Abstract
Prenatal stress can have profound long-term influences on physiological function throughout the course of life. We hypothesized that focused periods of moderate prenatal stress at discrete time points in late gestation have differential effects on hypothalamo–pituitary–adrenal (HPA) axis function in adult guinea pig offspring, and that changes in HPA axis function will be associated with modification of anxiety-related behaviour. Pregnant guinea pigs were exposed to a strobe light for 2 h on gestational days (GD) 50, 51, 52 (PS50) or 60, 61, 62 (PS60) (gestation length ∼70 days). A control group was left undisturbed throughout pregnancy. Behaviour was assessed in male offspring on postnatal day (PND)25 and PND70 by measurement of ambulatory activity and thigmotaxis (wall-seeking behaviour) in a novel open field environment. Subsequent to behavioural testing, male offspring were cannulated (PND75) to evaluate basal and activated HPA axis function. Body weight was significantly decreased in adult PS50 and PS60 offspring and this effect was apparent soon after weaning. The brain-to-body-weight ratio was significantly increased in adult PS50 males. Basal plasma cortisol levels were elevated in PS50 male offspring throughout the 24 h sampling period compared with controls. In response to an ACTH challenge and to exposure to an acute stressor, PS60 male offspring exhibited elevated plasma cortisol responses. Plasma testosterone concentrations were strikingly decreased in PS50 offspring. Thigmotaxis in the novel environment was increased in PS50 male offspring at PND25 and PND70, suggesting increased anxiety in these animals. In conclusion, prenatal stress during critical windows of neuroendocrine development programs growth, HPA axis function, and stress-related behaviour in adult male guinea pig offspring. Further, the nature of the effect is dependant on the timing of the maternal stress during pregnancy.
Epidemiological studies have revealed a relationship between low birth weight and an increased risk of mortality from cardiovascular disease and its associated risk factors including hypertension (Barker, 1995; Phillips et al. 2000). Low birth weight is used as a broad marker of disturbed fetal development, suggesting that impaired intrauterine growth has long-term effects. The mechanism underlying this relationship is largely unknown; however, exposure to excess glucocorticoids during critical windows of neuroendocrine development appears to be one of the primary causative factors (Matthews, 2002). Animal studies, together with retrospective studies in the human have shown that prenatal maternal stress is associated with reduced growth in utero and can lead to an increased risk for a number of pathologies, including impaired behavioural/emotional development and depression (Watson et al. 1999; O'connor et al. 2003).
Studies in rats have demonstrated profound effects of repeated prenatal stress on HPA axis function and behaviour in offspring, including elevated basal and stress-induced HPA axis activity and alterations in the circadian rhythm of corticosterone secretion (Weinstock et al. 1998; Koehl et al. 1999). However, rats are neuroanatomically immature in late gestation compared with humans. Studies in non-human primates have demonstrated altered HPA axis activity and stress-related behaviour in offspring of mothers stressed during pregnancy (Clarke et al. 1994; Coe et al. 2003). The stress protocols employed by non-human primate studies span a range of neurodevelopmental landmarks. Therefore, specific times of maximal fetal vulnerability and the long-term effects of prenatal stress during specific periods of brain development are currently unknown. The guinea pig, like humans, gives birth to neuroanatomically mature offspring and landmarks of brain and neuroendocrine growth are well characterized in this species (Dobbing & Sands, 1970). In the guinea pig, maximal brain growth occurs around GD50 (75% of gestation) and in the human this phase is initiated at 28–36 weeks (80%) (Dobbing & Sands 1970, 1979). In contrast, the phase of maximal brain growth in the rat does not occur until PND5–8 (Dobbing & Sands, 1979). Further, unlike other rodents the guinea pig has a similar pattern of placentation (haemomonochorial) to the human (Leiser & Kaufmann, 1994) and has a long gestation (∼70 days). The latter allows very specific aspects of fetal development to be targeted. Previous studies in our laboratory have demonstrated that short periods of nutrient restriction during the period of rapid fetal brain growth (GD50), results in male guinea pig offspring with decreased basal plasma ACTH and cortisol levels (Lingas & Matthews, 2001). However, since nutrient restriction is a severe metabolic stressor, other maternal and fetal physiological pathways are probably activated in addition to the HPA axis and sympathetic nervous system. An early study in the guinea pig demonstrated that exposure to a strobe light for 3 h on GD60 and GD67 increased maternal and fetal plasma ACTH and cortisol concentrations (Dauprat et al. 1984), and subsequently resulted in decreased plasma ACTH and cortisol responses to stress in juvenile and adult mixed-sex offspring (Cadet et al. 1986).
In the current study, we wished to assess the effect of short bouts of moderate maternal psychological stress at very specific periods of fetal brain development on HPA axis activity, anxiety-related behaviour and blood pressure in offspring. The developmental windows chosen for this study were the period of rapid fetal brain growth (GD50) and the period of maximal myelination and glial cell formation (GD60) (Dobbing & Sands, 1970). Such focused targeting of specific periods of brain development has not been undertaken in previous prenatal stress studies, but is essential for understanding the mechanisms of prenatal brain programming. We hypothesized that: (1) focused periods of moderate prenatal psychological stress will have effects on HPA axis function in adult guinea pig offspring; however, the magnitude and direction of the effect will be dependent upon the timing of the prenatal stress, and (2) there will be associated effects on behaviour in juvenile and adult offspring.
Methods
Animals
Female guinea pigs (400–500 g) (Hartley strain, Charles River Canada, St Constant, PQ, Canada) were mated in our animal facility as previously described (Dean & Matthews, 1999). This method produces accurately time-dated pregnant guinea pigs. Food (Guinea Pig Chow 5025, Ralston Purina International, Leis Pet Distributing Inc. Wellesley, ON, Canada) and water were available ad libitum. The animals were kept in a 12: 12 h light–dark cycle, with lights off at 19.00 h. Room temperature was 23°C. All studies were performed according to protocols approved by the Animal Care Committee at the University of Toronto, in accordance with the Canadian Council for Animal Care.
An initial pilot study was undertaken to determine the plasma cortisol responses of pregnant guinea pigs to a high frequency strobe light. While it had previously been determined that this stimulus results in a robust HPA response in pregnant guinea pigs (Cadet et al. 1986) we wished to confirm that this was the case in our laboratory before embarking on these developmental studies. An indwelling arterial catheter was surgically implanted in pregnant animals (n = 4) on GD45 as previously described (Banjanin et al. 2004). For the current study, however, animals were anaesthetized using Isoflurane (2–3%, IsoFlo®, USP, Abbott Laboratories, Limited, Saint-Laurent, Quebec). Maternal plasma cortisol response to a strobe light (Micro Strobelite, Poppy Industries) was assessed on GD54 and 60. Repeated measures ANOVA revealed a significant effect of the strobe light on maternal plasma cortisol levels for each exposure (P < 0.05), with an increase in plasma cortisol levels of 20 and 40%, respectively.
For the main study, pregnant guinea pigs were exposed to the strobe light for 2 h, from 09.00 to 11.00 h, on GD50, 51 and 52 (PS50, n = 12) or GD60, 61 and 62 (PS60, n = 9). A control group of pregnant guinea pigs (n = 12) was left undisturbed throughout gestation except for routine maintenance. All animals were allowed to deliver normally. Normal litter size is two to three fetuses. After birth, guinea pig offspring were weighed on PND5, 10 and 20. Animals were weaned on PND25, weighed, subjected to a 30 min behaviour test and then placed into individual clear polycarbonate cages. Animals were within visual, auditory and olfactory contact of at least two other animals at all times. Offspring remained undisturbed except for biweekly cage maintenance, weighing (PND30, 40, 50, 60 and 70) and behavioural testing.
Behavioural testing
Ambulatory activity and thigmotaxis in a novel environment (30 min) was determined twice using an Opto-Max animal activity meter (Columbus Instruments, Columbus, OH, USA) on PND25 and PND70. Horizontal (ambulatory) spontaneous locomotor activity values of individual offspring (1 from each litter) at PND25 and PND70 were determined at 5 min intervals for a 30 min period using an Opto-Varimex® monitor (Columbus Instruments; Columbus, OH, USA). This apparatus was equipped with an array of 15 infrared beams positioned 3 cm above the floor to measure horizontal spontaneous locomotor activity. Successive beam breaks in a 42 cm × 42 cm openfield arena and thigmotaxis, defined as the time spent in the outer 14 cm from the perimeter into the centre of the box, was quantified. Behavioural analysis of the individual offspring was performed between 08.00 and 10.00 h in a room with ambient temperature of 23°C and standard fluorescent lighting, in which noise was minimized. This behavioural testing system has previously been used in guinea pigs (Gibson et al. 2000).
Endocrine tests
On PND75, catheters were surgically implanted in the carotid artery under isoflurane anaesthesia as described above. Catheters were attached to a swivel system (Lomir Biomedical Inc., Notre-Dame-de-I'lle-Perot, PQ, Canada) above the cage, as previously described (Liu et al. 2001; Banjanin et al. 2004). This allowed full rotation of the catheter and unrestricted movement of the guinea pig. Repeated sampling of animals catheterized in this way does not result in activation of the HPA axis (Liu & Matthews, 1999; Liu et al. 2001). Catheters were filled with heparinized saline and flushed daily. Animals were left to recover a minimum of 3 days following surgery then exposed to a series of tests of HPA activity which were carried in the same order in each animal. Guinea pigs were allowed 48 h between tests.
Circadian rhythm
Twenty-four hour blood sampling was commenced at 07.00 h, samples were then taken every 2 h (200 μl) until 05.00 h the following day.
Adrenal sensitivity
ACTH1-24 (human; 2.0 μg kg−1i.a.) challenge commenced at 13.00 h and blood samples (100 μl) were taken at −30, 0, 30, 60 and 120 min. An average of the −30 and 0 samples was used as measurement of basal ACTH and cortisol levels prior to testing. We have previously characterized the use of this dose of ACTH in the adult guinea pig (Liu & Matthews, 1999).
HPA response to acute stress
Exposure (30 min) to a strobe light was undertaken. This challenge was commenced at 13.00 h and blood samples (200 μl) were taken at −30, 0, 5, 15, 30, 45, 60, 90 and 120 min. An average of the −30 and 0 samples was used as measurement of basal ACTH and cortisol levels prior to testing. After each blood sample, catheters were flushed with a heparinized saline solution. Blood was collected into EDTA–Trasylol, and plasma was separated by centrifugation and stored at −20°C. Mean arterial pressure (MAP) and heart rate were measured on at least two different days in each animal via the carotid artery cannula, as previously described (Banjanin et al. 2004). Upon completion of endocrine tests, animals were left undisturbed for at least 72 h prior to killing by decapitation. Organs were collected and weighed.
Endocrine analysis
Double-antibody and coated tube radioimmunoassay kits (ICN Biomedical Inc., Costa Mesa, CA, USA) were used to determine plasma ACTH, cortisol and testosterone concentrations. These assays have been previously used in the guinea pig (Mccabe et al. 2001; Owen et al. 2001; Banjanin et al. 2004). All samples from within each test were run in the same assay to negate interassay bias.
Statistical analysis
All data are expressed as mean ± standard error of the mean (s.e.m.). For all tests, significance was set at P < 0.05. Data were statistically analysed using multivariate analysis of variance (ANOVA), followed by Newman-Keuls method of post hoc comparison. Gestation length, litter size, MAP, heart rate, ambulatory activity, inner zone time and plasma testosterone concentrations were analysed by one-way (prenatal treatment) ANOVA. Maternal weight gain, offspring weight gain, 24 h sampling, ACTH challenge and the strobe light exposure were analysed by two-way (prenatal treatment × time) repeated measures ANOVA as well as one-way (prenatal treatment) ANOVA. Twenty-four hour plasma ACTH and cortisol data were also analysed by one-way (treatment) ANOVA on the area under the curve (AUC).
Results
Pregnancy outcome, weights and blood pressure
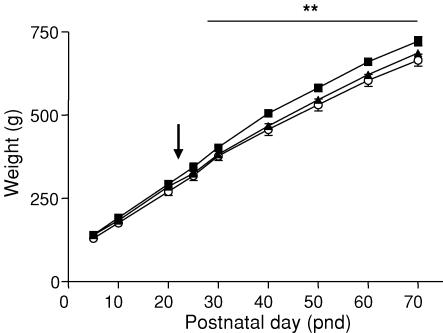
There was no significant effect of prenatal stress on maternal body weight or weight gain during pregnancy (Table 1). There was no effect on gestational length (mean ± s.e.m.: control, 69.82 ± 0.43 days; PS50, 69.13 ± 0.20 days; PS60, 69.89 ± 0.33 days) or litter size (mean ± s.e.m.: control, 2.94 ± 0.22; PS50, 3.06 ± 0.20; PS60, 3.15 ± 0.28). Prenatal stress did not cause a reduction in birth weight or body weight in the pre-weaning period (Fig. 1). However, PS60 offspring demonstrated a significant reduction (P < 0.01) in body weight during the post-weaning period (PND30–70) and PS50 offspring demonstrated a trend towards this effect (P = 0.086). On PND70 offspring (PS50) exhibited significantly increased brain-to-body-weight ratios compared with controls (P < 0.05), but this effect was not observed in PS60 offspring (Table 2). There was no significant effect of prenatal stress on other organ-to-body-weight ratios (Table 2), mean arterial pressure or heart rate (Table 3).
Table 1.
Body weights (g, mean ± s.e.m.) of guinea pig dams during pregnancy
| Day of pregnancy | ||||||||
|---|---|---|---|---|---|---|---|---|
| Prenatal treatment | 30 | 35 | 40 | 45 | 50 | 55 | 60 | 65 |
| Control (12) | 767.4 ± 27.51 | 798.5 ± 25.62 | 838.9 ± 25.52 | 885.5 ± 27.61 | 951.4 ± 28.58 | 993.1 ± 36.05 | 1035.1 ± 38.48 | 1108.9 ± 40.85 |
| PS50 (12) | 781.0 ± 14.90 | 816.0 ± 16.93 | 856.0 ± 17.66 | 893.3 ± 17.62 | 960.8 ± 22.06 | 1008.0 ± 27.56 | 1043.4 ± 27.05 | 1122.6 ± 21.69 |
| PS60 (9) | 780.4 ± 22.83 | 821.0 ± 25.76 | 871.8 ± 27.72 | 912.8 ± 29.15 | 975.6 ± 35.57 | 1019.2 ± 40.30 | 1034.7 ± 48.07 | 1122.4 ± 49.48 |
Numbers in brackets indicate number of animals in each group.
Figure 1. Body weight.
Body weight (mean ± s.e.m.) of male offspring born to mothers left undisturbed throughout pregnancy (control, ▪, n = 14) or exposed to a strobe light (2 h day−1) on GD50–52 (PS50, ▴, n = 15) or GD60–62 (PS60, ○, n = 14). Arrow indicates weaning (PND25). **P < 0.01 PS60 versus control.
Table 2.
Organ weights expressed as organ-to-body weight (brain, pituitary, adrenal and peripheral) or organ-to-brain weight (hippocampus) ratios (mean± s.e.m.) in adult male offspring whose mothers had been left unstressed throughout pregnancy (control) or exposed to a strobe light on GD50–52 (PS50) or GD60–62 (PS60)
| HPA axis | ||||
|---|---|---|---|---|
| Prenatal treatment | Hippocampus | Pituitary | Brain | Adrenal |
| Control | 2.5 ± 0.2(11) | 5.5 ± 0.9(11) | 5.7 ± 0.2(11) | 3.1 ± 0.2(11) |
| PS50 | 2.5 ± 0.2(14) | 3.9 ± 0.2(14) | 6.5 ± 0.3(15)* | 3.2 ± 0.3(15) |
| PS60 | 2.8 ± 0.2(10) | 4.7 ± 0.5(10) | 5.9 ± 0.1(10) | 3.1 ± 0.2(10) |
| Peripheral | ||||
| Prenatal treatment | Heart | Lung | Kidney | Gonad |
| Control | 3.9 ± 0.4(11) | 3.2 ± 0.3(11) | 5.3 ± 0.3(11) | 2.1 ± 0.1(11) |
| PS50 | 4.2 ± 0.5(15) | 3.7 ± 0.3(15) | 5.8 ± 0.3(15) | 2.2 ± 0.1(15) |
| PS60 | 4.5 ± 0.6(10) | 3.1 ± 0.24(10) | 5.6 ± 0.4(10) | 2.3 ± 0.1(10) |
Ratios were multiplied by 102 (hippocampus), 103 (pituitary, brain and peripheral) and 104 (adrenal).
P < 0.05 indicates significant difference from controls. Numbers in brackets indicate numbers of animals in each group.
Table 3.
Mean arterial blood pressure (MAP) and heart rate (mean ± s.e.m.) in adult male offspring born to mothers that had been left undisturbed throughout pregnancy (control) or exposed to a strobe light on GD50–52 (PS50) or GD60–62 (PS60)
| Prenatal treatment | MAP (mmHg) | Heart rate (beats min−1) |
|---|---|---|
| Control | 38.9 ± 2.8 (6) | 239.0 ± 12.4 (6) |
| PS50 | 40.7 ± 2.6 (11) | 270.9 ± 10.7 (11) |
| PS60 | 37.0 ± 3.2 (10) | 265.9 ± 9.4 (10) |
Numbers in brackets indicate numbers of animals in each group.
Behaviour
Ambulatory activity
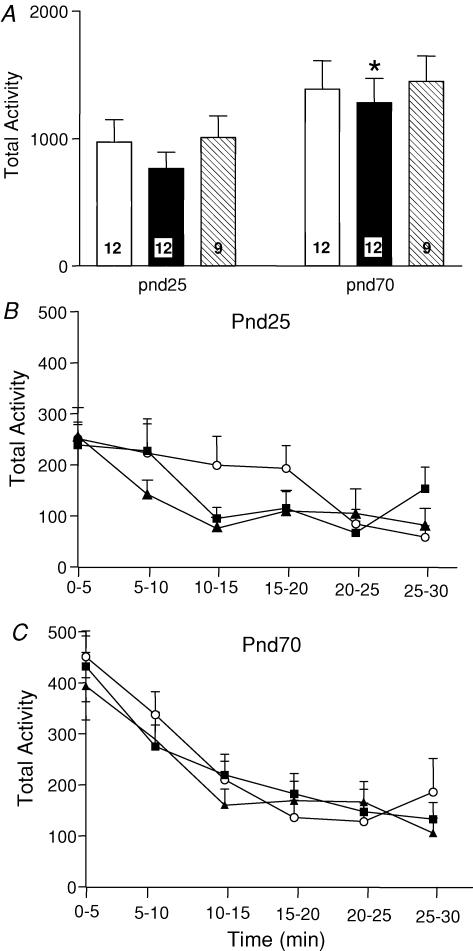
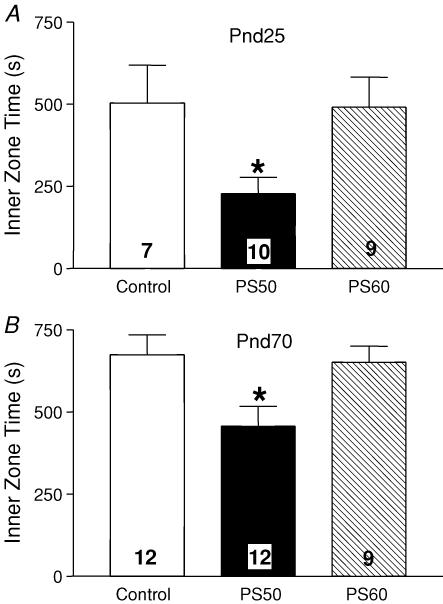
There was no significant difference in overall ambulatory activity at PND25 and PND70 between prenatal stress groups and controls (Fig. 2). However, an overall trend for increased ambulatory activity between PND25 and PND70 was observed and this was significant in the PS50 animals (P < 0.05; Fig. 2A). There was no significant difference in the profile of the 30 min of ambulatory activity when broken down into 5 min intervals at either PND25 or PND70 (Fig. 2B and C). At both PND25 and PND70, PS50 male offspring spent significantly less time in the inner zone of the activity box than control offspring (P < 0.05; Fig. 3); however, there were no differences between PS60 and control offspring at either age.
Figure 2. Ambulatory activity.
A, ambulatory activity (mean ± s.e.m.) over 30 min in an open field of male offspring at PND25 and PND70 born to mothers that were undisturbed throughout pregnancy (control, open bars), exposed to a strobe light on GD50–52 (PS50, filled bars) or GD60–62 (PS60, hatched bars). Numbers in each bar denote the number of animals in each group. *P < 0.05 compared with PND25 of same prenatal treatment group. B, ambulatory activity (30 min) divided into 5 min intervals of PND25 male offspring born to mothers that were undisturbed throughout pregnancy (control, ▪) or exposed to a strobe light on GD50–52 (PS50, ▴) or GD60–62 (PS60, ○). C, ambulatory activity (30 min) divided into 5 min intervals of the same groups of animals at PND70.
Figure 3. Time spent in the inner zone.
A, time spent in the inner zone of the open field (mean ± s.e.m.) by PND25 male offspring born to mothers that were undisturbed throughout pregnancy (control, open bars), exposed to a strobe light on GD50–52 (PS50, filled bars) or GD60–62 (PS60, hatched bars). Numbers in each bar denote the number of animals in each group. *P < 0.05 PS50 versus control. B, time spent in the inner zone of an open field (mean ± s.e.m.) by male offspring at PND70. *P < 0.05 PS50 versus control.
Endocrine
Circadian HPA rhythm
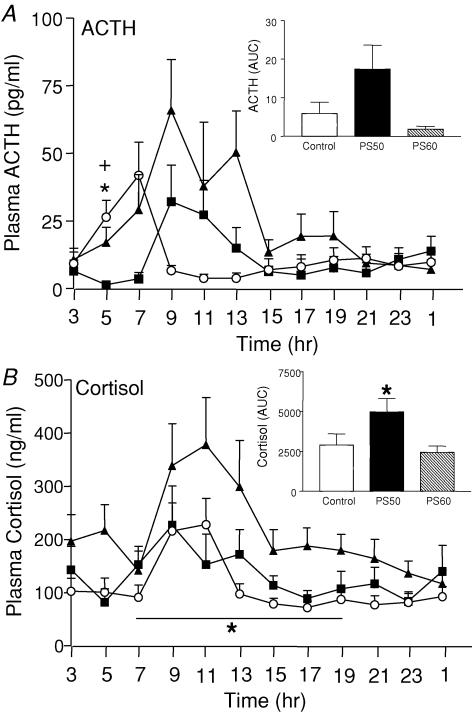
There was a significant effect of time on plasma ACTH (P < 0.05), and cortisol (P < 0.001) concentrations (Fig. 4); levels were generally higher during the subjective day (peak ∼09.00–11.00 h) and low throughout the night. Analysis of plasma ACTH levels revealed a significant interaction between prenatal treatment and time during the dark period (19.00–07.00 h). Further analysis revealed a significant effect of time on plasma ACTH levels in PS50 (P < 0.05) and PS60 (P < 0.001) offspring but not the control offspring. Post hoc analysis demonstrated significantly elevated plasma ACTH levels compared with controls (P < 0.05) at 05.00 h in PS50 and PS60 male offspring (Fig. 4A). In PS50 offspring, this appears to be a general increase in plasma ACTH levels during the morning, whereas, in the PS60 offspring, the increase at 05.00 h appears due to a phase shift of the circadian rhythm of ACTH secretion. Repeated measures ANOVA revealed a significant elevation in plasma cortisol concentrations during the light period (07.00–19.00 h) in PS50 offspring (P < 0.05; Fig. 4B) compared with controls. Post hoc analysis revealed that this effect on cortisol was most pronounced at 17.00 h (P < 0.05). Total cortisol levels over 24 h were also significantly greater in PS50 offspring as determined by AUC analysis (P < 0.05; Fig. 4B inset).
Figure 4. Twenty-four hour ACTH and cortisol concentrations.
A, 24 h plasma ACTH concentrations (mean ± s.e.m.) in male offspring born to mothers that were undisturbed throughout pregnancy (control, ▪), exposed to a strobe light on GD50–52 (PS50, ▴) or GD60–62 (PS60, ○). Inset, area under curve (AUC) for the entire 24-h period (03.00–01.00 h). Number of animals ranges from 7 to 14. *P < 0.05 PS50 versus control, +P < 0.05 PS60 versus control. B, 24 h plasma cortisol concentrations (mean ± s.e.m.) in male offspring born to mothers that were undisturbed throughout pregnancy (control, ▪), exposed to a strobe light on GD50–52 (PS50, ▴) or GD60–62 (PS60, ○). Inset, area under curve (AUC) for the entire 24-h period (03.00–01.00 h). Number of animals ranges from 7 to 14. *P < 0.05 PS50 versus control.
ACTH challenge
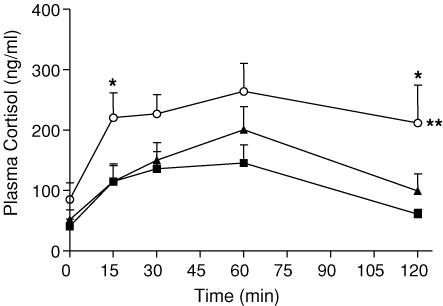
ACTH challenge (2.0 μg kg−1) significantly increased plasma cortisol concentrations over time (P < 0.001; Fig. 5). The plasma cortisol response to the challenge was significantly elevated in PS60 offspring compared with controls (P < 0.01). These differences were greatest at 15 and 120 min (P < 0.05). There was no significant difference in the plasma cortisol response to the ACTH challenge between the PS50 and control offspring.
Figure 5. Plasma cortisol concentrations.
Plasma cortisol concentrations (mean ± s.e.m.) in response to an ACTH (2.0 μg kg−1) challenge in male offspring born to mothers that were left undisturbed throughout pregnancy (control, ▪), exposed to a strobe light on GD50–52 (PS50, ▴) or GD60–62 (PS60, ○). ACTH was administered after the time 0 blood sample was taken. Number of animals ranges from 6 to 9. **P < 0.01, PS60 response over time versus control, *P < 0.05 PS60 versus control at a specific time point.
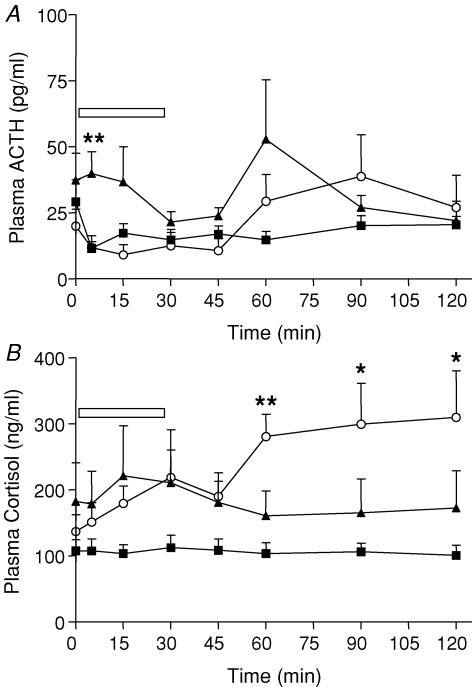
Strobe light exposure
Repeated measures ANOVA revealed no significant effect of time or prenatal treatment on plasma ACTH concentrations following exposure to the strobe light (Fig. 6A). At 5 min, PS50 males exhibited higher ACTH concentrations compared with control offspring (P < 0.01), though this was associated with higher baseline ACTH levels in this group and a decrease in ACTH levels in control offspring at this time point. There was a significant effect of the strobe light exposure (time) on plasma cortisol levels in PS60 offspring (P < 0.01; Fig. 6B) but not in PS50 or control offspring. PS60 offspring exhibited significantly greater plasma cortisol levels at 60 (P < 0.01), 90 (P < 0.05) and 120 (P < 0.05) minutes following challenge.
Figure 6. Plasma ACTH and cortisol concentrations.
A, plasma ACTH concentrations (mean ± s.e.m.) in response to exposure to a strobe light (30 min indicated by box) in adult male offspring born to mothers that were undisturbed throughout pregnancy (control, ▪), exposed to a high frequency strobe light on GD50–52 (PS50, ▴) or GD60–62 (PS60, ○). Number of animals ranges from 6 to 8. **P < 0.01 PS50 versus control. B, plasma cortisol concentrations (mean ± s.e.m.) in response to exposure to a strobe light. **P < 0.01, *P < 0.05 PS60 versus control.
Testosterone
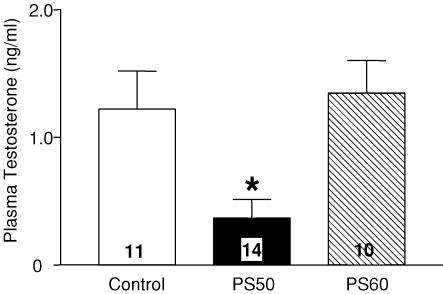
Plasma from the 09.00, 11.00, 13.00 and 15.00 h samples from 24 h circadian sampling were pooled to determine plasma testosterone concentrations. Levels of plasma testosterone were significantly reduced in PS50 offspring (P < 0.05; 30% of controls, Fig. 7). There was no effect of prenatal stress at GD60 on plasma testosterone concentrations in adult offspring.
Figure 7. Plasma testosterone concentrations.
Plasma testosterone concentrations (mean ± s.e.m.) in male offspring born to mothers that were undisturbed throughout pregnancy (control, open bars), exposed to a strobe light on GD50–52 (PS50, filled bars) or GD60–62 (PS60, hatched bars). Number in bar indicates the number of animals in each group. *P < 0.05 PS50 versus control.
Discussion
The current study has demonstrated that moderate prenatal psychological stress has profound effects on growth, behaviour and endocrine function (HPA axis activity and testosterone) in male offspring. Further, the results of this study highlight the importance of the timing of the prenatal stress. The period of rapid fetal brain growth (GD50), in which DNA accumulation is occurring most rapidly, appears to be a particularly vulnerable developmental window. This process begins at 28–36 weeks of pregnancy in humans (Dobbing & Sands, 1979). Stress during the period of maximal fetal myelination (GD60), when brain cholesterol accumulation is greatest renders offspring particularly sensitive to challenges of the HPA axis.
Exposure to a high frequency strobe light (2 h) resulted in an increase in maternal plasma cortisol concentrations though the effect was greater on GD60 (40%) than on GD50 (20%). There are very significant developmental changes in fetal HPA activity between GD50 and GD60 (Owen & Matthews, 2003; Owen et al. 2005). This, taken together with the differential effects of the same stress on maternal cortisol concentrations at GD50 and GD60, would indicate that fetal exposure to glucocorticoids following maternal stress may be quite different. Under basal, non-stressed conditions, fetal plasma ACTH levels are rising at GD50, though plasma cortisol concentrations remain low (Owen & Matthews, 2003; Owen et al. 2005). This suggests that the central drive of the HPA axis is increasing in the fetus, but that the adrenal is relatively insensitive to ACTH at this time in gestation. We have previously shown that maternal food withdrawal (48 h) at GD50 results in significant increases in maternal HPA axis activity and fetal plasma cortisol (2.5-fold), but no change in fetal plasma ACTH levels (Lingas & Matthews, 1999). This would indicate that the increase in fetal cortisol results from transfer of maternal cortisol to the fetus, and it is likely that the same occurred during maternal stress in the present study at GD50. In contrast, at GD60, there is a (500–1000%) increase in basal fetal plasma cortisol compared with GD50, with little further increase in plasma ACTH, suggesting a significant increase in fetal adrenal sensitivity at this age (Owen & Matthews, 2003; Andrews et al. 2004). At GD60, maternal stress (strobe light exposure) has been shown to result in a 3-fold increase in fetal plasma cortisol concentrations (Dauprat et al. 1990). We have shown that 11 beta-hydroxysteroid dehydrogenase 2 (11(β-HSD2) decreases between GD50 and GD60 in the guinea pig placenta (Sampath-Kumar et al. 1998), indicating that increased maternal–fetal transfer probably occurs at GD60 compared with GD50. Notwithstanding this, we have shown the most profound endocrine and behavioural outcome occurred in the PS50 offspring which suggests that this developmental window is more susceptible to insult. Further studies are required to determine the extent of fetal glucocorticoid exposure following maternal stress at different gestational ages.
Under normal physiological conditions, HPA axis activity is pulsatile, with increased amplitude in the early morning for diurnal, and in the evening for nocturnal species. In the present study, the cortisol secretion profile for control offspring demonstrated no robust morning or evening peak, though levels were higher during the day than during the night. This reinforces suggestions from early studies that guinea pigs are crepuscular, exhibiting increased activity at dusk and dawn with no clear sleep–wake pattern (Wagner, 1970). Male guinea pig offspring exposed to stress during the period of rapid brain growth (PS50) exhibited increased plasma cortisol levels throughout the entire 24-h period compared with control offspring. Additionally, the profile of cortisol secretion in PS50 males was different from that of controls, with significantly increased plasma cortisol levels during the light period. In line with the plasma cortisol concentrations, ACTH levels were significantly higher in PS50 male offspring at 05.00 h with a trend towards an increase over the 24-h period (under the curve analysis). PS60 offspring also demonstrated a significant increase in plasma ACTH at 05.00 h; however, the increase in this group appears to be due to advancement of the morning peak of ACTH secretion rather than an increase in absolute levels. Interestingly, the earlier rise in plasma ACTH was not associated with an advance in the morning rise in plasma cortisol in this group. Rat offspring born to mothers exposed to restraint stress during pregnancy exhibited a phase advance in the evening increase in corticosterone levels (Koehl et al. 1997). They also exhibited higher levels of total and free corticosterone at the end of the light period, with no diurnal fluctuation in ACTH.
Plasma cortisol responses to ACTH challenge were significantly elevated in PS60 offspring and this could reflect a difference in adrenal sensitivity and/or steroidogenesis. To our knowledge, no studies have investigated the effect of prenatal stress on adrenal steroidogenic capacity and further molecular studies examining the expression of MC2R (ACTH receptor) and key adrenal steroidogenic enzymes are required. The PS60 male offspring also exhibited elevated plasma cortisol response to the strobe light exposure. This finding is consistent with other prenatal stress studies in rats and primates (Clarke et al. 1994; Henry et al. 1994). The elevated and prolonged plasma cortisol response to challenge (ACTH and strobe light) probably results from decreased glucocorticoid receptor (GR) feedback sensitivity (De Kloet & Reul, 1987). There may also be differences in the perception of stress and altered neuronal/neurotransmitter input into the PVN in animals born to mothers stressed during pregnancy. Indeed, a recent study has demonstrated differences in neurotransmitter levels in brain regions involved in control of the HPA axis in prenatally stressed rat offspring (Bowman et al. 2004). Studies are required to determine whether this is the case in the guinea pig.
Hypercortisolaemia, as observed in the PS50 offspring, is one of the defining features of the metabolic syndrome (Phillips et al. 2000). This disease is associated with increased central obesity, high blood pressure, glucose intolerance, depression, emotional irritability and cognitive deficits (Phillips et al. 2000). In the current study, however, elevated blood pressure was not observed. Similarly, in a study of nutrient restriction during the period of rapid fetal brain growth in pregnant guinea pigs, there was no effect on blood pressure in offspring (Lingas & Matthews, 2001). However, in a model of chronic prenatal nutrient restriction, male guinea pig offspring exhibited elevated blood pressure (Kind et al. 2002), again illustrating the importance of timing and nature of the prenatal insult. There was a slight trend towards increased heart rate in the PS50 and PS60 male offspring and studies in rat and sheep models have demonstrated differences in baroreflex function following a prenatal nutritional challenge (Gardner et al. 2004; Pladys et al. 2004). The baroreceptor is critical for adapting to immediate changes in blood pressure and is expressed as change in heart rate as a function of blood pressure. In the current study, however, only resting blood pressure was measured, so we were unable to assess baroreceptor activity.
Prenatal stress had no effect on litter size, gestation length or birth weight. There was no difference in maternal body weight during pregnancy suggesting that prenatal stress did not affect food intake in these animals. However, after weaning, there was a significant reduction in body weight gain in male offspring born to mothers stressed during pregnancy. In rats, daily restraint stress over the last week of gestation (∼21 days) has been shown to have no effect (Bowman et al. 2004; McMillen et al. 2005) or decrease body weight in young male offspring (Vallee et al. 1996). The present study is consistent with the latter study, but further, indicates that discreet periods of moderate maternal stress can have a negative impact on adult body weight. Another recent study demonstrated that prenatal stress resulted in aged male rat offspring (24 months) that exhibit hyperglycaemia, glucose intolerance and decreased basal leptin but no difference in body weight (Lesage et al. 2004). In the current study, and that of Vallee et al. (1996), offspring were individually housed post-weaning (a requirement for catheterization). It is possible that prenatal stress causes male offspring to react more severely to the stress of weaning, inducing temporary hypophagia similar to that reported in models of chronic stress (Harris et al. 2002). The fact that body weight does not recover, might suggest that a permanent resetting of homeostatic metabolic mechanisms has occurred. In humans, low birth weight, which is considered an index of an adverse fetal environment, has been associated with the development of obesity and altered body composition in later life (Challis et al. 2004; Laitinen et al. 2004; McMillen et al. 2005). While prenatal stress in rodent models has no effect or reduces adult body weight, changes in body composition may occur but may not manifest until later in life.
An increased brain-to-body-weight ratio was observed in PS50 but not PS60. Whether this reflects brain sparing in utero is unclear as organ measurements were taken in adulthood. Brain sparing is often observed in cases of intrauterine growth restriction (IUGR) in both animals and humans (Lumbers et al. 2001; Dressino et al. 2002). In the present study it is of interest that the effect was only observed in the PS50 and not the PS60 offspring, because maximal brain growth is known to occur at GD50 in the guinea pig (Dobbing & Sands, 1970).
In humans, prenatal stress is a risk factor for the development of major depression and dysregulation of the HPA axis is present in approximately 20–30% of patients experiencing depression (Watson et al. 1999). Additionally, significantly higher cortisol levels are observed in patients with bipolar disorder (Cervantes et al. 2001; Young et al. 2001). Juvenile and adult PS50 male offspring spent less time in the inner zone of the activity arena, suggesting that these animals are more anxious. Thigmotaxis is a crude indicator of anxiety in rodent models including the guinea pig (Tzavara et al. 2002) (Catlin et al. 1993). Increased anxiety-related behaviour following prenatal stress has been reported in rats and primates (Schneider, 1992; Clarke & Schneider, 1993; Clarke et al. 1994; Welberg et al. 2000; Coe et al. 2003). Adult rats exposed to excess endogenous glucocorticoids in utero display decreased grooming and rearing in an open field and increased immobility in a forced swim test (Welberg et al. 2000). Pregnant rhesus monkeys exposed daily to extended periods of stress delivered offspring that exhibited decreased exploratory behaviour compared with controls (Schneider, 1992; Coe et al. 2003). As juveniles, these animals displayed abnormal social behaviour under basal and stressful situations and this was associated with heightened HPA activity (Clarke & Schneider, 1993; Clarke et al. 1994). The findings of the present study are novel because we have identified that the timing of prenatal stress exposure has dramatic effects on behavioural outcome.
The amygdala also plays an important role in fear and anxiety (Heilig et al. 1994). Corticotropin releasing hormone (CRH) is released from the amygdala in response to stress and administration of CRH increases anxiety-related behaviours (Gray & Bingaman, 1996). Amygdala CRH release is up-regulated by glucocorticoids via GR (Gray & Bingaman, 1996; Schulkin, 1999). Therefore, it is possible that the elevated basal plasma cortisol levels observed in PS50 offspring is linked to the increased fear response. Clearly, further studies are required to tease apart the mechanisms involved in increased anxiety behaviour following prenatal stress.
Plasma testosterone was very significantly decreased in PS50 offspring. Testosterone has an inhibitory effect on HPA axis function by decreasing arginine vasopressin (AVP) levels in the median eminence (Viau & Meaney, 1996). It is possible that the elevation in basal adrenocortical activity in PS50 offspring is in part mediated by the decrease in plasma testosterone in these animals. Testosterone also has anxiolytic effects (Edinger & Frye, 2004) and blocking testosterone metabolism in the hippocampus prevents these effects (Frye & Edinger, 2004). Therefore, the decrease in testosterone in the PS50 offspring may also play a role in increasing anxiety-related behaviour. To our knowledge, no studies have examined the effect of prenatal stress on fertility in human or animal models. Given the dramatic effects of prenatal stress on male testosterone levels, this is an area that clearly requires further consideration.
In conclusion, brief periods (3 × 2 h) of moderate stress during pregnancy have long-term effects on growth, HPA axis activity, HPG function and stress-related behaviour in adult male guinea pig offspring. Further, the magnitude and direction of these effects are dependent upon the timing of prenatal stress. Stress during the period of maximal fetal brain growth results in offspring that exhibit heightened basal plasma cortisol levels, decreased plasma testosterone concentrations, and increased anxiety-related behaviour. In contrast, maternal stress near term results in male offspring that exhibit heightened adrenocortical responsiveness to challenge. This indicates that the timing of prenatal insult is critical in determining the endocrine and behavioural phenotype in offspring. Dysregulation of the HPA axis and abnormal behaviour in response to a stressful situation is a prominent feature of patients with mental illness. It is also becoming increasingly clear that maternal stress during pregnancy can influence childhood behaviour (O'connor et al. 2003). Further studies are required to determine the mechanisms involved and to identify potential targets that maybe utilised to reverse the influences of maternal adversity during pregnancy.
Acknowledgments
We would like to thank Dr Marcus Andrews, Sonja Banjanin, Alice Kostaki and Elaine Setiawan for their assistance with this study. This project was funded by the Natural Sciences and Engineering Research Council (NSERC).
References
- Andrews MH, Kostaki A, Setiawan E, Mccabe L, Owen D, Banjanin S, Matthews SG. Developmental regulation of the 5-HT7 serotonin receptor and transcription factor NGFI-A in the fetal guinea-pig limbic system: influence of GCs. J Physiol. 2004;555:659–670. doi: 10.1113/jphysiol.2003.056705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banjanin S, Kapoor A, Matthews SG. Prenatal glucocorticoid exposure alters hypothalamic–pituitary–adrenal function and blood pressure in mature male guinea pigs. J Physiol. 2004;558:305–318. doi: 10.1113/jphysiol.2004.063669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. Intrauterine programming of adult disease. Mol Med Today. 1995;1:418–423. doi: 10.1016/s1357-4310(95)90793-9. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Maclusky NJ, Sarmiento Y, Frankfurt M, Gordon M, Luine VN. Sexually dimorphic effects of prenatal stress on cognition, hormonal responses, and central neurotransmitters. Endocrinology. 2004;145:3778–3787. doi: 10.1210/en.2003-1759. [DOI] [PubMed] [Google Scholar]
- Cadet R, Pradier P, Dalle M, Delost P. Effects of prenatal maternal stress on the pituitary adrenocortical reactivity in guinea-pig pups. J Dev Physiol. 1986;8:467–475. [PubMed] [Google Scholar]
- Catlin MC, Abdollah S, Brien JF. Dose-dependent effects of prenatal ethanol exposure in the guinea pig. Alcohol. 1993;10:109–115. doi: 10.1016/0741-8329(93)90089-7. [DOI] [PubMed] [Google Scholar]
- Cervantes P, Gelber S, Kin FN, Nair VN, Schwartz G. Circadian secretion of cortisol in bipolar disorder. J Psychiatry Neurosci. 2001;26:411–416. [PMC free article] [PubMed] [Google Scholar]
- Challis BG, Luan J, Keogh J, Wareham NJ, Farooqi IS, O'rahilly S. Genetic variation in the corticotrophin-releasing factor receptors: identification of single-nucleotide polymorphisms and association studies with obesity in UK Caucasians. Int J Obes Relat Metab Disord. 2004;28:442–446. doi: 10.1038/sj.ijo.0802564. [DOI] [PubMed] [Google Scholar]
- Clarke AS, Schneider ML. Prenatal stress has long-term effects on behavioral responses to stress in juvenile rhesus monkeys. Dev Psychobiol. 1993;26:293–304. doi: 10.1002/dev.420260506. [DOI] [PubMed] [Google Scholar]
- Clarke AS, Wittwer DJ, Abbott DH, Schneider ML. Long-term effects of prenatal stress on HPA axis activity in juvenile rhesus monkeys. Dev Psychobiol. 1994;27:257–269. doi: 10.1002/dev.420270502. [DOI] [PubMed] [Google Scholar]
- Coe CL, Kramer M, Czeh B, Gould E, Reeves AJ, Kirschbaum C, Fuchs E. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol Psychiatry. 2003;54:1025–1034. doi: 10.1016/s0006-3223(03)00698-x. [DOI] [PubMed] [Google Scholar]
- Dauprat P, Monin G, Dalle M, Delost P. The effects of psychosomatic stress at the end of pregnancy on maternal and fetal plasma cortisol levels and liver glycogen in guinea-pigs. Reprod Nutr Dev. 1984;24:45–51. doi: 10.1051/rnd:19840105. [DOI] [PubMed] [Google Scholar]
- Dauprat P, Dalle M, Delost P. Effects of neurotrophic stress on maternal metabolism and binding of plasma cortisol in late pregnant guinea-pigs and their fetuses. J Dev Physiol. 1990;13:13–16. [PubMed] [Google Scholar]
- Dean F, Matthews SG. Maternal dexamethasone treatment in late gestation alters glucocorticoid and mineralocorticoid receptor mRNA in the fetal guinea pig brain. Brain Res. 1999;846:253–259. doi: 10.1016/s0006-8993(99)02064-8. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Reul JM. Feedback action and tonic influence of corticosteroids on brain function: a concept arising from the heterogeneity of brain receptor systems. Psychoneuroendocrinology. 1987;12:83–105. doi: 10.1016/0306-4530(87)90040-0. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Growth and development of the brain and spinal cord of the guinea pig. Brain Res. 1970;17:115–123. doi: 10.1016/0006-8993(70)90311-2. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Dressino V, Orden B, Oyhenart EE. Sexual responses to intrauterine stress: body and brain growth. Clin Exp Obstet Gynecol. 2002;29:100–102. [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Testosterone's analgesic, anxiolytic, and cognitive-enhancing effects may be due in part to actions of its 5alpha-reduced metabolites in the hippocampus. Behav Neurosci. 2004;118:1352–1364. doi: 10.1037/0735-7044.118.6.1352. [DOI] [PubMed] [Google Scholar]
- Frye CA, Edinger KL. Testosterone's metabolism in the hippocampus may mediate its anti-anxiety effects in male rats. Pharmacol Biochem Behav. 2004;78:473–481. doi: 10.1016/j.pbb.2004.04.019. [DOI] [PubMed] [Google Scholar]
- Gardner DS, Pearce S, Dandrea J, Walker R, Ramsay MM, Stephenson T, Symonds ME. Peri-implantation undernutrition programs blunted angiotensin II evoked baroreflex responses in young adult sheep. Hypertension. 2004;43:1290–1296. doi: 10.1161/01.HYP.0000126991.67203.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson MA, Butters NS, Reynolds JN, Brien JF. Effects of chronic prenatal ethanol exposure on locomotor activity, and hippocampal weight, neurons, and nitric oxide synthase activity of the young postnatal guinea pig. Neurotoxicol Teratol. 2000;22:183–192. doi: 10.1016/s0892-0362(99)00074-4. [DOI] [PubMed] [Google Scholar]
- Gray TS, Bingaman EW. The amygdala: corticotropin-releasing factor, steroids, and stress. Crit Rev Neurobiol. 1996;10:155–168. doi: 10.1615/critrevneurobiol.v10.i2.10. [DOI] [PubMed] [Google Scholar]
- Harris RB, Mitchell TD, Simpson J, Redmann SM, Jr, Youngblood BD, Ryan DH. Weight loss in rats exposed to repeated acute restraint stress is independent of energy or leptin status. Am J Physiol Regul Integr Comp Physiol. 2002;282:R77–R88. doi: 10.1152/ajpregu.2002.282.1.R77. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF, Ekman R, Britton KT. Corticotropin-releasing factor and neuropeptide Y: role in emotional integration. Trends Neurosci. 1994;17:80–85. doi: 10.1016/0166-2236(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Henry C, Kabbaj M, Simon H, Le Moal M, Maccari S. Prenatal stress increases the hypothalamo-pituitary-adrenal axis response in young and adult rats. J Neuroendocrinol. 1994;6:341–345. doi: 10.1111/j.1365-2826.1994.tb00591.x. [DOI] [PubMed] [Google Scholar]
- Kind KL, Simonetta G, Clifton PM, Robinson JS, Owens JA. Effect of maternal feed restriction on blood pressure in the adult guinea pig. Exp Physiol. 2002;87:469–477. doi: 10.1111/j.1469-445x.2002.tb00060.x. [DOI] [PubMed] [Google Scholar]
- Koehl M, Barbazanges A, Le Moal M, Maccari S. Prenatal stress induces a phase advance of circadian corticosterone rhythm in adult rats which is prevented by postnatal stress. Brain Res. 1997;759:317–320. doi: 10.1016/s0006-8993(97)00394-6. [DOI] [PubMed] [Google Scholar]
- Koehl M, Darnaudery M, Dulluc J, Van Reeth O, Le Moal M, Maccari S. Prenatal stress alters circadian activity of hypothalamo-pituitary-adrenal axis and hippocampal corticosteroid receptors in adult rats of both gender. J Neurobiol. 1999;40:302–315. [PubMed] [Google Scholar]
- Laitinen J, Pietilainen K, Wadsworth M, Sovio U, Jarvelin MR. Predictors of abdominal obesity among 31-y-old men and women born in Northern Finland in 1966. Eur J Clin Nutr. 2004;58:180–190. doi: 10.1038/sj.ejcn.1601765. [DOI] [PubMed] [Google Scholar]
- Leiser R, Kaufmann P. Placental structure: in a comparative aspect. Exp Clin Endocrinol. 1994;102:122–134. doi: 10.1055/s-0029-1211275. [DOI] [PubMed] [Google Scholar]
- Lesage J, Del-Favero F, Leonhardt M, Louvart H, Maccari S, Vieau D, Darnaudery M. Prenatal stress induces intrauterine growth restriction and programmes glucose intolerance and feeding behaviour disturbances in the aged rat. J Endocrinol. 2004;181:291–296. doi: 10.1677/joe.0.1810291. [DOI] [PubMed] [Google Scholar]
- Lingas R, Dean F, Matthews SG. Maternal nutrient restriction (48 h) modifies brain corticosteroid receptor expression and endocrine function in the fetal guinea pig. Brain Res. 1999;846:236–242. doi: 10.1016/s0006-8993(99)02058-2. [DOI] [PubMed] [Google Scholar]
- Lingas RI, Matthews SG. A short period of maternal nutrient restriction in late gestation modifies pituitary-adrenal function in adult guinea pig offspring. Neuroendocrinology. 2001;73:302–311. doi: 10.1159/000054647. [DOI] [PubMed] [Google Scholar]
- Liu L, Li A, Matthews SG. Maternal glucocorticoid treatment programs HPA regulation in adult offspring: sex-specific effects. Am J Physiol Endocrinol Metab. 2001;280:E729–E739. doi: 10.1152/ajpendo.2001.280.5.E729. [DOI] [PubMed] [Google Scholar]
- Liu L, Matthews SG. Adrenocortical response profiles to corticotrophin-releasing hormone and adrenocorticotrophin challenge in the chronically catheterized adult guinea-pig. Exp Physiol. 1999;84:971–977. [PubMed] [Google Scholar]
- Lumbers ER, Yu ZY, Gibson KJ. The selfish brain and the barker hypothesis. Clin Exp Pharmacol Physiol. 2001;28:942–947. doi: 10.1046/j.1440-1681.2001.03554.x. [DOI] [PubMed] [Google Scholar]
- Mccabe L, Marash D, Li A, Matthews SG. Repeated antenatal glucocorticoid treatment decreases hypothalamic corticotropin releasing hormone mRNA but not corticosteroid receptor mRNA expression in the fetal guinea-pig brain. J Neuroendocrinol. 2001;13:425–431. doi: 10.1046/j.1365-2826.2001.00649.x. [DOI] [PubMed] [Google Scholar]
- McMillen IC, Adam CL, Muhlhausler BS. Early origins of obesity: programming the appetite regulatory system. J Physiol. 2005;565:9–17. doi: 10.1113/jphysiol.2004.081992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews S. Early programming of the hypothalamo-pituitary-adrenal axis. Trends Endocrinol Metab. 2002;13:373. doi: 10.1016/s1043-2760(02)00690-2. [DOI] [PubMed] [Google Scholar]
- O'Connor TG, Heron J, Golding J, Glover V. Maternal antenatal anxiety and behavioural/emotional problems in children: a test of a programming hypothesis. J Child Psychol Psychiatry. 2003;44:1025–1036. doi: 10.1111/1469-7610.00187. [DOI] [PubMed] [Google Scholar]
- Owen D, Banjanin S, Gidrewicz D, Mccabe L, Matthews SG. Central regulation of the hypothalamic-pituitary-adrenal axis during fetal development in the guinea-pig. J Neuroendocrinol. 2005;17:220–226. doi: 10.1111/j.1365-2826.2005.01294.x. [DOI] [PubMed] [Google Scholar]
- Owen D, Banjanin S, Matthews SG. Society for Neuroscience 31st Annual Meeting. San Diego,CA,USA: 2001. Sex differences in corticosteroid receptor development in the brain and pituitary of the guinea pig. [Google Scholar]
- Owen D, Matthews SG. Glucocorticoids and sex-dependent development of brain glucocorticoid and mineralocorticoid receptors. Endocrinology. 2003;144:2775–2784. doi: 10.1210/en.2002-0145. [DOI] [PubMed] [Google Scholar]
- Phillips DI, Walker BR, Reynolds RM, Flanagan DE, Wood PJ, Osmond C, Barker DJ, Whorwood CB. Low birth weight predicts elevated plasma cortisol concentrations in adults from 3 populations. Hypertension. 2000;35:1301–1306. doi: 10.1161/01.hyp.35.6.1301. [DOI] [PubMed] [Google Scholar]
- Pladys P, Lahaie I, Cambonie G, Thibault G, Le NL, Abran D, Nuyt AM. Role of brain and peripheral angiotensin II in hypertension and altered arterial baroreflex programmed during fetal life in rat. Pediatr Res. 2004;55:1042–1049. doi: 10.1203/01.PDR.0000127012.37315.36. [DOI] [PubMed] [Google Scholar]
- Sampath-Kumar R, Matthews SG, Yang K. 11beta-hydroxysteroid dehydrogenase type 2 is the predominant isozyme in the guinea pig placenta: decreases in messenger ribonucleic acid and activity at term. Biol Reprod. 1998;59:1378–1384. doi: 10.1095/biolreprod59.6.1378. [DOI] [PubMed] [Google Scholar]
- Schneider ML. Prenatal stress exposure alters postnatal behavioral expression under conditions of novelty challenge in rhesus monkey infants. Dev Psychobiol. 1992;25:529–540. doi: 10.1002/dev.420250706. [DOI] [PubMed] [Google Scholar]
- Schulkin J. Corticotropin-releasing hormone signals adversity in both the placenta and the brain: regulation by glucocorticoids and allostatic overload. J Endocrinol. 1999;161:349–356. doi: 10.1677/joe.0.1610349. [DOI] [PubMed] [Google Scholar]
- Tzavara ET, Monory K, Hanoune J, Nomikos GG. Nicotine withdrawal syndrome: behavioural distress and selective up-regulation of the cyclic AMP pathway in the amygdala. Eur J Neurosci. 2002;16:149–153. doi: 10.1046/j.1460-9568.2002.02061.x. [DOI] [PubMed] [Google Scholar]
- Vallee M, Mayo W, Maccari S, Le Moal M, Simon H. Long-term effects of prenatal stress and handling on metabolic parameters: relationship to corticosterone secretion response. Brain Res. 1996;712:287–292. doi: 10.1016/0006-8993(95)01459-4. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. The inhibitory effect of testosterone on hypothalamic-pituitary-adrenal responses to stress is mediated by the medial preoptic area. J Neurosci. 1996;16:1866–1876. doi: 10.1523/JNEUROSCI.16-05-01866.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JE, Manning PJ. The Biology of the Guinea Pig. New York: Academic Press; 1970. [Google Scholar]
- Watson JB, Mednick SA, Huttunen M, Wang X. Prenatal teratogens and the development of adult mental illness. Dev Psychopathol. 1999;11:457–466. doi: 10.1017/s0954579499002151. [DOI] [PubMed] [Google Scholar]
- Weinstock M, Poltyrev T, Schorer-Apelbaum D, Men D, Mccarty R. Effect of prenatal stress on plasma corticosterone and catecholamines in response to footshock in rats. Physiol Behav. 1998;64:439–444. doi: 10.1016/s0031-9384(98)00056-0. [DOI] [PubMed] [Google Scholar]
- Welberg LA, Seckl JR, Holmes MC. Inhibition of 11beta-hydroxysteroid dehydrogenase, the foeto-placental barrier to maternal glucocorticoids, permanently programs amygdala GR mRNA expression and anxiety-like behaviour in the offspring. Eur J Neurosci. 2000;12:1047–1054. doi: 10.1046/j.1460-9568.2000.00958.x. [DOI] [PubMed] [Google Scholar]
- Young EA, Carlson NE, Brown MB. Twenty-four-hour ACTH and cortisol pulsatility in depressed women. Neuropsychopharmacology. 2001;25:267–276. doi: 10.1016/S0893-133X(00)00236-0. [DOI] [PubMed] [Google Scholar]