Abstract
The central cholinergic system plays a crucial role in synaptic plasticity and spatial attention; however, the roles of the individual cholinergic receptors involved in these activities are not well understood at present. In the present study, we show that acetylcholine (ACh) can facilitate or depress synaptic transmission in occipital slices of mouse visual cortex. The precise nature of the ACh effects depends on the ACh concentration, and is input specific, as shown by stimulating different synaptic pathways. Pharmacological blockade of muscarinic receptor (mAChR) subtypes and the use of M1–M5 mAChR-deficient mice showed that specific mAChR subtypes, together with the activity of the cholinesterases (ChEs), mediate facilitation or depression of synaptic transmission. The present data suggest that local ACh, acting through mAChRs, regulates the cortical dynamics making cortical circuits respond to specific stimuli.
Cholinergic transmission plays an essential role in the modulation of memory, attention and neuronal plasticity in the CNS (Everitt & Robbins, 1997; Sarter & Bruno, 1997; Segal & Auerbach, 1997; Kirkwood et al. 1999; Linster et al. 2003; Warburton et al. 2003). Acetylcholine (ACh) in the cerebral cortex serves as a neuromodulator rather than as a classical neurotransmitter (Krnjevic, 2004). Indeed, in sensory cortices, ACh influences cortical neurones by modulating responses to sensory inputs. In the visual cortex of cats in vivo, iontophoretic application of ACh induces changes of cell responses to visual stimuli (Sillito & Kemp, 1983; Sato et al. 1987). Several studies have shown that changes in the concentration of ACh in the hippocampus and cortex correlate with learning and cognitive function (Fadda et al. 1996; Ragozzino et al. 1996; Hironaka et al. 2001; Chang & Gold, 2003).
To clarify the cellular mechanisms of cholinergic effects, a great number of studies have been conducted using in vitro slices of different brain areas. Most of these studies reported an increase of neuronal excitability following application of cholinomimetic drugs (Krnjevic & Phillis, 1963; McCormick & Prince, 1987). However, discrepant results were obtained regarding the effects of cholinomimetic drugs on glutamatergic transmission. Some authors reported a decrease in synaptic efficacy when ACh or cholinergic agonists were applied to the cortex and to hippocampus slices or cultured cells (Huerta & Lisman, 1993; Vidal & Changeux, 1993; Hasselmo & Cekic, 1996; Gil et al. 1997; Kimura & Baughman, 1997). However, others showed an increase in glutamatergic (Cox et al. 1994; Marino et al. 1998) or synaptic transmission (Gil et al. 1997) after application of ACh. Additional experiments conducted in the piriform cortex and hippocampus advanced the idea that the action of ACh on synaptic transmission is region- and input specific (Hasselmo & Bower, 1992; Hasselmo & Schnell, 1994; Kimura et al. 1999).
Taken together, the reported studies suggest that ACh has numerous and specific actions on neural networks. However, the roles of the individual cholinergic receptors involved in these various actions of ACh are not well understood at present.
In the present study we have examined the functional role of ACh in synaptic transmission using in vitro electrophysiology and a combination of genetic and pharmacological approaches on visual cortex slices. We found that varying the concentrations of ACh is critical for determining the type of modulation of the synaptic response elicited by electric stimulation of white matter (WM), layer IV and layer II/III in visual cortex slices. Indeed, high and low concentrations of ACh induced depression and facilitation of synaptic responses, respectively. Modulation of synaptic transmission by ACh is mediated by multiple muscarinic receptors (mAChRs), as shown using pharmacological tools and M1–M5 mAChR knockout (KO) mice (for a review see Wess, 2004). Cholinergic modulation of synaptic transmission changed when different synaptic pathways were stimulated, suggesting that the effects of ACh are input specific. These results indicate that local ACh modulates the functional dynamics of the cortical network.
Methods
Slice preparation
Primary visual cortex slices were prepared from adult mice. Pharmacological experiments were performed in SLJ mice crossed with C57BL/6J mice, SJL–C57BL/6J, unless otherwise stated. Animals were deeply anaesthetized by intraperitoneal injection of urethane (0.7 ml/100 mg in 20% physiological solution) and then decapitated. The brain was rapidly removed and 400-μm-thick coronal sections of the occipital poles were sliced with a vibratome. All steps were performed in ice-cold artificial cerebrospinal fluid (ACSF) solution (mm: NaCl, 119; KCl, 2.5; CaCl2, 2.5; MgSO4, 1.3; NaH2PO4, 1; NaHCO3, 26.2; and glucose, 11) bubbled with 95% O2/5% CO2. Prior to recording, slices were stored for at least 1 h in a recovery chamber containing oxygenated ACSF solution, at 33 ± 1°C. During electrophysiological recordings, slices were perfused at 3–4 ml min−1 with oxygenated ACSF, at 33 ± 1°C (see also Pesavento et al. 2000).
Electrophysiological recordings
Extracellular field potentials (FPs) were evoked via a tungsten concentric bipolar stimulating electrode placed in three different sites: WM/layer VI border, layer IV and layer II/III. The recording electrode was filled with ACSF solution and placed in layer II/III. In order to isolate the horizontal from the vertical synaptic pathways, a vertical cut under the stimulating electrode was made when the stimulating electrode was placed in layer II/III. The amplitude of the FPs in layer II/III was used as a measure of the evoked population excitatory current as reported previously (Mitzdorf & Singer, 1978; Domenici et al. 1995). Baseline responses were obtained with a stimulation intensity that yielded 50–60% of maximal amplitude. All FPs had a peak latency from time of stimulation ranging from 4 to 7 ms, and a maximal amplitude of at least −0.6 mV. FP amplitudes were monitored every 20 s, and averaged every three responses using an on-line data acquisition software (Anderson & Collingridge, 2001).
At least 10 min of stable basal FPs were recorded before application of cholinergic drugs. To check for involvement of the cholinergic system we employed different concentrations of ACh (10 μm to 1 mm), antagonists of cholinergic receptors (1 μm atropine, 3 μm mecamylamine), and an agonist of cholinergic receptors (0.5–10 μm muscarine). In addition, we used edrophonium (1–10 μm) to block cholinesterase (ChE) activity. All compounds were purchased from Sigma (St Louis, MO, USA). The role of mAChRs in synaptic transmission was investigated by using different antagonists purchased from Tocris (Bristol, UK); M1 receptors were preferentially blocked by using pirenzepine (10 nm–2 μm); M4 receptors were preferentially blocked by PD102807 (0.5–1 μm). Cholinergic drugs were dissolved in ACSF solution and delivered through slice perfusion.
For each kind of treatment, drug effects on FP amplitudes were measured by averaging the FP amplitudes of the last three minutes of drug application, normalized with respect to the average of FP amplitudes of the last three minutes of basal stimulation (relative amplitude with respect to the baseline).
M1–M5 mAChR KO mice
The generation of homozygous M1–M5 single receptor KO mice (genetic background: 129/SvEv × CF1 (M1, M3, M4, and M5), or 129J1 × CF1 (M2)) has been previously described (Gomeza et al. 1999a, 1999b; Yamada et al. 2001a, 2001b; Fisahn et al. 2002). Similarly, M1/M3 (Gautam et al. 2004), M2/M4 (Duttaroy et al. 2002), and M1/M4 (Gautam et al. 2004) double KO mice were obtained as previously described. The M1/M3 and M1/M4 receptor double KO mice had the same mixed genetic background (129SvEv (50%) × CF1 (50%)). The M2/M4 receptor double KO mice had the following genetic background: 129J1 (25%) × 129SvEv (25%) × CF1 (50%). For each KO strain, wild-type (WT) mice of the same mixed genetic background were used in parallel as controls (named M4 WT for M1, M3, M4 and M5 single KO and M2 WT for the M2 KO single KO mice). All experiments were carried out with mice that were at least 6 weeks old.
RT-PCR
Anaesthetized SLJ-C57BL/6J wild-type mice were decapitated and the visual cortex was immediately removed, frozen in dry ice, and kept at −80°C until processed. Total RNA was extracted using the Trizol reagent (Invitrogen, Carlsbad, CA, USA), treated with DNAse I (Ambion, Austin, TX, USA), and tested by PCR in order to ensure the absence of genomic DNA in the sample. Five micrograms of total RNA were reverse transcribed using the SuperScript First-Strand Synthesis System for RT-PCR kit (Invitrogen), according to manufacturer's indications, using random hexamers to start the reaction. Amplification of specific domains of the different muscarinic receptor cDNAs were carried out using the following primers, specific for each gene: M1S1: 5′-cca aca tca ccg tct tgg cac-3′ M1A1: 5′-agt gcc aat gat gag atc agc-3′ M2-A6: 5′-gct att acc agt cct tac aag aca-3′ M2-B5: 5′-cca gag gat gaa gga aag aac c-3′ M3-A3: 5′-aag acc aca gta gca gtg-3′ M3-B: 5′-ctc tct aca tcc ata gtc cc-3′ M4-A: 5′-gga gaa gaa ggc caa gac tct gg-3′ M4-B: 5′-ggc agt cac aca ttc act gcc tg-3′ M5A7: 5′-tcc gtc atg acc ata ctc ta-3′ M5B6: 5′-ccc gtt gtt gag gtg ctt cta c-3′.
Two different programmes were used to amplify these sequences: PCR programme A was used to amplify the M1, M3, and M5 receptor cDNAs (consisting in an initial denaturing step at 94°C for 10 min, followed by 30 cycles at 94°C for 30 s, 55°C for 30 s and, 72°C for 2 min, which were followed by a final step at 72°C for 8 min). Programme B was used to amplify the M2 and M4 receptor cDNAs (essentially as programme A, except that the 30 amplification cycles consisted of 94°C for 30 s, 60°C for 30 s and, 72°C for 1 min). The specificity of the amplified bands was assessed by restriction endonuclease digestion.
Statistics
Statistical comparison between FP amplitudes measured during baseline, and FP amplitudes measured during bath application of pharmacological compounds was performed by applying Student's t test. The t test was also used for statistical comparisons among different groups. Differences were considered significant with P < 0.05.
Results
The amplitude of FPs evoked by stimulation of WM is modulated in an opposing way by different concentrations of ACh
To understand the role of ACh in cortical synaptic transmission we measured the changes in amplitude of FPs evoked by stimulation of WM and recording in cortical layer II/III, using bath application of different pharmacological compounds in slices of SJL–C57BL/6J mice. FPs recorded in layer II/III represent a current sink reflecting the strength of excitatory synaptic connections (Mitzdorf & Singer, 1978; Mitzdorf, 1985; Bode-Greuel et al. 1987; Lee et al. 1991), and correlate with variation in intracellular EPSP response (Kirkwood & Bear, 1994)
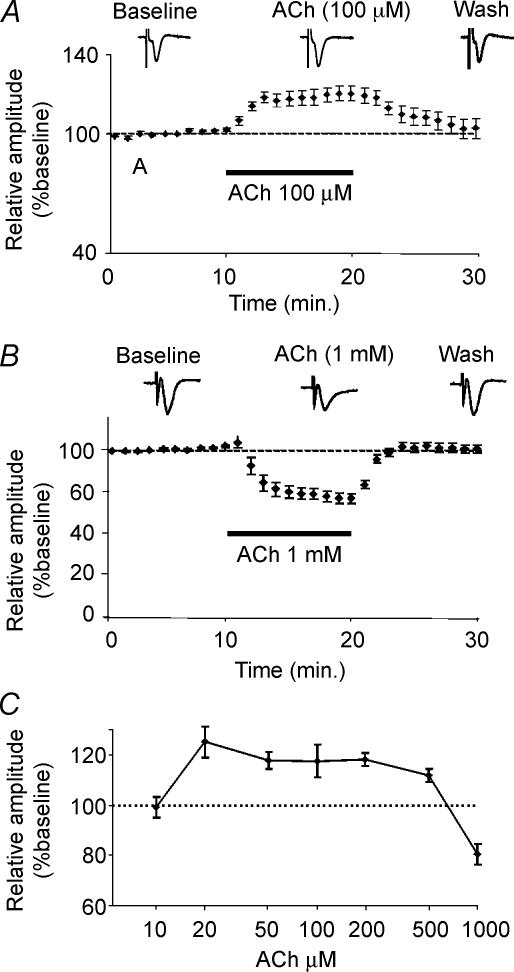
Application of 100 μm ACh for 10 min through general perfusion induced a significant increase in amplitude of FPs (120 ± 3.5% of baseline, n = 15; Fig. 1A). This facilitation reached a plateau within a few minutes and returned to basal values after washout of ACh. A longer application of ACh (20 min) led to similar results (121 ± 3.2%, n = 11; P < 0.001; data not shown). In contrast, when 1 mm ACh was applied, a significant decrease in the amplitude of FPs was observed (72 ± 3.5% of baseline, n = 15; Fig. 1B). Also in this case the amplitude of FPs returned to basal levels after washout. In order to obtain a concentration–response curve, we tested different concentrations of ACh. We found that 10 μm ACh did not induce any changes in the amplitude of FPs (99 ± 3.4% of baseline, n = 6; Fig. 1C). Concentrations of 20, 50, 200 and 500 μm ACh induced a significant facilitation that was not significantly different from that obtained with 100 μm ACh (relative changes from baseline: 125 ± 6, n = 5; for 20 μm; 118 ± 3.4, n = 6, for 50 μm; 118 ± 2.6, n = 6, for 200 μm; 112 ± 2.6, n = 7; for 500 μm; Fig. 1C). Thus, ACh enhances or inhibits synaptic transmission depending on the concentration used.
Figure 1. ACh modulates the amplitude of FPs in layer II/III of mouse (SLJ–C57BL/6J) visual cortex during stimulation of WM.
A, bath application of 100 μm ACh induces an increase of FP amplitudes that return to baseline after washout (n = 15, symbols represent the mean relative values ± s.e.m.). B, bath application of 1 mm ACh induces a decrease in amplitude of FPs that returns to baseline after washout (n = 15, symbols represent the mean relative values ± s.e.m.). C, concentration–response curve of ACh bath application (20 μm, n = 5; 50 μm, n = 6; 100 μm, n = 15; 200 μm, n = 6; 500 μm, n = 7). Horizontal bars depict the period of application of ACh. Inset: representative traces. Calibration bar = 0.2 mV/5 ms. In C circles represent the mean amplitude of FPs during the last three minutes of ACh application normalized with respect to the mean FP amplitudes during the last three minutes of baseline (s.e.m. is shown as a vertical bar for each symbol).
Muscarinic receptor subtypes mediate Ach-dependent facilitation and depression of synaptic transmission
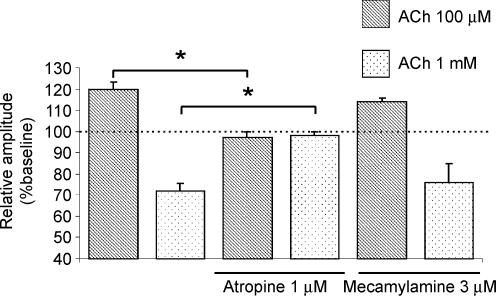
In order to dissect the roles of the different types of cholinergic receptors in modulating cortical synaptic transmission, we employed nicotinic and muscarinic antagonists. Ten minutes of bath application of the mAChR antagonist atropine (1 μm) did not significantly modify basal FP responses (SJL–C57BL/6J slices, 99 ± 1% of baseline, n = 6, data not shown). However, 1 μm atropine prevented both FP facilitation induced by 100 μm ACh (97 ± 3% of baseline, n = 5; Fig. 2) and depression of FPs induced by 1 mm ACh (98 ± 2% of baseline, n = 5; Fig. 2). The nicotinic receptor antagonist mecamylamine (3 μm, 10 min) did not modify basal FP responses (101 ± 1% of baseline, n = 4, data not shown). In contrast to atropine, facilitation and depression induced by 100 μm ACh (114 ± 1.9% of baseline, n = 5; Fig. 2) and 1 mm ACh (76 ± 9% of baseline, n = 6; Fig. 2), respectively, were not affected by mecamylamine. These data suggest that ACh-induced modifications in the amplitude of FPs are mediated by muscarinic but not nicotinic receptors.
Figure 2. The modulatory effects of ACh in visual cortex slices are mediated by mAChRs.
Atropine or mecamylamine were bath-applied 10 min before ACh application; 1 μm atropine prevents both 100 μm ACh-induced facilitation (n = 5) and 1 mm ACh-induced depression (n = 5) of the amplitude of the FPs elicited by WM stimulation. Mecamylamine (3 μm) failed to prevent 100 μm ACh-induced facilitation (n = 5) and 1 mm-induced depression (n = 6) of the amplitude of the FPs elicited by WM stimulation. Columns represent the mean FP amplitudes during the last three minutes of drug application normalized with respect to the mean amplitude of FPs with s.e.m. during the last 3 min of baseline. *P < 0.05.
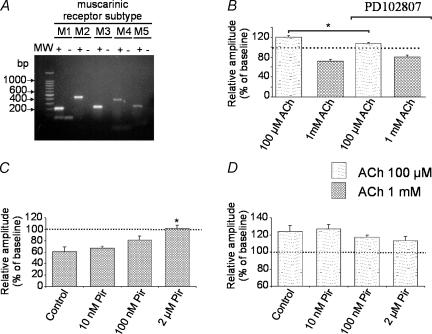
The analysis of total RNA extracted from the visual cortex of wild-type mice (strain SJL–C57BL/6J) revealed the presence of all five mAChR transcripts (Fig. 3A). Receptor subtype-specific primers were used for cDNA amplification. As shown in Fig. 3A, these reactions resulted in the expected PCR product sizes for each mAChR gene (M1: 197 bp; M2: 435 bp; M3: 226 bp; M4: 367 bp; M5: 227 bp).
Figure 3. Effects of muscarinic receptor antagonists on ACh modulation of synaptic transmission.
Pharmacological antagonists of mAChRs were bath applied in visual cortex slices of SLJ–C57BL/6J mice. A, agarose gel electrophoresis showing the products from reverse transcription and PCR of total RNA from visual cortex of SLJ–C57BL/6J wild-type mice using M1, M2, M3, M4, and M5 mAChR gene-specific primers. Amplification of cDNA from reverse transcription reactions is indicated as +. Control reactions to check for contamination with genomic DNA were run without reverse transcribing the RNA samples (indicated with –). All mAChR genes are expressed in the visual cortex of control mice. B, in the presence of the selective M4 mAChR antagonist PD102807 (0.5–1 μm), there was a significant reduction of 100 μm ACh-induced (n = 13) facilitation without affecting 1 mm ACh-induced depression in FP amplitudes (n = 7).(C and D). The mAChR antagonist pirenzepine was bath-applied at three different concentrations (10 nm, 100 nm, and 2 μm). Application of pirenzepine for 10 min did not modify basal FP amplitudes (data not shown). C, depression of FPs induced by 1 mm ACh was prevented by 2 μm but not by 10 and 100 nm pirenzepine. *P < 0.01; other conventions as in Fig. 2. D, pirenzepine (Pir) at the concentrations used did not significantly change the facilitation of FP amplitudes induced by 100 μm ACh.
In order to test whether different mAChRs play differential roles in the ACh-induced modulation of FPs, we used two different experimental approaches. First, we used pharmacological tools with the aim of preferentially blocking specific mAChR subtypes. Second, we studied preparations from M1–M5 receptor KO mice.
Use of muscarinic antagonists
As pharmacological tools, we used the M4 receptor-preferring antagonist, PD102807, and the M1/M4 receptor-preferring antagonist, pirenzepine (Caulfield & Birdsall, 1998). The presence of the M4 receptor-preferring antagonist PD102807 (0.5–1 μm) did not prevent depression induced by 1 mm ACh (79 ± 4% of baseline, n = 7; Fig. 3B) in SJL–C57BL/6J mice. However, ACh (200 μm) induced only a small facilitation in the amplitude of FPs (107 ± 3.3% of baseline, n = 13; Fig. 3B), which was significantly lower than that obtained in ACh alone (P = 0.009, Fig. 3B).
To further investigate the contribution of different mAChRs to ACh modulation of synaptic transmission, we used three different concentrations of pirenzepine (10 nm, 100 nm, and 2 μm). According to Caulfield & Birdsall (1998), 10 nm pirenzepine blocks about 50% of M1 receptors, 100 nm pirenzepine blocks most M1 and part of M4 and M3 receptors, and 2 μm blocks most M1 and M4 and a considerable portion of M2, M3, M5 receptors. Ten minutes of bath application of pirenzepine (2 μm) did not modify basal FP amplitudes (SJL–C57BL/6J slices, 100 ± 1.6% of baseline, n = 9; data not shown). 1 mm ACh induced a significant depression of FPs (61 ± 8% of baseline, n = 9, controls SJL–C57BL/6J mice, Fig. 3C), whose amplitude was not significantly reduced by the presence of 10 and 100 nm pirenzepine (67 ± 4% of baseline, n = 8 and 81 ± 7% of baseline, n = 7, respectively; Fig. 3C). ACh-dependent depression was completely blocked only when pirenzepine was applied at the highest concentration (102 ± 5% of baseline, n = 5, 2 μm pirenzepine; P < 0.01 compared to control, Fig. 3C). Interestingly, when ACh-dependent depression was blocked by 2 μm pirenzepine, 1 mm ACh failed to elicit facilitation (Fig. 3C).
Facilitation induced by 100 μm ACh was not changed by 10 nm pirenzepine; higher concentrations of pirenzepine, 100 nm and 2 μm, slightly reduced ACh-dependent facilitation, although the level of reduction was not significant (relative changes with respect to baseline: 124 ± 7%, n = 9, control SJL–C57BL/6J; 127 ± 5%, n = 6, 10 nm pirenzepine; 117 ± 3%, n = 7, 100 nm pirenzepine; 113 ± 5%, n = 6, 2 μm pirenzepine; Fig. 3D).
M1–M5 receptor KO mice
M1–M5 mAChR single KO and M1/M3, M1/M4 and M2/M4 mAChR double KO mice were used for the preparation of occipital slices. WT control mice with the same genetic background as the individual KO mice were studied in parallel (for details, see Methods). Mice prepared for electrophysiology were genotyped by PCR using DNA extracted from tail biopsies.
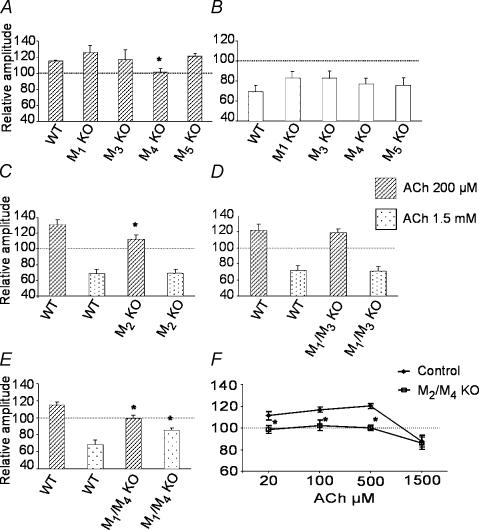
We found that 200 μm ACh induced facilitation of FPs in both WT strains used as controls for mAChR single KO mice (relative changes with respect to baseline: M4 WT, 115 ± 1%, n = 10, Fig. 4A; M2 WT, 131 ± 6%, n = 6, Fig. 4C). A concentration of 1.5 mm ACh was necessary to obtain a significant depression of FPs in both M2 and M4 WT mice (relative changes with respect to baseline: 69 ± 6%, n = 7, for M4 WT, Fig. 4B; and 68 ± 6%, n = 6, for M2 WT, Fig. 4C). In M1, M3 and M5 KO mice, 200 μm ACh induced facilitation of FPs whose amplitude was not significantly different from that measured in control animals (relative changes with respect to baseline: 126 ± 8%, n = 7, for M1 KO; 116 ± 12%, n = 7, for M3 KO and 121 ± 4%, n = 8, for M5 KO Fig. 4A). In contrast, facilitation was absent or significantly reduced in M4 and M2 KO mice (M4 KO, 102 ± 5% of baseline, n = 7 P < 0.05 compared to control, Fig. 4A; M2 KO, 112 ± 5% of baseline, n = 8, P < 0.05 compared to WT mice, Fig. 4C). In all M1–M5 KO animals, 1.5 mm ACh induced a significant depression of FPs, whose amplitude was not significantly different with respect to that measured in WT controls (relative changes with respect to baseline: M1 KO, 65 ± 8%, n = 6; M3 KO, 82 ± 7%, n = 5; M4 KO, 77 ± 6%, n = 6; M5 KO, 76 ± 8%, n = 7; M2 KO, 69 ± 5%, n = 7; Fig. 4B and C).
Figure 4. Modulatory effects of ACh on FPs elicited in visual cortex slices from M1–M5 mAChR KO mice.
A, bath application of 200 μm ACh. Facilitation of FPs was absent in M4 KO mice while it was retained in M1, M3 and M5 KO mice (WT, n = 10; M4 KO, n = 7; M3 KO, n = 8; M5 KO, n = 8; mean FP amplitudes were significantly different between M4 KO and WT mice). B, bath application of 1.5 mm ACh. Depression of FPs was not affected in M1, M3, M4, and M5 KO mice (WT, n = 7; M1 KO, n = 6; M2 KO, n = 7; M3 KO, n = 5; M4 KO, n = 6; M5 KO, n = 7; mean FP amplitudes were not significantly different between KO and WT mice). C, M2 KO mice showed a significant decrease of facilitation induced by 200 μm ACh (WT, n = 6; M2 KO, n = 8; mean FP amplitudes were significantly different between KO and WT mice). Depression of FPs induced by 1.5 mm ACh was normal in M2 KO mice. D, M1/M3 double KO mice showed normal facilitation and depression of synaptic transmission after bath application of ACh at different concentrations (200 μm and 1.5 mm) (M1/M3 WT, n = 10; M1/M3 KO, n = 10; mean FP amplitudes were not significantly different between KO and WT mice). E, M1/M4 double KO mice showed a significant decrease of FP depression induced by 1.5 mm ACh (M1/M4 WT, n = 6; M1/M4 KO, n = 6) and absence of facilitation induced by 200 μm ACh (M1/M4 WT, n = 8; M1/M4 KO, n = 5). F, concentration–response curves in M2/M4 double KO and WT mice. In KO animals, FP facilitation was absent at all ACh concentrations, while depression was induced only by 1.5 mm ACh; the amplitude of FPs was not significantly different between KO and WT mice (see also Table 1).The different KO animals were compared with their corresponding WT mice having the same genetic background (see Methods). Columns in (A–E) and symbols in (F) represent the mean amplitude of FPs during the last 3 min of ACh application, normalized with respect to the mean amplitude of FPs during the last 3 min of baseline. s.e.m. is shown as a vertical bar. *P < 0.05.
Since ACh-induced depression was not sensitive to the lack of single mAChRs, ACh effects were further investigated in mAChR double KO mice. Analysis of M1/M3 mAChR KO mice showed that neither facilitation induced by 200 μm ACh (121 ± 8% of baseline in WT, n = 10; 119 ± 4% of baseline in M1–M3 KO, n = 10) nor depression due to 1.5 mm ACh (71 ± 6% of baseline in WT, n = 10; 71 ± 6% of baseline in M1–M3 KO, n = 10;) were affected (Fig. 4D). In M1/M4 mAChR KO mice, 200 μm ACh was unable to induce FP facilitation (WT, 115 ± 3% of baseline, n = 8; M1/M4 KO, 99 ± 4% of baseline, n = 5; P < 0.05 between groups, Fig. 4E), in agreement with results obtained in M4 single KO mice (see Fig. 4A). Remarkably, FP depression produced by 1.5 mm ACh was significantly reduced in M1/M4 mAChR double KO mice (WT, 68 ± 6% of baseline, n = 6; M1/M4 KO, 85 ± 3% of baseline, n = 6; P < 0.05 between groups; Fig. 4E). Finally, we found that ACh-dependent facilitation but not depression was impaired in M2/M4 mAChR double KO mice (Table 1; Fig. 4F). In these mutant mice, ACh concentrations of less than 1.5 mm failed to induce depression. Moreover, the magnitude of the depression induced by 1.5 mm was similar between control and KO animals. Thus, the relationship between ACh concentration and depression was not changed when ACh-dependent facilitation was blocked.
Table 1.
Relative changes of FP amplitudes in WT and M2/M4 mAChR KO mice following bath application of different ACh concentrations
| ACh | 20 μm | 100 μm | 500 μm | 1.5 mm |
|---|---|---|---|---|
| Control (WT) | 111 ± 4% (6) | 117 ± 2% (8) | 120 ± 2% (5) | 87 ± 5% (6) |
| M2/M4 KO | 98 ± 4% (6) | 102 ± 5% (6) | 100 ± 3% (6) | 86 ± 6% (8) |
Values of n are given in parentheses. Each value represents the mean amplitude of FPs ± s.e.m. during the last 3 min of ACh application normalized with respect to the mean amplitude of FPs during the last 3 min of baseline.
The overall results obtained using mAChR KO mice and mAChR antagonists indicate that low concentrations of ACh facilitate synaptic transmission via activation of M2 and M4 mAChRs. Depression induced by high ACh concentrations appears to be mediated by multiple mAChRs, including the combined action of M1 and M4 receptors, as suggested by experiments using pirenzepine and M1/M4 double KO mice.
Cholinesterase activity regulates the modulatory action of ACh
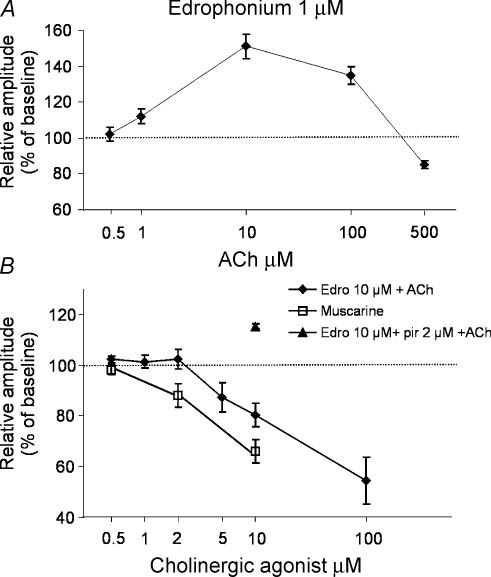
When examining the physiological effects induced by ACh, attention must be paid to the role of ChEs, the enzymes which promote the hydrolysis of ACh. In the primary visual cortex, ChE enzymes show a characteristic pattern of expression throughout the cortical layers (Zilles et al. 1984; Maffei et al. 1992). We investigated whether modulation of FP amplitudes by ACh was influenced by ChEs. To this end, we used edrophonium, an inhibitor of ChEs. Bath application of 1 μm edrophonium did not modify basal FP amplitudes (102 ± 4% of baseline, n = 5, data not shown). In the presence of 1 μm edrophonium, however, we observed a shift of the ACh concentration–response curve to the left (compare Fig. 1C with Fig. 5A). Indeed, a significant depression of FPs was induced by 500 μm ACh (85 ± 2% of baseline, n = 5; Fig. 5A), while a significant facilitation was observed both with 100 μm ACh (135 ± 5% of baseline, n = 5; P < 0.01; Fig. 2A) and 10 μm ACh (151 ± 7% of baseline, n = 5; Fig. 5A). A significant facilitation, although reduced in magnitude, was also seen with 1 μm ACh (112 ± 4% of baseline; n = 6, Fig. 5A), but was no longer observed with 0.5 μm ACh (102 ± 4% of baseline, n = 4; Fig. 5A).
Figure 5. ChE influences cholinergic modulation of synaptic transmission in visual cortex slices.
A, concentration–response curve of ACh effects on FP amplitudes is shifted towards lower values in the presence of 1 μm edrophonium (0.5 μm, n = 4; 1 μm, n = 6; 10 μm, n = 5; 100 μm, n = 5; 500 μm, n = 5). B, in the presence of 10 μm edrophonium (Edro), a depression of FP amplitudes was observed at ACh concentrations ranging from 5–100 μm (5 μm, n = 10; 10 μm, n = 11; 100 μm, n = 5), while no changes were found at ACh concentrations ranging from 0.5–2 μm (0.5 μm, n = 5; 1 μm, n = 13; 2 μm, n = 9). Pirenzepine (pir; 2 μm) unmasked ACh-induced facilitation of FPs in the presence of 10 μm edrophonium (10 μm ACh, n = 8). Bath application of muscarine produced an effect similar to that observed with ACh in the presence of 10 μm edrophonium. In A and B, symbols represent the mean amplitude of FPs during the last 3 min of agonist application, normalized with respect to the mean amplitude of FPs during the last 3 min before drug application. s.e.m. is shown as a vertical bar for each symbol.
A small but significant increase of FP responses was produced by bath application of 10 μm edrophonium (108 ± 2% of baseline, n = 5, P < 0.05, data not shown). In addition, 10 μm edrophonium caused a dramatic change of the ACh concentration–response curve. Indeed, a significant depression of FP amplitudes was observed with 5–100 μm ACh (100 μm, 54 ± 9% of baseline, n = 5; 10 μm, 80 ± 5% of baseline, n = 11; 5 μm, 87 ± 6% of baseline, n = 10; Fig. 5B). In contrast, no statistically significant changes were found with 0.5–2 μm ACh (2 μm, 102 ± 4% of baseline, n = 9; 1 μm, 101 ± 4% of baseline, n = 13; 0.5 μm, 97 ± 2% of baseline, n = 5; Fig. 5B). Since only FP depression was observed in the presence of 10 μm edrophonium, we examined whether facilitation can be induced in the absence of depression. To this end, a low concentration of ACh was applied in the simultaneous presence of 10 μm edrophonium and a pirenzepine concentration able to prevent ACh-dependent depression (2 μm, see Fig. 3C). Under these experimental conditions, 10 μm ACh induced a significant facilitation of FP responses (115 ± 1% of pre ACh, P < 0.05, n = 8; Fig. 5B). These data suggest that ChE activity determines the range of ACh concentrations that induce enhancement or depression of FPs.
As blockade of ChEs enhances ACh-dependent depression over facilitation, we predicted that the use of cholinergic agonists resistant to ChEs would mimic this activity. We found that the concentration–response curve produced by bath application of muscarine was similar to that induced by ACh in the presence of 10 μm edrophonium, with the absence of facilitatory effects even at low agonist concentrations (muscarine 10 μm, 66 ± 5% of baseline, n = 7; muscarine 2 μm, 88 ± 5% of baseline, n = 5; muscarine 0.5 μm 98 ± 2% of baseline, n = 4; Fig. 5B).
Effects of ACh on cortical synaptic transmission upon stimulation of different cortical synaptic pathways
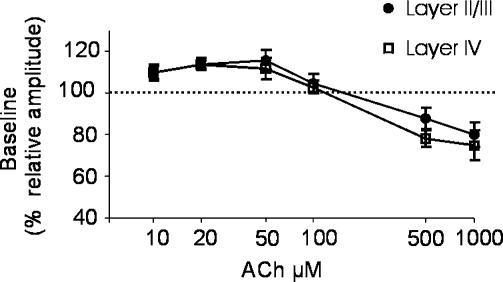
To study whether the modulatory action of ACh is input specific, we applied ACh while recording FPs induced by stimulation of different synaptic pathways of the primary visual cortex. The recording electrode was placed in layer II/III as usual, while the stimulating electrode was placed either in layer IV in order to stimulate layer IV–II/III vertical intracortical transmission, or in layer II//III, at the same level of the recording electrode, to stimulate horizontal intracortical connections (Domenici et al. 1995). We found that bath application of 10–50 μm ACh significantly facilitates FPs induced by stimulation of layer II–III (110 ± 3% of baseline, n = 5, for 10 μm ACh; 114 ± 2% of baseline, n = 5, 20 μm ACh; 116 ± 5% of baseline, n = 5, 50 μm ACh; Fig. 6) and layer IV (110 ± 4% of baseline, n = 5, for 10 μm ACh; 114 ± 3% of baseline, n = 6, for 20 μm ACh; 112 ± 5% of baseline, n = 7, for 50 μm ACh; Fig. 6). Application of 100 μm ACh did not modify either FPs induced by stimulation of layer II/III (105 ± 3.9% of baseline, n = 6; Fig. 6) or those induced by stimulation of layer IV (103 ± 3.2% of baseline, n = 8; Fig. 6). When 500 μm and 1 mm ACh were applied, a significant depression in amplitude of FPs was observed for stimulation of both layers, i.e. layer II/III (88 ± 5% of baseline, n = 14, 500 μm ACh; 80 ± 5.9% of baseline, n = 5, 1 mm ACh; Fig. 6) and layer IV (78 ± 7.4% of baseline, n = 6, 500 μm ACh; 75 ± 6.7% of baseline, n = 5, 1 mm ACh, Fig. 6). These data show that different concentrations of ACh enhance or inhibit synaptic transmission for stimulation of intracortical pathways. However, ACh concentrations were shifted towards lower values when stimulating layer II–III and layer IV with respect to stimulation of WM (compare Fig. 6 with Fig. 1C), suggesting that cholinergic modulation of synaptic transmission is input specific.
Figure 6. ACh modulation of FPs elicited in visual cortex slices via stimulation of intracortical pathways.
ACh concentration–response curve of FP elicited by stimulation of layer II/III (•) or layer IV (□). Symbols represent the mean relative amplitude of FPs during the last three minutes of ACh application, normalized with respect to the mean FP amplitudes during the last 3 min of baseline s.e.m. is shown as a vertical bar for each symbol.
Discussion
In the present work, we investigated the role of ACh in modulating cortical synaptic transmission. We found that (i) different concentrations of ACh coupled with ChE activity enhance or inhibit cortical synaptic transmission of vertical input from WM to layer II/III (ii) the modulatory action of ACh is exerted through mAChRs with M2 and M4 mAChRs mediating enhancement of synaptic transmission and multiple mAChR (including M1 and M4 mAChRs) mediating depression, and (iii) the action of ACh is input specific.
Cholinergic modulation of synaptic transmission has been widely discussed in the literature; a large number of methodologies have been used, sometimes leading to opposing results. In particular, while there is general agreement that ACh increases intrinsic excitability of cortical neurones (Krnjevic & Phillis, 1963; McCormick & Prince, 1987), some authors report an ACh-mediated suppression of glutamatergic inputs, while others report an enhancement (Huerta & Lisman, 1993; Cox et al. 1994; Hasselmo & Cekic, 1996; Gil et al. 1997).
In the present study, we showed that different concentrations of exogenously supplied ACh resulted in opposite effects on the amplitude of FPs evoked by stimulating WM. Concentrations of ACh ranging from 20 to 500 μm facilitate FPs, increasing their amplitude, while ACh concentrations in the millimolar range depress FPs.
The effects of ACh are due to stimulation of mAChRs since they are blocked by an antagonist of muscarinic (atropine) but not of nicotinic (mecamylamine) receptors. Facilitation of extracortical input has previously been shown to be induced by ACh through the activation of nicotinic receptors in layer II/III of the barrel cortex (Gil et al. 1997). In the present study, we provide new evidence that ACh is able to enhance and inhibit synaptic transmission of the vertical pathway from WM to layer II/III in visual cortex, through activation of mAChRs, independent of nicotinic receptors.
It has been reported that different mAChR subtypes are expressed in the visual cortex, with characteristic patterns of distribution throughout the cortical layers (Levey et al. 1991; Mrzljak et al. 1993; Aubert et al. 1996; Tigges et al. 1997). Transcripts for all five mAChRs (M1–M5) are expressed in the adult mouse visual cortex, as shown in the present paper. To identify the specific mAChR subtypes controlling ACh-dependent facilitation and depression of synaptic responses, we used a combination of pharmacological tools and M1–M5 KO mice (Gomeza et al. 1999a, b; Yamada et al. 2001a, 2001b; Fisahn et al. 2002). We showed that different mAChR subtypes are involved in facilitation/depression of FPs evoked by stimulation of WM. In particular, M2 and M4 mAChRs are necessary for the enhancement of synaptic transmission by low ACh concentrations, as shown by the results obtained with M2 and M4 KO mice and the use of an M4 receptor-preferring antagonist. In contrast, the depression of synaptic transmission induced by high ACh concentrations is not modified in M1–M5 mAChR single KO mice. Since ACh-dependent depression was normal in mAChR single KO mice, we also analysed a series of mAChR double KO mice. We found that M1/M4 double KO mice showed reduced depression at high ACh concentrations. However, since the ACh-induced depression was reduced but not abolished in M1/M4 double KO mice, additional mAChRs must be involved in mediating this activity. In agreement with this idea, we found that a high concentration of pirenzepine (2 μm) that blocks most M1 and M4 and a portion of M2, M3, and M5 receptors (Caulfield & Birdsall, 1998) completely suppresses ACh-dependent depression.
One important question is whether the facilitatory and inhibitory effects of ACh on FPs overlap. We investigated this issue by examining the effects of ACh under experimental conditions characterized by the absence of either depression or facilitation. M4 KO mice lacking ACh-mediated facilitation did not show a depressing effect at low ACh concentrations. Analogously, when ACh-dependent depression was blocked by 2 μm pirenzepine, high ACh concentrations failed to induce facilitatory effects. Thus, ACh-induced facilitatory and inhibitory effects do not overlap at the ACh concentrations used.
Partial blockade of ChE activity by 1 μm edrophonium modified the modulatory effects of ACh, shifting the concentrations of ACh inducing depression towards lower values. Increasing the concentration of edrophonium up to 10 μm resulted in a dramatic shift to the left of the ACh concentration–response curve, with a very low concentration of ACh (5 μm) still inducing depression of synaptic responses. A critical question is whether inhibition of ChE activity directly affects ACh-dependent facilitation or simply masks the facilitatory effect by enhancing depression. To address this issue, ACh-mediated depression was blocked by 2 μm pirenzepine. Under these conditions, low concentrations of ACh were able to elicit facilitation even in the presence of 10 μm edrophonium, suggesting that inhibition of ChE activity favours ACh-dependent depression, thus masking the facilitatory effects of ACh.
In control experiments, we showed that the ACh-induced facilitatory and inhibitory effects do not overlap. However, when ChE activity is blocked, facilitatory and depressing effects appear to overlap, with depression being the predominant response. This suggests that the activity of ChEs plays an essential role in determining the precise nature of the ACh response.
What are the potential mechanisms underlying ACh-dependent facilitation and depression? Our data suggest that ACh modulation of synaptic transmission relies on ChE activity and preferential activation of different mAChRs subtypes. We showed that low levels of ACh induce facilitation of synaptic transmission by activating M4 and M2 receptors. At higher ACh levels, multiple mAChRs are activated. Indeed, the combined lack of M1 and M4 receptors impairs depression of synaptic transmission. At a molecular level, the ACh-dependent depression may be due to differences in the subcellular distribution of different mAChR subtypes, including presynaptic versus postsynaptic sites, and the activation of multiple intracellular signalling pathways. Our data suggest that ChE activity determines whether ACh induces facilitation or depression of FPs. At low ACh levels, activation of M2 and M4 mAChRs leads to facilitatory responses. When ACh levels exceed the saturation threshold of local ChE activity (Silver, 1974), additional mAChRs are activated leading to depression of FPs. Due to the high hydrolytic activity of ChEs (Rosenberry, 1975; Quinn, 1987; for reviews see Descarries et al. 1997; Massoulie et al. 1999), these enzymes would be able to regulate ACh levels and thereby determine which mAChR subtypes are activated. This hypothesis is supported by data showing that the affinity of ACh is higher for M2 and M4, i.e. the receptors involved in ACh-dependent facilitation, than for M1, M3 and M5 receptors (Lazareno & Birdsall, 1995; Page et al. 1995). Based on this concept, the different distribution of ChE (Rotundo & Carbonetto, 1987) and mAChRs (Levey et al. 1991; Buwalda et al. 1995) within the same cortical areas and in different cortical areas could contribute to generating the input specificity in the response to ACh observed in previous reports (Hasselmo & Bower, 1992; Hasselmo & Schnell, 1994; Kimura et al. 1999).
In agreement with this idea, we showed that the modulatory action of ACh on FP amplitude is input specific. We found that ACh concentration–response curves obtained by stimulation of layer II/III and layer IV intracortical pathways were shifted towards lower ACh concentrations with respect to those obtained by WM stimulation. In particular, different ranges of ACh concentrations were able to modulate cortical synaptic transmission. High ACh concentrations, in the millimolar range, inhibit synaptic transmission of both intracortical pathways and WM. Since WM contains extracortical connections, we suggest that at high ACh concentrations there is a suppression of both intracortical and extracortical inputs. Intermediate ACh concentrations (500 μm) facilitated responses to extracortical input, while inhibiting intracortical inputs. Considering that about 80% of excitatory synapses in the cortex are of intracortical origin (Douglas & Martin, 1991), specific cholinergic enhancement of extracortical connections appears to be a suitable mechanism to increase the influence of extracortical input on the cortical network. ACh in the range of 20–50 μm facilitated both extracortical and intracortical inputs and, finally, concentrations lower than 20 μm favoured only intracortical connections. Therefore changes of local ACh levels, together with the activity of ChE and the stimulation of different mAChR subtypes, appear to be responsible for the fine adjustment of cortical responsiveness to different inputs in primary visual cortex.
The two intracortical pathways, i.e. the layer II–III and layer IV intracortical pathways, were modulated in a similar way by the different ACh concentrations. This could be due to a similar ACh responsiveness of cortical neurones activated by the stimulation of either pathway, probably due to similar mAChR and ChE expression and distribution patterns. However, we cannot exclude minor differences among the ACh responsiveness of the two intracortical pathways at ACh concentrations not used in the present study.
Microdialysis studies showed that cortical ACh levels are in the range of tens to hundredths of micromolar (Mitsushima et al. 1996; Acquas et al. 1998; Diez-Ariza et al. 2002), i.e. one order of magnitude lower than necessary to induce depression of synaptic transmission under our experimental conditions. However, microdialysis studies use large volumes of extracellular liquid that accumulate over minutes, and most of the results have been obtained in the presence of ChE inhibitors with the consequence that this technique cannot be used to determine actual local ACh concentrations. Thus, it remains to be investigated whether local ACh concentrations, under physiological conditions, can reach the ACh levels used in this study.
We showed that in occipital slices it is possible to modulate the amplitude of basal FPs when ChE activity is drastically reduced by 10 μm edrophonium, leading to an increase in endogenous ACh levels. This observation, together with data showing that mAChR antagonists did not change FP amplitudes, suggests that endogenous ACh is present but at a relatively low level, which is not sufficient to modulate cortical responses.
Concluding remarks
We showed that changes of local ACh, together with the activity of ChE and the stimulation of different mAChR subtypes, appear to be responsible for the fine adjustment of cortical responsiveness to different inputs in primary visual cortex. Since the activity of basal forebrain cholinergic nuclei regulates cortical efflux of ACh (for reviews see Hasselmo & McGaughy, 2004; Pepeu & Giovannini, 2004), we propose that local ACh concentrations acting through specific mAChR subtypes modulate the cortical flow of information. This mechanism may play a key role in switching the cortex through different cognitive states associated with high or low activity of the cholinergic system (Sarter et al. 1996; Passetti et al. 2000; Dalley et al. 2001; Pepeu & Giovannini, 2004). Consistent with this concept, Hasselmo & McGaughy (2004) proposed a model where high levels of ACh favour encoding of sensory information by enhancing extracortical over intracortical inputs, while low levels of ACh promote memory consolidation inducing enhancement of intracortical versus extracortical inputs.
Cholinergic impairment is associated with age-related cognitive deficits such as those occurring in sporadic Alzheimer's disease (AD) (Auld et al. 2002). ChE inhibitors, which increase ACh levels in the brain, are used for the treatment of AD. However, the present data suggest that ChE regulates local ACh concentrations to induce either enhancement or suppression of synaptic inputs. As discussed above the physiological activity of the cholinergic system depends on selective activation of specific mAChR subtypes. Therefore, it would be interesting to study new pharmacological tools targeted at specific mAChRs, for example allosteric ligands that can change the binding affinity of ACh for distinct mAChR subtypes (Tucek & Proska, 1995; Jakubik et al. 1997). This strategy, together with the use of therapeutic agents that increase the bio-availability of ACh, might be particularly useful to halt the cognitive impairment in neurodegenerative diseases affecting the cholinergic system.
Acknowledgments
We are grateful to Giancarlo Pepeu, Enrico Cherubini and Alessandro Treves for critically reading the manuscript. We acknowledge W.W. Anderson and C. Orsini for on-line acquisition software. This work was partially supported by a grant from the Friuli Venezia Giulia Region (NEUR.291, Convenzione 2003 FVG/SISSA).
References
- Acquas E, Wilson C, Fibiger HC. Pharmacology of sensory stimulation-evoked increases in frontal cortical acetylcholine release. Neuroscience. 1998;85:73–83. doi: 10.1016/s0306-4522(97)00546-0. [DOI] [PubMed] [Google Scholar]
- Anderson WW, Collingridge GL. The LTP Program: a data acquisition program for on-line analysis of long-term potentiation and other synaptic events. J Neurosci Meth. 2001;108:71–83. doi: 10.1016/s0165-0270(01)00374-0. [DOI] [PubMed] [Google Scholar]
- Aubert I, Cecyre D, Gauthier S, Quirion R. Comparative ontogenic profile of cholinergic markers, including nicotinic and muscarinic receptors, in the rat brain. J Com Neurol. 1996;369:31–55. doi: 10.1002/(SICI)1096-9861(19960520)369:1<31::AID-CNE3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Auld DS, Kornecook TJ, Bastianetto S, Quirion R. Alzheimer's disease and the basal forebrain cholinergic system: relations to beta-amyloid peptides, cognition, and treatment strategies. Prog Neurobiol. 2002;68:209–245. doi: 10.1016/s0301-0082(02)00079-5. [DOI] [PubMed] [Google Scholar]
- Bode-Greuel KM, Singer W, Aldenhoff JB. A current source density analysis of field potentials evoked in slices of visual cortex. Exp Brain Res. 1987;69:213–219. doi: 10.1007/BF00247044. [DOI] [PubMed] [Google Scholar]
- Buwalda B, de Groote L, Van der Zee EA, Matsuyama T, Luiten PG. Immunocytochemical demonstration of developmental distribution of muscarinic acetylcholine receptors in rat parietal cortex. Brain Res Dev Brain Res. 1995;84:185–191. doi: 10.1016/0165-3806(94)00170-5. [DOI] [PubMed] [Google Scholar]
- Caulfield MP, Birdsall NJ. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998;50:279–290. [PubMed] [Google Scholar]
- Chang Q, Gold PE. Switching memory systems during learning: changes in patterns of brain acetylcholine release in the hippocampus and striatum in rats. J Neurosci. 2003;23:3001–3005. doi: 10.1523/JNEUROSCI.23-07-03001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CL, Metherate R, Ashe JH. Modulation of cellular excitability in neocortex: muscarinic receptor and second messenger-mediated actions of acetylcholine. Synapse. 1994;16:123–136. doi: 10.1002/syn.890160206. [DOI] [PubMed] [Google Scholar]
- Dalley JW, McGaughy J, O'Connell MT, Cardinal RN, Levita L, Robbins TW. Distinct changes in cortical acetylcholine and noradrenaline efflux during contingent and noncontingent performance of a visual attentional task. J Neurosci. 2001;21:4908–4914. doi: 10.1523/JNEUROSCI.21-13-04908.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descarries L, Gisiger V, Steriade M. Diffuse transmission be acetylcholine in the CNS. Prog Neurobiol. 1997;53:603–625. doi: 10.1016/s0301-0082(97)00050-6. [DOI] [PubMed] [Google Scholar]
- Diez-Ariza M, Garcia-Alloza M, Lasheras B, Del Rio J, Ramirez MJ. GABA (A) receptor antagonists enhance cortical acetylcholine release induced by 5-HT3 receptor blockade in freely moving rats. Brain Res. 2002;956:81–85. doi: 10.1016/s0006-8993(02)03483-2. [DOI] [PubMed] [Google Scholar]
- Domenici L, Harding GW, Burkhalter A. Patterns of synaptic activity in forward and feedback pathways within rat visual cortex. J Neurophysiol. 1995;74:2649–2664. doi: 10.1152/jn.1995.74.6.2649. [DOI] [PubMed] [Google Scholar]
- Douglas RJ, Martin KA. A functional microcircuit for cat visual cortex. J Physiol. 1991;440:735–769. doi: 10.1113/jphysiol.1991.sp018733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duttaroy A, Gomeza J, Gan JW, Siddiqui N, Basile AS, Harman WD, Smith PL, Felder CC, Levey AI, Wess J. Evaluation of muscarinic agonist-induced analgesia in muscarinic acetylcholine receptor knockout mice. Mol Pharmacol. 2002;62:1084–1093. doi: 10.1124/mol.62.5.1084. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu Rev Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Fadda F, Melis F, Stancampiano R. Increased hippocampal acetylcholine release during a working memory task. Eur J Pharmacol. 1996;307:R1–R2. doi: 10.1016/0014-2999(96)00289-0. [DOI] [PubMed] [Google Scholar]
- Fisahn A, Yamada M, Duttaroy A, Gan J-W, Deng C-X, McBain CJ, Wess J. Muscarinic induction of hippocampal gamma oscillations requires coupling of the M1 receptor to two mixed cation channels. Neuron. 2002;33:615–624. doi: 10.1016/s0896-6273(02)00587-1. [DOI] [PubMed] [Google Scholar]
- Gautam D, Heard TS, Cui Y, Miller G, Bloodworth L, Wess J. Cholinergic stimulation of salivary secretion studied with M1 and M3 muscarinic receptor single- and double-knockout mice. Mol Pharmacol. 2004;66:260–267. doi: 10.1124/mol.66.2.260. [DOI] [PubMed] [Google Scholar]
- Gil Z, Connors BW, Amitai Y. Differential regulation of neocortical synapses by neuromodulators and activity. Neuron. 1997;19:679–686. doi: 10.1016/s0896-6273(00)80380-3. [DOI] [PubMed] [Google Scholar]
- Gomeza J, Shannon H, Kostenis E, Felder C, Zhang L, Brodkin J, Grinberg A, Sheng H, Wess J. Pronounced pharmacologic deficits in M2 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci U S A. 1999a;96:1692–1697. doi: 10.1073/pnas.96.4.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomeza J, Zhang L, Kostenis K, Felder C, Bymaster F, Brodkin J, Shannon H, Xia B, Deng C, Wess J. Enhancement of D1 dopamine receptor-mediated locomotor stimulation in M4 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci U S A. 1999b;96:10483–10488. doi: 10.1073/pnas.96.18.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Bower JM. Cholinergic suppression specific to intrinsic not afferent fiber synapses in rat piriform (olfactory) cortex. J Neurophysiol. 1992;67:1222–1229. doi: 10.1152/jn.1992.67.5.1222. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Cekic M. Suppression of synaptic transmission may allow combination of associative feedback and self-organizing feedforward connections in the neocortex. Behav Brain Res. 1996;79:153–161. doi: 10.1016/0166-4328(96)00010-1. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, McGaughy J. High acetylcholine sets circuit dynamics for attention and encoding; low acetylcholine sets dynamics for consolidation. Prog Brain Res. 2004;145:207–231. doi: 10.1016/S0079-6123(03)45015-2. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Schnell E. Laminar selectivity of the cholinergic suppression of synaptic transmission in rat hippocampal region CA1: computational modeling and brain slice physiology. J Neurosci. 1994;14:3898–3914. doi: 10.1523/JNEUROSCI.14-06-03898.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hironaka N, Tanaka K, Izaki Y, Hori K, Nomura M. Memory-related acetylcholine efflux from rat prefrontal cortex and hippocampus: a microdialysis study. Brain Res. 2001;901:143–150. doi: 10.1016/s0006-8993(01)02338-1. [DOI] [PubMed] [Google Scholar]
- Huerta PT, Lisman JE. Heightened synaptic plasticity of hippocampal CA1 neurons during a cholinergically induced rhythmic state. Nature. 1993;364:723–725. doi: 10.1038/364723a0. [DOI] [PubMed] [Google Scholar]
- Jakubik J, Bacakova L, El-Fakahany EE, Tucek S. Positive cooperativity of acetylcholine and other agonists with allosteric ligands on muscarinic acetylcholine receptors. Mol Pharmacol. 1997;52:172–179. doi: 10.1124/mol.52.1.172. [DOI] [PubMed] [Google Scholar]
- Kimura F, Baughman RW. Distinct muscarinic receptor subtypes suppress excitatory and inhibitory synaptic responses in cortical neurons. J Neurophysiol. 1997;77:709–716. doi: 10.1152/jn.1997.77.2.709. [DOI] [PubMed] [Google Scholar]
- Kimura F, Fukuda M, Tsumoto T. Acetylcholine suppresses the spread of excitation in the visual cortex revealed by optical recording: possible differential effect depending on the source of input. Eur J Neurosci. 1999;1:3597–3609. doi: 10.1046/j.1460-9568.1999.00779.x. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Bear MF. Hebbian synapses in visual cortex. J Neurosci. 1994;14:1634–1645. doi: 10.1523/JNEUROSCI.14-03-01634.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Rozas C, Kirkwood J, Perez F, Bear MF. Modulation of long-term synaptic depression in visual cortex by acetylcholine and norepinephrine. J Neurosci. 1999;19:1599–1609. doi: 10.1523/JNEUROSCI.19-05-01599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjevic K. Synaptic mechanisms modulated by acetylcholine in cerebral cortex. Prog Brain Res. 2004;145:81–93. [PubMed] [Google Scholar]
- Krnjevic K, Phillis JW. Acetylcholine-sensitive cells in the cerebral cortex. J Physiol. 1963;166:296–327. doi: 10.1113/jphysiol.1963.sp007106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazareno S, Birdsall NJ. Detection, quantitation, and verification of allosteric interactions of agents with labeled and unlabeled ligands at G protein-coupled receptors: interactions of strychnine and acetylcholine at muscarinic receptors. Mol Pharmacol. 1995;48:362–378. [PubMed] [Google Scholar]
- Lee SM, Weisskopf MG, Ebner FF. Horizontal long-term potentiation of responses in rat somatosensory cortex. Brain Res. 1991;544:303–310. doi: 10.1016/0006-8993(91)90069-8. [DOI] [PubMed] [Google Scholar]
- Levey AI, Kitt CA, Simonds WF, Price DL, Brann MR. Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci. 1991;11:3218–3226. doi: 10.1523/JNEUROSCI.11-10-03218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster C, Maloney M, Patil M, Hasselmo ME. Enhanced cholinergic suppression of previously strengthened synapses enables the formation of self-organized representations in olfactory cortex. Neurobiol Learn Mem. 2003;80:302–314. doi: 10.1016/s1074-7427(03)00078-9. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Prince DA. Actions of acetylcholine in the guinea-pig and cat medial and lateral geniculate nuclei, in vitro. J Physiol. 1987;392:147–165. doi: 10.1113/jphysiol.1987.sp016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei L, Berardi N, Domenici L, Parisi V, Pizzorusso T. Nerve growth factor (NGF) prevents the shift in ocular dominance distribution of visual cortical neurons in monocularly deprived rats. J Neurosci. 1992;12:4651–4662. doi: 10.1523/JNEUROSCI.12-12-04651.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino MJ, Rouse ST, Levey AI, Potter LT, Conn PJ. Activation of the genetically defined m1 muscarinic receptor potentiates N-methyl-d-aspartate (NMDA) receptor currents in hippocampal pyramidal cells. Proc Natl Acad Sci U S A. 1998;95:11465–11470. doi: 10.1073/pnas.95.19.11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massoulie J, Anselmet A, Bon S, Krejci E, Legay C, Morel N. The polymorphism of acetylcholinesterase post-translational processing, quaternary association and localization. Chem Biol Interact. 1999;118:29–42. doi: 10.1016/s0009-2797(99)00011-3. [DOI] [PubMed] [Google Scholar]
- Mitsushima D, Mizuno T, Kimura F. Age-related changes in diurnal acetylcholine release in the prefrontal cortex of male rats as measured by microdialysis. Neuroscience. 1996;72:429–434. doi: 10.1016/0306-4522(95)00572-2. [DOI] [PubMed] [Google Scholar]
- Mitzdorf U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol Rev. 1985;65:37–100. doi: 10.1152/physrev.1985.65.1.37. [DOI] [PubMed] [Google Scholar]
- Mitzdorf U, Singer W. Basic patterns of synaptic activity in visual cortex of normal and monocularly deprived cats: a current source density analysis of electrically evoked potentials. J Physiol. 1978;284:120. [PubMed] [Google Scholar]
- Mrzljak L, Levey AI, Goldman Rakic PS. Association of m1 and m2 muscarinic receptor proteins with asymmetric synapses in the primate cerebral cortex: morphological evidence for cholinergic modulation of excitatory neurotransmission. Proc Natl Acad Sci U S A. 1993;90:5194–5198. doi: 10.1073/pnas.90.11.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page KM, Curtis CA, Jones PG, Hulme EC. The functional role of the binding site aspartate in muscarinic acetylcholine receptors, probed by site-directed mutagenesis. Eur J Pharmacol. 1995;289:429–437. doi: 10.1016/0922-4106(95)90151-5. [DOI] [PubMed] [Google Scholar]
- Passetti F, Dalley JW, O'Connell MT, Everitt BJ, Robbins TW. Increased acetylcholine release in the rat medial prefrontal cortex during performance of a visual attentional task. Eur J Neurosci. 2000;12:3051–3058. doi: 10.1046/j.1460-9568.2000.00183.x. [DOI] [PubMed] [Google Scholar]
- Pepeu G, Giovannini MG. Changes in acetylcholine extracellular levels during cognitive processes. Learn Mem. 2004;11:21–27. doi: 10.1101/lm.68104. [DOI] [PubMed] [Google Scholar]
- Pesavento E, Margotti E, Righi M, Cattaneo A, Domenici L. Blocking the NGF–TrkA interaction rescues the developmental loss of LTP in the rat visual cortex: role of the cholinergic system. Neuron. 2000;25:165–175. doi: 10.1016/s0896-6273(00)80880-6. [DOI] [PubMed] [Google Scholar]
- Quinn DM. Acetylcholinesterase: enzyme structure, reaction dynamics and virtual translation states. Chem Re. 1987;87:955–979. [Google Scholar]
- Ragozzino ME, Unick KE, Gold PE. Hippocampal acetylcholine release during memory testing in rats: augmentation by glucose. Proc Natl Acad Sci U S A. 1996;93:4693–4698. doi: 10.1073/pnas.93.10.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberry TL. Catalysis by acetylcholinesterase; evidence that the rate-limiting step for acylation with certain substrates precedes general acid–base catalysis. Proc Natl Acad Sci U S A. 1975;72:3834–3838. doi: 10.1073/pnas.72.10.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotundo RL, Carbonetto ST. Neurons segregate clusters of membrane-bound acetylcholinesterase along their neurites. Proc Natl Acad Sci U S A. 1987;84:2063–2067. doi: 10.1073/pnas.84.7.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Bruno JP. Cognitive functions of cortical acetylcholine: toward a unifying hypothesis. Brain Res Brain Res Rev. 1997;23:28–46. doi: 10.1016/s0165-0173(96)00009-4. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP, Givens B, Moore H, McGaughy J, McMahon K. Neuronal mechanisms mediating drug-induced cognition enhancement: cognitive activity as a necessary intervening variable. Brain Res Cogn Brain Res. 1996;3:329–343. doi: 10.1016/0926-6410(96)00018-3. [DOI] [PubMed] [Google Scholar]
- Sato H, Hata Y, Masui H, Tsumoto T. A functional role of cholinergic innervation to neurons in the cat visual cortex. J Neurophysiol. 1987;58:765–780. doi: 10.1152/jn.1987.58.4.765. [DOI] [PubMed] [Google Scholar]
- Segal M, Auerbach JM. Muscarinic receptors involved in hippocampal plasticity. Life Sci. 1997;60:1085–1091. doi: 10.1016/s0024-3205(97)00051-9. [DOI] [PubMed] [Google Scholar]
- Sillito AM, Kemp JA. Cholinergic modulation of the functional organization of the cat visual cortex. Brain Res. 1983;289:143–155. doi: 10.1016/0006-8993(83)90015-x. [DOI] [PubMed] [Google Scholar]
- Silver A. The biology of cholinesterase. In: Neuberger A, Tatum EL, editors. Frontiers of Biology. Vol 36. Amsterdam: Elsevier; 1974. [Google Scholar]
- Tigges M, Tigges J, Rees H, Rye D, Levey AI. Distribution of muscarinic cholinergic receptor proteins m1 to m4 in area 17 of normal and monocularly deprived rhesus monkeys. J Comp Neurol. 1997;388:130–145. [PubMed] [Google Scholar]
- Tucek S, Proska J. Allosteric modulation of muscarinic acetylcholine receptors. Trends Pharmacol Sci. 1995;16:205–212. doi: 10.1016/s0165-6147(00)89023-9. [DOI] [PubMed] [Google Scholar]
- Vidal C, Changeux JP. Nicotinic and muscarinic modulations of excitatory synaptic transmission in the rat prefrontal cortex in vitro. Neuroscience. 1993;56:23–32. doi: 10.1016/0306-4522(93)90558-w. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Koder T, Cho K, Massey PV, Duguid G, Barker GR, Aggleton JP, Bashir ZI, Brown MW. Cholinergic neurotransmission is essential for perirhinal cortical plasticity and recognition memory. Neuron. 2003;38:987–996. doi: 10.1016/s0896-6273(03)00358-1. [DOI] [PubMed] [Google Scholar]
- Wess J. Muscarinic acetylcholine receptor knockout mice: novel phenotypes and clinical implications. Annu Rev Pharmacol Toxicol. 2004;44:423–450. doi: 10.1146/annurev.pharmtox.44.101802.121622. [DOI] [PubMed] [Google Scholar]
- Yamada M, Lamping KG, Duttaroy A, Zhang W, Cui Y, Bymaster FP, McKinzie DL, Felder CC, Deng CX, Faraci FM, Wess J. Cholinergic vasodilation of cerebral blood vessels is abolished in M5 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci U S A. 2001b;98:14096–14101. doi: 10.1073/pnas.251542998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Miyakawa T, Duttaroy A, Yamanaka A, Moriguchi T, Makita R, Ogawa M, Chou CJ, Xia B, Crawley JN, Felder CC, Deng CX, Wess J. Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nature. 2001a;410:207–212. doi: 10.1038/35065604. [DOI] [PubMed] [Google Scholar]
- Zilles K, Wree A, Schleider A, Divac I. The monocular and binocular subfields of the rat's primary visual cortex: a quantitative morphological approach. J Comp Neurol. 1984;226:391–402. doi: 10.1002/cne.902260308. [DOI] [PubMed] [Google Scholar]