a stepwise increase (black trace) or the train of pulsed increases (red trace) in NO concentration (10 μm) displayed on an expanded time scale. koff = 2 × 10−5 s−1.
Kinetics of HBR–GFP fluorescence intensity change
Figure 2C shows the time courses of the changes in the fluorescence intensity of HBR–GFP upon the addition of NOC7 at various concentrations. The time courses of the concentration of NO liberated from NOC7 were determined in parallel experiments using a NO electrode (Fig. 2B). The NO concentration plateaued at 100–300 s after NOC7 addition. During the plateau phase of NO concentration, the fluorescence intensity of HBR–GFP increased exponentially (a exp[–kobst]+b; circles in Fig. 2C show the best-fit exponential curves). Thus, the reaction scheme for HBR–GFP can be written as follows.
| Scheme (1) |
The on-rate constant (kon) can be obtained from the plot of kobs against the steady-state NO concentration (Fig. 2D). This plot should have a slope of kon and an intercept of koff. From the slope, we estimated kon≈ 2.08 × 103m−1 s−1, while koff seemed to be ∼1 × 10−3 s−1 or lower. We constructed a mathematical model of Scheme (1) (see Methods) and examined its output in response to the NO concentration changes that are shown in Fig. 2B, assuming that the fluorescence intensity of NO HBR–GFP was 14% higher than that of HBR–GFP. We varied the off-rate constant koff between 0 and 1 × 10−3 s−1. Fair agreement between the measured HBR–GFP fluorescence intensity changes (Fig. 2C) and the predicted changes using the model was obtained with koff≤ 2 × 10−4 s−1 (Fig. 2E, solid and broken lines). The predicted time courses seem to level off earlier than the observations when koff = 1 × 10−3 s−1 (Fig. 2E, broken curves). On the basis of these results, we estimated koff to be at most 1 × 10−3 s−1, and more likely less than 2 × 10−4 s−1. Therefore, the unbinding of NO from HBR–GFP seems to be extremely slow. Thus, HBR–GFP is not an instantaneous indicator of NO, but an integrator of NO concentration changes. Using this mathematical model, we estimated the time course of fluorescence intensity change in response to a stepwise increase in NO concentration (Fig. 2F, black traces). These estimations are almost insensitive to the variation in koff up to 100 s after NO addition, and indicate that the rate of increase in HBR–GFP fluorescence intensity depends on the NO concentration. In neurones, NO may be generated as a train of brief spikes in response to intracellular Ca2+ transients during nerve activities. HBR–GFP may be able to integrate such a train of brief NO transients. As shown by the red trace in Fig. 2F, the model indeed responds to a train of 350-ms rectangular NO pulses at 1 Hz with a peak size of 10 μm (time-averaged concentration = 3.5 μm). Therefore, we can estimate the time-averaged concentration of NO using HBR–GFP.
NO imaging in cerebellar Purkinje cells
We then used HBR–GFP to image NO dynamics in cerebellar slices. Sindbis virus particles carrying the gene encoding HBR–GFP were injected into the cerebellar cortex of mice. On the following day, a clear fluorescence signal of HBR–GFP was observed in parasagittal cerebellar slices (Fig. 3A). Most of the fluorescence signals were detected in Purkinje cells, due to the higher affinity of this virus for neurones than for glial cells (Gwag et al. 1998; Ehrengruber et al. 1999; Okubo et al. 2001) and the much larger surface area of Purkinje cells than that of other neurones in the cerebellar cortex (Fig. 3B). The fluorescence signal images of Purkinje cells expressing HBR–GFP in cerebellar slices were obtained at 15 s intervals using a laser-scanning confocal microscope. A homogeneous increase in the fluorescence intensity of Purkinje cell dendrites was observed upon NOC7 addition (Fig. 3C), indicating that HBR–GFP responds to NO within Purkinje cells.
We then examined whether HBR–GFP responds to NO generated by neuronal activities. Burst stimulations (BS, each BS = 5 pulses at 50 Hz), which mimic physiological firing patterns of PFs (Chadderton et al. 2004), were repetitively carried out (1 Hz for 60 s) to stimulate a fine beam of PFs, which run perpendicular to the plane of innervating Purkinje cells. Within 15 s after the onset of the electric stimulation, HBR–GFP fluorescence intensity focally increased beneath the stimulating electrode (Fig. 4A). With continued stimulation, the HBR–GFP response became stronger. Since PF stimulation induces an increase in [Ca2+]i and an acidification within Purkinje cells (Finch & Augustine, 1998; Takechi et al. 1998; Willoughby & Schwiening, 2002), we studied the Ca2+ and pH dependence of HBR–GFP. The fluorescence intensity of HBR–GFP was not sensitive to a wide range of Ca2+ concentrations, and was slightly sensitive to pH around neutral pH (Supplemental Fig. 1). To further clarify the effect of pH, we studied the change in the fluorescence intensity of EGFP expressed in Purkinje cells. There was no detectable change in the EGFP fluorescence intensity during PF stimulation (Supplemental Fig. 2B and C). The PF-induced response of HBR–GFP was abolished when the slices were pretreated with the NO synthase inhibitor NG–nitro-l-arginine (l-NAME, 100 μm) (Supplemental Fig. 2A and C). These results demonstrate that the HBR–GFP response upon electrical stimulation of PFs is due to an increase in NO concentration within Purkinje cells.
The PF-induced change was confined to small areas of dendrites (Fig. 4A). This suggests that the mobility of HBR–GFP is low within Purkinje cell dendrites. We examined this possibility by fluorescence recovery after photobleaching. The average fluorescence intensity recovered at a time constant of 161 ± 45 s (mean ± s.d., n = 4) after photobleaching an area (12.2 ± 3.2 μm2) of a dendrite with intense laser light. The apparent diffusion coefficient and mobile fraction were estimated to be 0.011 ± 0.005 μm2 s−1 and 1.01 ± 0.08 (mean ± s.d., n = 4), respectively. Thus, HBR–GFP diffusion is limited, albeit not completely inhibited within Purkinje cell dendrites. Although the HBR–GFP diffusion is slow, repetitive measurements may be carried out if there is sufficient time for the replenishment of naive HBR–GFP from peripheral regions. Indeed, we were able to observe repetitive responses within the same dendritic area when the same PFs were stimulated at 10–15 min intervals (Supplemental Fig. 3).
To examine whether NO concentration is buffered by expressed HBR–GFP within Purkinje cells, we analysed the correlation between the peak fluorescence intensity change (ΔF/F0) elicited by the 60 s PF stimulation, and HBR–GFP expression level estimated from the initial fluorescence intensity (F0). No significant correlation was found between ΔF/F0 and F0 (correlation coefficient = 0.04, n = 22). Thus, at the present HBR–GFP expression levels, NO buffering does not pose a problem.
Spatial distribution of NO signalling at the PF–Purkinje cell synapse
We then studied the spatial distribution of NO signals upon PF stimulation. Concentric circles with radii of multiples of 2.5 μm were placed on the images around the centre of response. The fluorescence intensities of dendrites were averaged within the innermost circle or within each doughnut-like region between the two adjacent concentric circles at the end of the 60 s electric stimulation. The averaging was carried out only within the area occupied by dendrites. Results from five experiments were averaged, and the spatial distribution of HBR–GFP responses within dendrites could be fitted by a Gaussian curve of the half peak width (diameter) of <10 μm (Fig. 4B). The F/F0 within the innermost circle (radius of 2.5 μm) increased with the duration of PF stimulation reaching ∼1.06 after the 60 s stimulation (60 BS at 1 Hz, Fig. 4C).
We then compared the spatial distribution of NO signals with that of electrically stimulated synapses. PF–Purkinje cell synaptic transmission causes a Ca2+ concentration increase in postsynapses (Finch & Augustine, 1998; Takechi et al. 1998). A fluorescent Ca2+ indicator was introduced into Purkinje cells via a patch pipette and the PF-induced increase in Ca2+ concentration was analysed (Fig. 4E). The spatial distribution of Ca2+ responses could be fitted by a Gaussian curve of the half peak width of <10 μm (Fig. 4D). This distribution of synaptically evoked Ca2+ signals was similar to that of NO signals.
Property and spatial distribution of NO-dependent synaptic plasticity
It has been shown that either the application of a NO donor to cerebellar slices, or the uncaging of a caged NO inside Purkinje cells produces LTP of PF–Purkinje cell synapses (Lev-Ram et al. 2002). To study whether the NO release from PFs similarly induces synaptic plasticity, we examined the effect of PF stimulation on PF–Purkinje cell transmission. After 60 BSs repeated at 1 Hz, the amplitude of EPSCs of PF synapses was indeed markedly potentiated and maintained stable at ∼200% of the prestimulation level for at least 30 min (Fig. 5A, black symbols). The 1 Hz BS LTP was NO dependent, because it was not induced in the presence of the NOS inhibitor l-NAME (Fig. 5A, red symbols).
It has been shown that single-pulse stimulation of PF at 1 Hz for 5 min generates LTP (1 Hz SP LTP) (Lev-Ram et al. 2002; Coesmans et al. 2004). Because 1 Hz SP LTP is indicated to be also NO dependent (Lev-Ram et al. 2002; Coesmans et al. 2004), we further characterized the property of the 1 Hz BS LTP, and compared it with that of the previously reported 1 Hz SP LTP. Lev-Ram et al. (2002) indicated that 1 Hz SP LTP is postsynaptic in origin, because it was not associated with a change in paired-pulse ratio (PPR), an index for the change in release probability at presynaptic terminals (Zucker & Regehr, 2002). In addition, 1H-(1,2,4)oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), an inhibitor of sGC, did not prevent 1 Hz SP LTP in the previous study (Lev-Ram et al. 2002), whereas LTD was significantly blocked by ODQ (Lev-Ram et al. 1997). To elucidate the locus of the BS-induced LTP observed in this study, we examined whether the PPR of PF-EPSCs was changed after the BS. As shown in Fig. 5B, the LTP induced by 60 BSs at 1 Hz was not accompanied by a significant change in PPR at any interpulse intervals examined. The result indicates that the locus of the 1 Hz BS LTP in this study is also postsynaptic. To examine the involvement of sGC in the BS-induced LTP, ODQ (1 μm) was applied to the bathing solution from 1 h before the BS. Application of ODQ did not affect the basic transmission properties of the PF synapse (data not shown). Furthermore, ODQ had no effect on 1 Hz BS LTP (Fig. 5C), suggesting that activation of sGC by NO is not essential for the BS-induced LTP. Although the stimulation pulse protocols and durations are not the same between the two forms (1 Hz SP and 1 Hz BS) of LTP, they share the same characteristics in terms of their NO requirement, ODQ insensitivity and postsynaptic expression, and may utilize the same downstream mechanisms.
We next studied the synaptic specificity of 1 Hz BS LTP. Two stimulating electrodes (S1 and S2) were closely placed to alternately stimulate two separate beams of PFs innervating the same Purkinje cell for EPSC measurements. The degree of overlap of the two inputs was estimated by the cross facilitation, that is paired-pulse facilitation of two successive stimuli from different electrodes coupled in a 50 ms interval (Nishiyama et al. 2000) (Fig. 5E, right). The cross facilitation decreased with increasing distance between S1 and S2, and there was no cross facilitation (no functional overlap) between S1 and S2 when the two electrodes were separated by 23–43 μm. Under those conditions, after the application of BSs at 1 Hz for 60 s to one of the electrodes (S1), LTP was observed in the synapses stimulated by the S1 electrode but not in the synapses stimulated by the S2 electrode (Fig. 5D). With shorter S1–S2 distance (<20 μm), the magnitude of LTP increased in a similar manner to the cross facilitation of pared inputs (Fig. 5E, left). Thus, the NO-dependent LTP is confined to the synapses that received 1 Hz BS.
Dependence of NO signalling on PF activity patterns
We next examined how NO release depends on the frequency of PF stimulation. Because PFs show intrinsic frequency of firing in response to sensory stimulation (Chadderton et al. 2004), we altered the interval of repetitive application of BSs rather than the interval between each pulse. When 60 BSs were applied at 0.25 Hz, there was only a very small change in the HBR–GFP signal (Fig. 6A). The same number of BSs at 0.5 Hz resulted in a small increase in NO concentration. NO release was further increased when 60 BSs were repetitively applied at 1 Hz (Fig. 6A). Unexpectedly, the NO release was decreased when the BS frequency exceeded 1 Hz, and the NO release was greatly reduced at 4 Hz (Fig. 6A). Thus, NO release at PF synapses had a bell-shaped BS frequency dependence with an optimal frequency at 1 Hz (Fig. 6B). The failure in NO release by 4 Hz BS was not due to the insufficient duration of stimulation, because 240 BSs applied at 4 Hz for 60 s did not elicit a significant NO signal (data not shown).
Figure 6. Bell-shaped frequency dependence of NO release.
A, time courses of NO signal intensity changes at the PF–Purkinje cell synapse in response to 60 BSs applied at 0.25, 1 or 4 Hz. Representative result of five experiments. B, peak values of NO release after 60 BSs applied at different frequencies. Mean ± s.e.m. (n = 5). C, increases in intracellular Ca2+ concentration within the fine dendrites of Purkinje cells at the end of 60 BSs at various frequencies (n = 7).
We then determined whether the strength of the postsynaptic response to the synaptic transmission also depends similarly on the frequency. Since the glutamatergic transmission at this synapse generates postsynaptic Ca2+ signals, we measured the intracellular Ca2+ concentration in fine dendrites of the Purkinje cells at the end of 60 BSs applied at different frequencies. Unlike the NO signal intensity, the postsynaptic Ca2+ signal intensity increased with the frequency (Fig. 6C). This result emphasizes the unique property of the NO signalling.
Because nNOS activity is dependent on the intracellular Ca2+ concentration, a decrease in Ca2+ influx through the voltage-dependent Ca2+ channel (VDCC) at the PF terminals may underlie the reduction in NO release at higher BS frequencies. Thus, we examined NO release at an increased extracellular Ca2+ concentration to enhance Ca2+ influx via the presynaptic VDCC. Indeed, NO release induced by 4 Hz BS was significantly potentiated in 8 mm Ca2+ compared to 2 mm Ca2+ (Fig. 7A). On the other hand, there was no significant effect of increasing Ca2+ on NO release by 1 Hz BS (Fig. 7A), indicating that the level of NO release induced by 1 Hz BS was saturated at 2 mm Ca2+. These results suggest that the decrease in NO release at higher frequencies of stimulation is due to a decrease in Ca2+ influx at the presynaptic terminals. We examined possible involvement of the NMDA receptor in the NO signalling, using an NMDA antagonist, 3-((R)-2-carboxypiperazine-4-yl)-propyl-1-phosphonic acid (R-CPP). No effect of 10 μm R-CPP was observed on the peak F/F0 of HBR–GFP after 60 BSs at 1 Hz (control: 1.064 ± 0.006, n = 5; R-CPP: 1.064 ± 0.008, n = 5).
Figure 7. Mechanism of inhibition of NO release by 4 Hz BS.
A, effects of extracellular Ca2+ (2 mm and 8 mm) on NO release in response to 60 BSs repetitively applied at 1 Hz (open bars) and 4 Hz (filled bars). B, effects on the BS-induced NO release of specific antagonists of type I mGluR (CPCCOEt, 100 μm). C and D, peak values of NO release in response to 60 BSs applied at 1 Hz and 4 Hz in the absence and presence of the CB1 receptor antagonist AM281 (2 μm) or CB1 receptor agonist WIN55,212-1 (5 μm). Mean ± s.e.m. (n = 5).
The metabotropic glutamate receptor (mGluR) is activated by high-frequency synaptic inputs (Batchelor et al. 1994; Tempia et al. 1998). Type II and III mGluRs are localized at the PF terminal, and are suggested to mediate the inhibition of transmitter release (Kreitzer & Regehr, 2001). Thus, possible involvement of mGluR in the frequency-dependent inhibition of NO release was examined. Indeed, NO release in response to 60 BSs at 4 Hz was rescued in the presence of a broad-spectrum antagonist of mGluR, MCPG, but the inhibition was not relieved by either an antagonist of either type II mGluRs (LY341495) or type III mGluRs (UPB1112) (Supplemental Fig. 4). On the other hand, a specific antagonist of type I mGluRs (CPCCOEt) rescued NO release by 4 Hz BS (Fig. 7B). The type I mGluR is not localized at the presynaptic terminal but at the postsynaptic site of the PF–Purkinje cell synapse (Baude et al. 1993; Lujan et al. 1997). How then does activation of the type I mGluR in the postsynapse inhibit the presynaptic NO release?
Activation of type I mGluRs in Purkinje cells induces secretion of endocannabinoid (Maejima et al. 2001), and activation of the cannabinoid (CB1) receptor localized at the PF terminals reduces the Ca2+ influx at presynaptic terminals (Brown et al. 2004). Thus, possible involvement of the CB1 receptor was tested. In the presence of the CB1 receptor antagonist AM281, 4 Hz BS-induced NO release was significantly potentiated, although no effect was found during 1 Hz BS (Fig. 7C). Taken together, these results suggest that endocannabinoid is released during high-frequency stimulation and retrogradely inhibits the NOS activity at the PF terminal, probably due to inhibition of the VDCC. In accordance with this notion, the release of NO induced by 1 Hz BS was virtually abolished by the CB1 receptor agonist WIN 55,212-2 (Fig. 7D).
Impact of frequency-dependent NO release on synaptic function
We next examined the relationship between LTP and the frequency dependence of NO production. After 60 BSs repetitively applied either at 0.25 Hz or 4 Hz, where no significant NO production is observed, no LTP was induced (Fig. 8A and B, black symbols). Since NO generation by 4 Hz BS was rescued by the cannabinoid antagonist, we examined the effect of 4 Hz BS on the synaptic transmission in the presence of AM281. Indeed, LTP was induced by 4 Hz BS in the presence of the CB1 receptor antagonist (Fig. 8B and C, red symbols). This rescued LTP was inhibited by the NOS inhibitor, l-NAME (Supplemental Fig. 5). These results indicate that NO release is necessary for the LTP induction, and the optimal frequency of PF stimulation for inducing LTP (Fig. 8C) was determined by the biphasic dependence of NO release on the frequency of BS (Fig. 6B).
Figure 8. Dependence of LTP on frequency of PF stimulation.
A, absence of LTP after 60 BSs repetitively applied at 0.25 Hz. Mean ± s.e.m. (n = 4). B, LTP was not induced after 60 BSs applied at 4 Hz, but was rescued in the presence of the CB1 receptor antagonist AM281 (2 μm). Right panel, averaged amplitudes of PF-EPSC during 21–30 min after 4 Hz BS in the absence and presence of AM281. Mean ± s.e.m. (n = 5). C, summary of the BS frequency dependence of LTP. Mean ± s.e.m. (n = 4–5).
Discussion
Using a novel genetically encoded fluorescent NO indicator (HBR–GFP), we succeeded in observing local NO signalling and its dependence on the neuronal firing frequency at the PF–Purkinje cell synapse in brain slices. We showed that the NO signalling is concentrated at the active synapses but decays rapidly with the distance from the activated synapse in a similar manner to that of postsynaptic increase in intracellular Ca2+ concentration (Fig. 4B and D). We also showed that the NO signalling induced synapse-specific LTP at the PF synapse, which seems to be postsynaptic and sGC independent (Fig. 5). Interestingly, NO signalling had a biphasic dependence on the frequency of PF activity (Fig. 6B). The high-frequency inhibition of NO signalling was mediated by the cannabinoid receptor-dependent retrograde signalling. The frequency-dependent NO production offers a clear explanation for the characteristic frequency required for LTP generation at this synapse (Fig. 8C). Thus, the present study provided important new insights into the dynamics and the role of NO in the regulation of synaptic functions.
While the absorption spectra of HBR–GFP suggest very specific NO binding by the indicator (Fig. 1B), the off-rate constant of NO from HBR–GFP is extremely low (Fig. 2D), and it makes HBR–GFP an integrator of the NO signal rather than an instantaneous indicator of NO. HBR–GFP's parent molecule sGC (a heterodimer of α and β subunits) has a NO dissociation rate constant of ∼0.006 s−1, which increases to ∼0.2 s−1 in the presence of the enzyme's substrate MgGTP (Kharitonov et al. 1997; but see also Brandish et al. 1998). The deactivation kinetics of sGC activity within cells was reported to be even faster (3.7 s−1) (Bellamy & Garthwaite, 2001). Therefore, the parts of the sGC molecule outside HBR and possibly some additional cytosolic molecules are required for the fast kinetics of NO-dependent regulation of sGC activity. It has been shown that sGC makes an interaction with cell membranes or with other proteins (Zabel et al. 2002; Venema et al. 2003). It seems possible that HBR–GFP made similar interactions with intracellular components, and that such interactions might have altered the NO sensitivity of HBR–GFP and contributed to the slow diffusion of HBR–GFP in Purkinje cells. The elucidation of such a mechanism may help alter the kinetic property of HBR-based NO indicators in the future. HBR–GFP is a good starting point for such alteration, and is indeed the first useful indicator for detecting NO signals at the synaptic terminals.
We electrically stimulated the PFs with a pulse protocol (BS, five pulses at 50 Hz) that is assumed to be within the physiological range of PF activity (Takechi et al. 1998; Chadderton et al. 2004). Ca2+ measurements in PFs showed that each action potential generates a 300 nm peak increase in Ca2+ concentration with a decay time constant of 150 ms, and that the Ca2+ transients due to a train of action potentials summate linearly at least up to 10 pulses (Regehr & Atluri, 1995). Our pulse protocol, then, generates a peak Ca2+ concentration of ∼1.2 μm at ∼80 ms after the initiation of each five-pulse sequence, and decays with a time constant of 150 ms. Therefore, the Ca2+ concentration at the presynaptic terminal is elevated above 200 nm for ∼350 ms for each BS. The Km of Ca2+ for the activation of nNOS is ∼200 nm (Bredt & Snyder, 1990; Lee & Stull, 1998). The deactivation kinetics of nNOS upon withdrawal of Ca2+ are rapid, having a rate constant >10 s−1 (Persechini et al. 1996). The apparent on-rate constant should also be rapid at or above 200 nm Ca2+. These kinetic parameters suggest that the duration of nNOS activity should fairly closely follow that of the Ca2+ transients. These considerations suggest that NO was generated at the presynaptic terminals as a train of brief (a few hundred milliseconds' duration) transients at 1 Hz during our standard electrical stimulation protocol (1 Hz BS). HBR–GFP should be able to detect the NO signal during such a train of transients (Fig. 2F, red trace). The time course of F/F0 of HBR–GFP within Purkinje cells reached ∼1.06 after 60 s of 1 Hz BS. This indicates, based on the model calibration, that the average NO concentration during the PF stimulation at the centre of response was of the order of ∼5 μm. This NO concentration is higher than that required for the activation of sGC (see below), but may be required for the sGC-independent reaction of 1 Hz BS LTP.
Recent measurements of NO sensitivity of sGC indicate a Km of ∼1.7 nm (in vitro conditions) to ∼10 nm (in cellular context) (Mo et al. 2004). Although our measurement showed that the NO signal intensity sharply decays with the distance from active synapses, HBR–GFP does not effectively detect NO signals at the lower nanomolar range, and it is possible that the synaptic NO release may induce cGMP signals at a certain distance from the active synapses. The spreading distance of cGMP signalling should depend on the diffusion constant and lifetime of NO, as well as the activity of phosphodiesterase that inactivates cGMP within Purkinje cells. Intracellular cGMP imaging (Hartell et al. 2001; Honda et al. 2001) at the level of fine dendrites may elucidate this issue.
We found a bell-shaped dependence of NO release on the frequency of burst-like PF activity. This appears to be at variance with the result of Shibuki & Kimura (1997), who found, using a NO electrode, a monotonic increase in NO release from PFs with the stimulation frequency. However, the previous authors did not use BS, but equally spaced pulses, and the highest stimulation frequency was 20 Hz for 5 s. Although direct comparison between the two studies is not possible, it seems possible that higher frequency single-pulse stimulation was required to observe the inhibitory phase. We then looked into the mechanism of high-frequency BS-induced inhibition of NO release. Our results indicate that endocannabinoid is generated postsynaptically in response to the high-frequency PF inputs, and sends a retrograde signal to induce CB1 receptor-mediated inhibition of presynaptic Ca2+ influx via VDCCs. There was a rapid decline in EPSPs during the train of BSs at 4 Hz, while the EPSP amplitude was maintained at 4 Hz in the presence of the CB1 receptor antagonist (AM281) or at 1 Hz (unpublished observation). Therefore, our results suggest that CB1 receptor-mediated inhibition of the VDCC resulting in the reduction of the presynaptic Ca2+ transient amplitude decreases nNOS activation. Indeed, it has been shown that PF stimulation at 50 Hz (3–10 pulses) reduces presynaptic Ca2+ transients via the CB1 receptor (Brown et al. 2003). At present, one cannot exclude the possibility that there is an additional mechanism that inhibits nNOS activity downstream of the CB1 receptor.
It is known that certain frequencies of synaptic activities are optimal for the generation of synaptic plasticity. However, a clear explanation of the optimal frequency requirement has not been available, because signal intensity should increase at the postsynapse with an increase in the frequency of activity. Indeed, postsynaptic Ca2+ concentration during synaptic transmission increased progressively with the frequency (Fig. 6C). Our results, indicating that NO is generated optimally at 1 Hz BS, provided a straightforward explanation for the characteristic frequency required for the generation of NO-dependent LTP at the PF–Purkinje cell synapse. Purkinje cells receive two excitatory inputs, one from PFs and another from climbing fibres. Conjunctive stimulation of the two inputs at 1 Hz for 5 min generates Hebbian LTD (Karachot et al. 1994), which has been implicated in several forms of motor learning (Ito, 2001). Single-pulse stimulation of PFs at 1 Hz for 5 min without stimulation of climbing fibres generated 1 Hz SP LTP (Lev-Ram et al. 2002). Although the stimulation pulse protocol of 1 Hz SP LTP is not the same as that of 1 Hz BS LTP, these two forms of LTP share similar characteristics in terms of the requirement of NO, postsynaptic expression and resistance to the inhibitor of sGC (ODQ), and may employ the same downstream mechanism. The 1 Hz SP LTP has been proposed to be the mechanism that extinguishes the Hebbian LTD (Lev-Ram et al. 2002). Our results, indicating that NO signalling at PF synapse has a bell-shaped frequency dependence, imply that specific patterns of PF activity are required for NO-dependent LTP and possibly for extinguishing the LTD. Further studies are required to clarify the relationship between the 1 Hz BS LTP and the extinguishing mechanism of LTD.
Supplementary Material
Acknowledgments
This work was supported by Grants in Aid for Scientific Research and partly by the Advanced and Innovational Research Program in Life Science from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- Batchelor AM, Madge DJ, Garthwaite J. Synaptic activation of metabotropic glutamate receptors in the parallel fibre–Purkinje cell pathway in rat cerebellar slices. Neuroscience. 1994;63:911–915. doi: 10.1016/0306-4522(94)90558-4. [DOI] [PubMed] [Google Scholar]
- Baude A, Nusser Z, Roberts JD, Mulvihill E, McIlhinney RA, Somogyi P. The metabotropic glutamate receptor (mGluR1 a) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. doi: 10.1016/0896-6273(93)90086-7. [DOI] [PubMed] [Google Scholar]
- Bellamy TC, Garthwaite J. Sub-second kinetics of the nitric oxide receptor, soluble guanylyl cyclase, in intact cerebellar cells. J Biol Chem. 2001;276:4287–4292. doi: 10.1074/jbc.M006677200. [DOI] [PubMed] [Google Scholar]
- Brandish PE, Buechler W, Marletta MA. Regeneration of the ferrous heme of soluble guanylate cyclase from the nitric oxide complex: acceleration by thiols and oxyhemoglobin. Biochemistry. 1998;37:16898–16907. doi: 10.1021/bi9814989. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- Brown SP, Brenowitz SD, Regehr WG. Brief presynaptic bursts evoke synapse-specific retrograde inhibition mediated by endogenous cannabinoids. Nat Neurosci. 2003;6:1048–1057. doi: 10.1038/nn1126. [DOI] [PubMed] [Google Scholar]
- Brown SP, Safo PK, Regehr WG. Endocannabinoids inhibit transmission at granule cell to Purkinje cell synapses by modulating three types of presynaptic calcium channels. J Neurosci. 2004;24:5623–5631. doi: 10.1523/JNEUROSCI.0918-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadderton P, Margrie TW, Hausser M. Integration of quanta in cerebellar granule cells during sensory processing. Nature. 2004;428:856–860. doi: 10.1038/nature02442. [DOI] [PubMed] [Google Scholar]
- Coesmans M, Weber JT, De Zeeuw CI, Hansel C. Bidirectional parallel fiber plasticity in the cerebellum under climbing fiber control. Neuron. 2004;44:691–700. doi: 10.1016/j.neuron.2004.10.031. [DOI] [PubMed] [Google Scholar]
- Daniel H, Levenes C, Crepel F. Cellular mechanisms of cerebellar LTD. Trends Neurosci. 1998;21:401–407. doi: 10.1016/s0166-2236(98)01304-6. [DOI] [PubMed] [Google Scholar]
- Edwards FA, Konnerth A, Sakmann B, Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989;414:600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Ehrengruber MU, Lundstrom K, Schweitzer C, Heuss C, Schlesinger S, Gahwiler BH. Recombinant Semliki Forest virus and Sindbis virus efficiently infect neurons in hippocampal slice cultures. Proc Natl Acad Sci U S A. 1999;96:7041–7046. doi: 10.1073/pnas.96.12.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch EA, Augustine GJ. Local calcium signalling by inositol-1,4,5-trisphosphate in Purkinje cell dendrites. Nature. 1998;396:753–756. doi: 10.1038/25541. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Boulton CL. Nitric oxide signaling in the central nervous system. Annu Rev Physiol. 1995;57:683–706. doi: 10.1146/annurev.ph.57.030195.003343. [DOI] [PubMed] [Google Scholar]
- Gwag BJ, Kim EY, Ryu BR, Won SJ, Ko HW, Oh YJ, Cho YG, Ha SJ, Sung YC. A neuron-specific gene transfer by a recombinant defective Sindbis virus. Brain Res Mol Brain Res. 1998;63:53–61. doi: 10.1016/s0169-328x(98)00251-4. [DOI] [PubMed] [Google Scholar]
- Haley JE. Gases as neurotransmitters. Essays Biochem. 1998;33:79–91. doi: 10.1042/bse0330079. [DOI] [PubMed] [Google Scholar]
- Hartell NA, Furuya S, Jacoby S, Okada D. Intercellular action of nitric oxide increases cGMP in cerebellar Purkinje cells. Neuroreport. 2001;12:25–28. doi: 10.1097/00001756-200101220-00013. [DOI] [PubMed] [Google Scholar]
- Honda A, Adams SR, Sawyer CL, Lev-Ram V, Tsien RY, Dostmann WR. Spatiotemporal dynamics of guanosine 3′,5′-cyclic monophosphate revealed by a genetically encoded, fluorescent indicator. Proc Natl Acad Sci U S A. 2001;98:2437–2442. doi: 10.1073/pnas.051631298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro LJ, Kadowitz PJ. The pharmacological and physiological role of cyclic GMP in vascular smooth muscle relaxation. Annu Rev Pharmacol Toxicol. 1985;25:171–191. doi: 10.1146/annurev.pa.25.040185.001131. [DOI] [PubMed] [Google Scholar]
- Ito M. Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiol Rev. 2001;81:1143–1195. doi: 10.1152/physrev.2001.81.3.1143. [DOI] [PubMed] [Google Scholar]
- Jacoby S, Sims RE, Hartell NA. Nitric oxide is required for the induction and heterosynaptic spread of long-term potentiation in rat cerebellar slices. J Physiol. 2001;535:825–839. doi: 10.1111/j.1469-7793.2001.t01-1-00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakizawa S, Yamada K, Iino M, Watanabe M, Kano M. Effects of insulin-like growth factor I on climbing fibre synapse elimination during cerebellar development. Eur J Neurosci. 2003;17:545–554. doi: 10.1046/j.1460-9568.2003.02486.x. [DOI] [PubMed] [Google Scholar]
- Kakizawa S, Yamasaki M, Watanabe M, Kano M. Critical period for activity-dependent synapse elimination in developing cerebellum. J Neurosci. 2000;20:4954–4961. doi: 10.1523/JNEUROSCI.20-13-04954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karachot L, Kado RT, Ito M. Stimulus parameters for induction of long-term depression in in vitro rat Purkinje cells. Neurosci Res. 1994;21:161–168. doi: 10.1016/0168-0102(94)90158-9. [DOI] [PubMed] [Google Scholar]
- Kharitonov VG, Sharma VS, Magde D, Koesling D. Kinetics of nitric oxide dissociation from five- and six-coordinate nitrosyl hemes and heme proteins, including soluble guanylate cyclase. Biochemistry. 1997;36:6814–6818. doi: 10.1021/bi970201o. [DOI] [PubMed] [Google Scholar]
- Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Nagano T. Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal Chem. 1998;70:2446–2453. doi: 10.1021/ac9801723. [DOI] [PubMed] [Google Scholar]
- Konnerth A, Llano I, Armstrong CM. Synaptic currents in cerebellar Purkinje cells. Proc Natl Acad Sci U S A. 1990;87:2662–2665. doi: 10.1073/pnas.87.7.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Lancaster JR., Jr Simulation of the diffusion and reaction of endogenously produced nitric oxide. Proc Natl Acad Sci U S A. 1994;91:8137–8141. doi: 10.1073/pnas.91.17.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Stull JT. Calmodulin-dependent regulation of inducible and neuronal nitric-oxide synthase. J Biol Chem. 1998;273:27430–27437. doi: 10.1074/jbc.273.42.27430. [DOI] [PubMed] [Google Scholar]
- Lev-Ram V, Makings LR, Keitz PF, Kao JP, Tsien RY. Long-term depression in cerebellar Purkinje neurons results from coincidence of nitric oxide and depolarization-induced Ca2+ transients. Neuron. 1995;15:407–415. doi: 10.1016/0896-6273(95)90044-6. [DOI] [PubMed] [Google Scholar]
- Lev-Ram V, Nebyelul Z, Ellisman MH, Huang PL, Tsien RY. Absence of cerebellar long-term depression in mice lacking neuronal nitric oxide synthase. Learn Mem. 1997;4:169–177. doi: 10.1101/lm.4.1.169. [DOI] [PubMed] [Google Scholar]
- Lev-Ram V, Wong ST, Storm DR, Tsien RY. A new form of cerebellar long-term potentiation is postsynaptic and depends on nitric oxide but not cAMP. Proc Natl Acad Sci U S A. 2002;99:8389–8393. doi: 10.1073/pnas.122206399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan R, Roberts JD, Shigemoto R, Ohishi H, Somogyi P. Differential plasma membrane distribution of metabotropic glutamate receptors mGluR1 a, mGluR2 and mGluR5, relative to neurotransmitter release sites. J Chem Neuroanat. 1997;13:219–241. doi: 10.1016/s0891-0618(97)00051-3. [DOI] [PubMed] [Google Scholar]
- McGee AW, Topinka JR, Hashimoto K, Petralia RS, Kakizawa S, Kauer F, Aguilera-Moreno A, Wenthold RJ, Kano M, Bredt DS. PSD-93 knock-out mice reveal that neuronal MAGUKs are not required for development or function of parallel fiber synapses in cerebellum. J Neurosci. 2001;21:3085–3091. doi: 10.1523/JNEUROSCI.21-09-03085.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima T, Hashimoto K, Yoshida T, Aiba A, Kano M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron. 2001;31:463–475. doi: 10.1016/s0896-6273(01)00375-0. [DOI] [PubMed] [Google Scholar]
- Mo E, Amin H, Bianco IH, Garthwaite J. Kinetics of a cellular nitric oxide/cGMP/phosphodiesterase-5 pathway. J Biol Chem. 2004;279:26149–26158. doi: 10.1074/jbc.M400916200. [DOI] [PubMed] [Google Scholar]
- Namiki S, Hirose K, Iino M. Mapping of heme-binding domains in soluble guanylyl cyclase β1 subunit. Biochem Biophys Res Commun. 2001;288:798–804. doi: 10.1006/bbrc.2001.5836. [DOI] [PubMed] [Google Scholar]
- Nishiyama M, Hong K, Mikoshiba K, Poo MM, Kato K. Calcium stores regulate the polarity and input specificity of synaptic modification. Nature. 2000;408:584–588. doi: 10.1038/35046067. [DOI] [PubMed] [Google Scholar]
- Okubo Y, Kakizawa S, Hirose K, Iino M. Visualization of IP3 dynamics reveals a novel AMPA receptor-triggered IP3 production pathway mediated by voltage-dependent Ca2+ influx in Purkinje cells. Neuron. 2001;32:113–122. doi: 10.1016/s0896-6273(01)00464-0. [DOI] [PubMed] [Google Scholar]
- Persechini A, White HD, Gansz KJ. Different mechanisms for Ca2+ dissociation from complexes of calmodulin with nitric oxide synthase or myosin light chain kinase. J Biol Chem. 1996;271:62–67. doi: 10.1074/jbc.271.1.62. [DOI] [PubMed] [Google Scholar]
- Prast H, Philippu A. Nitric oxide as modulator of neuronal function. Prog Neurobiol. 2001;64:51–68. doi: 10.1016/s0301-0082(00)00044-7. [DOI] [PubMed] [Google Scholar]
- Regehr WG, Atluri PP. Calcium transients in cerebellar granule cell presynaptic terminals. Biophys J. 1995;68:2156–2170. doi: 10.1016/S0006-3495(95)80398-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuman EM, Madison DV. A requirement for the intercellular messenger nitric oxide in long-term potentiation. Science. 1991;254:1503–1506. doi: 10.1126/science.1720572. [DOI] [PubMed] [Google Scholar]
- Schweighofer N, Ferriol G. Diffusion of nitric oxide can facilitate cerebellar learning: a simulation study. Proc Natl Acad Sci U S A. 2000;97:10661–10665. doi: 10.1073/pnas.97.19.10661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuki K. An electrochemical microprobe for detecting nitric oxide release in brain tissue. Neurosci Res. 1990;9:69–76. doi: 10.1016/0168-0102(90)90048-j. [DOI] [PubMed] [Google Scholar]
- Shibuki K, Kimura S. Dynamic properties of nitric oxide release from parallel fibres in rat cerebellar slices. J Physiol. 1997;498:443–452. doi: 10.1113/jphysiol.1997.sp021870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuki K, Okada D. Endogenous nitric oxide release required for long-term synaptic depression in the cerebellum. Nature. 1991;349:326–328. doi: 10.1038/349326a0. [DOI] [PubMed] [Google Scholar]
- Snyder SH. Nitric oxide: first in a new class of neurotransmitters. Science. 1992;257:494–496. doi: 10.1126/science.1353273. [DOI] [PubMed] [Google Scholar]
- Stone JR, Marletta MA. Soluble guanylate cyclase from bovine lung: activation with nitric oxide and carbon monoxide and spectral characterization of the ferrous and ferric states. Biochemistry. 1994;33:5636–5640. doi: 10.1021/bi00184a036. [DOI] [PubMed] [Google Scholar]
- Stone JR, Marletta MA. Spectral and kinetic studies on the activation of soluble guanylate cyclase by nitric oxide. Biochemistry. 1996;35:1093–1099. doi: 10.1021/bi9519718. [DOI] [PubMed] [Google Scholar]
- Takechi H, Eilers J, Konnerth A. A new class of synaptic response involving calcium release in dendritic spines. Nature. 1998;396:757–760. doi: 10.1038/25547. [DOI] [PubMed] [Google Scholar]
- Tempia F, Miniaci MC, Anchisi D, Strata P. Postsynaptic current mediated by metabotropic glutamate receptors in cerebellar Purkinje cells. J Neurophysiol. 1998;80:520–528. doi: 10.1152/jn.1998.80.2.520. [DOI] [PubMed] [Google Scholar]
- Traylor TG, Sharma VS. Why NO. Biochemistry. 1992;31:2847. doi: 10.1021/bi00126a001. [DOI] [PubMed] [Google Scholar]
- Venema RC, Venema VJ, Ju H, Harris MB, Snead C, Jilling T, Dimitropoulou C, Maragoudakis ME, Catravas JD. Novel complexes of guanylate cyclase with heat shock protein 90 and nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2003;285:H669–H678. doi: 10.1152/ajpheart.01025.2002. [DOI] [PubMed] [Google Scholar]
- Willoughby D, Schwiening CJ. Electrically evoked dendritic pH transients in rat cerebellar Purkinje cells. J Physiol. 2002;544:487–499. doi: 10.1113/jphysiol.2002.027508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J, Garthwaite J. Models of the diffusional spread of nitric oxide: implications for neural nitric oxide signalling and its pharmacological properties. Neuropharmacology. 1994;33:1235–1244. doi: 10.1016/0028-3908(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Suzuki S, Nakahara A, Iwasaki H, Masuko M, Matsubara T. Spectral properties of nitric oxide complexes of cytochrome c' from Alcaligenes sp. NCIB 11015. Biochemistry. 1986;25:2436–2442. [Google Scholar]
- Zabel U, Kleinschnitz C, Oh P, Nedvetsky P, Smolenski A, Muller H, Kronich P, Kugler P, Walter U, Schnitzer JE, Schmidt HH. Calcium-dependent membrane association sensitizes soluble guanylyl cyclase to nitric oxide. Nat Cell Biol. 2002;4:307–311. doi: 10.1038/ncb775. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.