Abstract
Glycine receptors exhibit a biphasic sensitivity profile in response to Zn2+-mediated modulation, with low Zn2+ concentrations potentiating (< 10 μm), and higher Zn2+ concentrations inhibiting submaximal responses to glycine. Here, a substantial 30-fold increase in sensitivity to Zn2+-mediated inhibition was apparent for the homomeric glycine receptor (GlyR) α1 subunit compared to either GlyR α2 or α3 subtypes. Swapping the divergent histidine (H107) residue in GlyR α1, which together with the conserved H109 forms part of an intersubunit Zn2+-binding site, for the equivalent asparagine residue present in GlyR α2 and α3, reversed this phenotype. Co-expression of heteromeric GlyR α1 or α2 with the ancillary β subunit yielded receptors that maintained their distinctive sensitivities to Zn2+ inhibition. However, GlyR α2β heteromers were consistently 2-fold more sensitive to inhibition compared to the GlyR α2 homomer. Comparative studies to elucidate the specific residue in the β subunit responsible for this differential sensitivity revealed instead threonine 133 in the α1 subunit as a new vital component for Zn2+-mediated inhibition. Further studies on heteromeric receptors demonstrated that a mutated β subunit could indeed affect Zn2+-mediated inhibition but only from one side of the intersubunit Zn2+-binding site, equivalent to the GlyR α1 H107 face. This strongly suggests that the α subunit is responsible for Zn2+-mediated inhibition and that this is effectively transduced, asymmetrically, from the side of the Zn2+-binding site where H109 and T133 are located.
The glycine receptor (GlyR) is a prominent inhibitory synaptic receptor of the mammalian hindbrain and spinal cord (Aprison & Daly, 1978). This pentameric receptor consists of ligand-binding α subunits and homologous structural β subunits (Pfeiffer et al. 1982). To date, molecular cloning has revealed four subtypes of the α subunit (α1–4) and a single variant of the β subunit (Handford et al. 1996). The GlyR is a founder member of the Cys loop ion channel superfamily along with the homologous γ-aminobutyric acid type A (GABAA), nicotinic acetylcholine (nACh) and serotonin type 3 (5HT3) receptors (Grenningloh et al. 1987).
GlyRs are targets for a number of different modulators, including ethanol and anaesthetics (Celentano et al. 1988; Harrison et al. 1993), picrotoxin (Schmieden et al. 1989) and Zn2+ (Bloomenthal et al. 1994). This divalent cation exerts a complex biphasic modulation of recombinant α1, α2 and α1β GlyRs and also of native GlyRs from spinal cord neurones (Bloomenthal et al. 1994; Laube et al. 1995). Modulation by Zn2+ potentiates GlyR activation at low concentrations (0.1–10 μm) and attenuates the sensitivity to glycine at higher concentrations (> 10 μm). These actions could be physiologically relevant as Zn2+ is concentrated in selected nerve terminals and packaged into synaptic vesicles. Moreover, it may be released into the synaptic cleft or form a thin ‘Zn2+ veneer’ following nerve fibre stimulation (Assaf & Chung, 1984; Howell et al. 1984; Frederickson et al. 2000; Kay, 2003) resulting in multiple effects on neuronal excitability by modulating ion channels (Smart et al. 1994; Harrison & Gibbons, 1994; Smart et al. 2004).
An inhibitory Zn2+-binding site has been proposed on GlyR α1 that involves Zn2+ coordination by two histidine residues, H107 and H109 (Harvey et al. 1999) with the potential involvement of T112 (Laube et al. 2000). More recently, co-expression of mixed GlyR α1 point-mutated subunits suggested that the H107 and H109 residues are contributed from adjacent α subunits (Nevin et al. 2003) forming an intersubunit Zn2+-binding site, with T112 probably playing a less direct, general structural role. Sequence alignments of GlyR subunits reveal that the equivalent position to H107 in all other GlyR subtypes is occupied by an asparagine residue, though previously no difference in sensitivity to Zn2+-mediated inhibition has been detected between GlyR α1 and α2 (Laube et al. 1995). This contrasts with recombinant GABAA receptors, which demonstrate differential sensitivities to inhibitory Zn2+ determined by their subunit composition (Draguhn et al. 1990; Smart et al. 1991; Hosie et al. 2003).
In this study, we report a large difference in the potency of Zn2+-mediated inhibition at GlyR α1 compared to the GlyR α2 and α3 subtypes and additionally elucidate a novel residue, T133, in the GlyR α1 subunit that is critical for inhibition by Zn2+. Additionally, the role of the GlyR β subunit in influencing the effects of Zn2+-mediated inhibition revealed a functional asymmetry to the Zn2+-binding site with the GlyR α1 H109, T133 ‘face’ forming a vital transduction component required for the inhibitory modulation by Zn2+.
Methods
cDNA constructs
Wild-type cDNA constructs that were used included the human (h) GlyR α1L (long, or α1INS), hGlyR α2A and rat (r) GlyR α3S (short) splice variants, and the hGlyR β subunit. Site-directed mutagenesis was performed using the Stratagene Quikchange kit. The mutated sequences were confirmed by complete sequencing of the cDNA inserts using an ABI sequencer.
Cell culture and transfection
Human embryonic kidney (HEK) cells (ATCC CRL1573) were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS), 2 mm glutamine, 100 units ml−1 penicillin G and 100 μg ml−1 streptomycin, incubated at 37°C in 95% air−5% CO2 (Smart et al. 1991). HEK cells were transfected by electroporation at 400 V, infinite resistance and 125 μF, using a Biorad Gene Electropulser II. Plasmids were cotransfected in a 1: 1 ratio with a plasmid for the reporter, enhanced green fluorescent protein (GFP). To ensure co-expression of GlyR αβ heteromers, the GlyR β subunit expression construct was mixed with GlyR α subunit plasmids at a ratio of 30: 1. Cells were plated onto poly-l-lysine-coated coverslips (100 μg ml−1 poly-l-lysine) sufficient to achieve 20% confluence and used for electrophysiology the day after transfection.
Neuronal cell culture and acute spinal cord slice preparation
In accordance with UK leglislation, embryonic day 15 (E15) embryos were extracted from Sprague-Dawley rats by Caesarean section and placed in ice-cold phosphate-buffered saline (PBS). The spinal columns were excised and separated from the meninges and dorsal root ganglia. Spinal cords were cut into four sections and treated with 0.25% w/v trypsin in Earle's balanced salt solution (EBSS) for 15 min at 37°C. The tissue was then washed three times in EBSS to remove residual trypsin and sequentially triturated using fire-polished Pasteur pipettes of narrowing tip diameter. Spinal cord cell suspensions were then centrifuged at 500 g for 5 min and resuspended in DMEM plating medium supplemented per 100 ml with 5 ml FCS, 5 ml horse serum (HS), 0.6% w/v l-glucose (Sigma) and 0.04% w/v NaHCO3. Cells were plated at a density of 5 × 105 per coverslip precoated either with poly-l-lysine alone, or also with an astrocyte monolayer. After 4 days the primary culture medium was replaced with Neurobasal medium (Invitrogen) supplemented with 1% v/v B-27 supplement (Invitrogen) 0.25% v/v of 200 mml-glutamine, 1 ng ml−1 recombinant rat ciliary neurotrophic factor (CNTF, Peprotech, London, UK) and 100 pg ml−1 recombinant glial cell line-derived neurotrophic factor (GDNF; Peprotech). This medium was replenished twice weekly.
Acute spinal cord slices (350 μm thickness) were obtained from postnatal day P0–P1 or P17–P18 rats. Briefly, the animal was anaesthetized with an intraperitoneal injection of urethane (10% w/v, Sigma) and decapitated. After ventral laminectomy, the spinal cord (from mid-thoracic to lumbar region) was removed and fixed vertically to an agar block using tissue glue (Vetbond, WPI Scientific Instruments). The block was glued to the base of the slicing chamber of a Leica VT1000 vibratome and eight to ten slices were taken from the lumbar region spanning the L2–L5 segments. After 30 min of incubation at 37°C, the slices were allowed to cool to, and then maintained at, room temperature (20–22°C) for another 30 min. Individual slices were then transferred to the recording chamber and superfused with Krebs solution continuously gassed with 95% O2–5% CO2.
Solutions
For the cultured cells, the internal patch pipette solution contained (mm): KCl 140, MgCl2 2, CaCl2 1, Hepes 10, EGTA 11 and ATP 2; pH 7.11 for HEK cells and pH 7.3 (with NaOH) for spinal cord primary cultures (∼300 mosmol l−1). The Krebs solution consisted of (mm): NaCl 140, KCl 4.7, MgCl2 1.2, CaCl2 2.5, Hepes 10 and d-glucose 11; pH 7.4 (∼ 300 mosmol l−1). Primary cultured neuronal cells were superfused in Krebs solution containing: 0.5 μm tetrodotoxin (TTX), 10 μm bicuculline, 20 μm 2-amino-5-phosphovalerate (AP5) and 10 μm 6-cyano-2,3-nitroquinoxalinedione (CNQX) to abolish action potentials and synaptic GABAA and glutamate receptor activation.
The dissection and Krebs solutions used for the acute spinal cord slices were identical and composed of (mm): NaCl 113, KCl 3, NaHCO3 25, NaH2PO4 1, CaCl2 2, MgCl2 2 and d-glucose 11. The patch pipette solution used for the slices contained (mm): CsCl 140, NaCl 4, MgCl2 1, CaCl2 0.5, EGTA 5, Hepes 10 and Mg-ATP 2; pH adjusted to 7.3 using CsOH.
Electrophysiology
An Axopatch 200B amplifier (Axon Instruments) was used to record whole-cell currents from single HEK cells or spinal cord primary cultures using the patch-clamp technique. HEK cells exhibited resting potentials between −10 and −40 mV and were voltage clamped at −40 mV. Healthy spinal cord neurones were judged on the basis of robust dendritic networking, resting potentials of −50 to −70 mV and steady holding currents of < 10 pA. These cells were clamped at −70 mV and series resistance compensation of 70–90% was employed. All cells were visualized with a differential interference contrast Nikon microscope and an epifluorescence attachment was used to identify GFP-transfected HEK cells. A Y-tube was used to rapidly apply drugs and Krebs solution (exchange rate approximately 50–100 ms) to patch-clamped cells. Patch electrodes were fabricated using a Narashige PC-10 puller with resistances after polishing of 4–5 MΩ. All recordings were performed in constantly perfusing Krebs solution at room temperature.
Recordings from acute spinal cord slices were performed from motoneurones visually identified with infra-red differential interference contrast microscopy on the basis of their ventral location and morphology. Electrodes were pulled with 1–1.5 MΩ resistance and fire polished to a final resistance of approximately 2.5 MΩ. Cells were voltage clamped at −70 mV and only those cells with stable holding currents (< 40 pA) for the duration of the experiment were included for analysis. Series resistance (6–10 MΩ) was routinely compensated (70–90%). Glycine (30 μm, with or without Zn2+) was applied via a Y-tube at intervals of 2 min for the duration of the experiment before, during and after pre-incubation with 30 μm Zn2+. Cells were included in the analysis only if the response to glycine recovered after application of Zn2+, to within 90–110% of the control response. The Krebs solution contained 0.5 μm TTX, 5 μm SR95531 hydrobromide (GABAA receptor antagonist), 20 μm AP5 and 10 μm CNQX to pharmacologically isolate glycine currents.
Data acquisition and analysis
All currents were filtered at 3 kHz using a Bessel filter (−36 dB per octave). Data were recorded in 20 s acquisition episodes directly to a Pentium IV, 1.8 GHz computer into Clampex 8.0 via a Digidata 1322A (Axon Instruments) sampling at 200 μs intervals. Ligand-induced responses were assessed by sequentially applying a concentration of the test drug, twice, between control responses evoked by EC50 values of the agonist to assess the response stability during the experiments. For the pre-application experiments, Zn2+ was applied for 15 s prior to glycine application and then followed by a 2 min recovery in Krebs solution prior to further drug applications. If the control responses varied by less than 15% from each other, then the test responses were normalized by linear interpolation between the two surrounding control responses. Digitized current records were analysed off-line using Axoscope 8.2. Biphasic Zn2+ concentration–response curves were fitted as previously described (Miller et al. 2004) and the glycine concentration–response curves were fitted with the Hill equation:
where EC50 represents the concentration of glycine (A) inducing 50% of the maximal current evoked by a saturating concentration of glycine and n is the Hill coefficient. The glycine concentration–response data were normalized to the maximum response to glycine. The Zn2+ dose–response data were normalized to the control glycine response amplitude in the absence of Zn2+. The competitive-type inhibition caused by Zn2+ was analysed according to the method of Arunlakshana & Schild (1959). Full glycine concentration–response curves were obtained in each HEK cell and then at least one and usually more curves were obtained in the presence of 50, 100, 200 and 500 μm Zn2+. The curves were tested for parallelity and the dose ratios (DRs) for glycine were calculated from the respective glycine EC50 values. The mean dose ratios for each Zn2+ concentration (B) allowed the dissociation constant for Zn2+ (KB) to be determined using the transformed Schild equation:
The slope of the Schild plot of log (DR – 1) versus log(B) was not significantly different from unity (P < 0.05). The slope was then constrained to unity and the intercept on the abscissa used to determine the pA2 (=−log KB) for Zn2+. All statistical comparisons used an unpaired t test.
Modelling
The mature N-terminal extracellular domain (ECD) of the human GlyR α2 subunit, was modelled on the crystal structure of the acetylcholine binding protein (AChBP; Brejc et al. 2001) using SwissProt DeepView in accordance with a ClustalW protein alignment.
Results
Differential Zn2+ sensitivity between GlyR α1 and GlyR α2 subunits
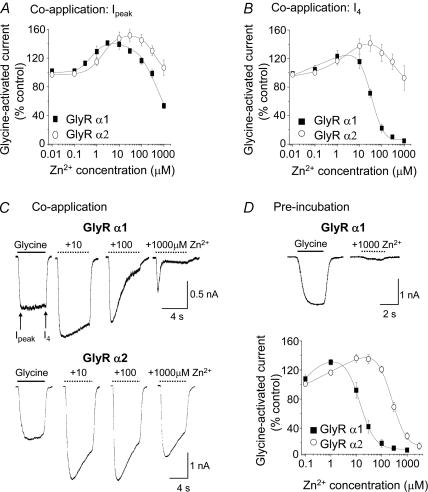
A comparison of the sensitivities of GlyRs to Zn2+-mediated inhibition was initiated because one of the key Zn2+-binding residues we reported previously, H107, is present in the GlyR α1 but not in the GlyR α2 subtype (Harvey et al. 1999). Modulation by Zn2+ was examined using two different protocols. The first involved co-application of varying concentrations of Zn2+ with a concentration of glycine equivalent to the EC50. The degree of inhibition was measured for the peak glycine response (Ipeak) and then 4 s after the co-application (I4) to reveal a delayed onset of inhibition (Fig. 1A–C). The second protocol utilized the pre-incubation of Zn2+ and only the peak responses to glycine were measured as pre-incubation allowed Zn2+ to equilibrate with the GlyR (Fig. 1D). Irrespective of the protocol, all Zn2+ concentration–response curves exhibited a biphasic shape due to the potentiating and inhibitory effects of Zn2+ on GlyRs. Using these procedures, a clear difference in the potency of inhibitory Zn2+ was observed between GlyR α1 and GlyR α2 subunits. Under pre-incubation conditions both receptors could be inhibited but there was a 25-fold reduction in Zn2+ potency for GlyR α2 (IC50, 360 ± 40 μm; n = 11) compared to GlyR α1 (IC50, 15 ± 2 μm; n = 5; P < 0.05; Table 1). All subsequent experiments utilized the pre-incubation protocol with Zn2+.
Figure 1. Differential inhibition of glycine-activated currents by Zn2+ at GlyR α1 and GlyR α2.
A, Zn2+ concentration–response curves for the modulation of peak (Ipeaak) glycine-evoked currents (EC50: GlyR α1, 20 μm; GlyR α2, 70 μm) following a 4 s co-application of glycine and Zn2+. B, Zn2+ concentration–response curves as in A with the current amplitudes measured at the end of the co-application (I4). C, membrane currents comparing glycine-activated Ipeak and I4 for GlyR α1 (upper traces) and GlyR α2 (lower traces) after applying glycine (EC50) in the absence and presence of co-applied 10, 100 and 1000 μm Zn2+. D, glycine-activated Ipeak following co-application of glycine (EC50) and Zn2+ after prior incubation for 15 s with an equivalent concentration of Zn2+ for GlyR α1 and GlyR α2. The inset shows typical glycine-activated currents for GlyR α1 in the absence and presence of 1000 μm Zn2+ under the pre-incubation protocol. n = 6–13 for all experiments. All points in this and succeeding figures represent the mean ± s.e.m.
Table 1.
GlyR sensitivities to glycine and Zn2+
| GlyR subtype | Gly EC50 (μm) | Imax (nA) | n | Zn2+ IC50 (μm) | n |
|---|---|---|---|---|---|
| α1 | 24 ± 5 | 4.5 ± 0.5 | 10 | 15 ± 2 | 5 |
| α2 | 66 ± 6 | 3.7 ± 0.5 | 13 | 360 ± 40 | 11 |
| α1 β | 18 ±3 | 4.5 ± 0.9 | 6 | 13 ± 2 | 3 |
| α2 β | 51 ± 4 | 5.6 ± 0.5 | 3 | 180 ± 30 | 13 |
| α1H107N | 24 ± 2 | 5.9 ± 0.3 | 4 | 230 ± 30 | 5 |
| α1H109F | 56 ± 5 | 5.1 ± 0.2 | 3 | > 1 mm | 3 |
| α1T133A | 130 ± 30 | 4.8 ± 0.7 | 5 | > 1 mm | 5 |
| α1T135A | 17 ± 2 | 3.3 ± 0.6 | 3 | 26 ± 6 | 3 |
| α1H107NβN130H | 28 ± 8 | 4.8 ± 0.8 | 3 | 24 ± 3 | 5 |
| α1H109FβS156T | 39 ± 4 | 5.2 ± 1.2 | 3 | > 1 mm | 3 |
| α1T133AβS156T | 90 ± 16 | 3.2 ± 0.9 | 3 | > 1 mm | 3 |
| α1 βS156A | 44 ± 9 | 5.9 ± 0.7 | 3 | 17 ± 7 | 3 |
| α2N114H | 58 ± 3 | 4.2 ± 0.7 | 3 | 29 ± 2 | 4 |
| α2 βG128S | 52 ± 5 | 5.0 ± 1.6 | 3 | 170 ± 20 | 4 |
| α2 βS156T | 47 ± 9 | 5.6 ± 1.4 | 3 | 210 ± 20 | 5 |
| α3 | 48 ± 3 | 5.3 ± 1.3 | 4 | 150 ± 10 | 4 |
| α3N107H | 53 ± 12 | 5.3 ± 0.8 | 5 | 26 ± 7 | 3 |
| sc 5 DIV | ∼ 40 | 3.1 ± 0.3 | 12 | 320 ± 50 | 8 |
| sc 7 DIV | ∼ 30 | 4.9 ± 0.7 | 12 | 110 ± 10 | 8 |
| sc 10–14 DIV | ∼ 30 | 4.3 ± 0.4 | 8 | 88 ± 21 | 8 |
The values indicate the glycine EC50 values for activating the recombinant and native GlyRs and also the Zn2+ IC50 values for modulating the half-maximal glycine-activated responses where Zn2+ is first pre-equilibrated with the receptor for 15 s before an equivalent concentration of Zn2+ is coapplied with glycine. All numbers are means ± s.e.m. from n cells. For the native GlyR in spinal cord (sc) neurones, the EC50 values were estimated from linear segments of the dose–response curves.
Histidine 107 is responsible for differential Zn2+ sensitivity between GlyR α subunits
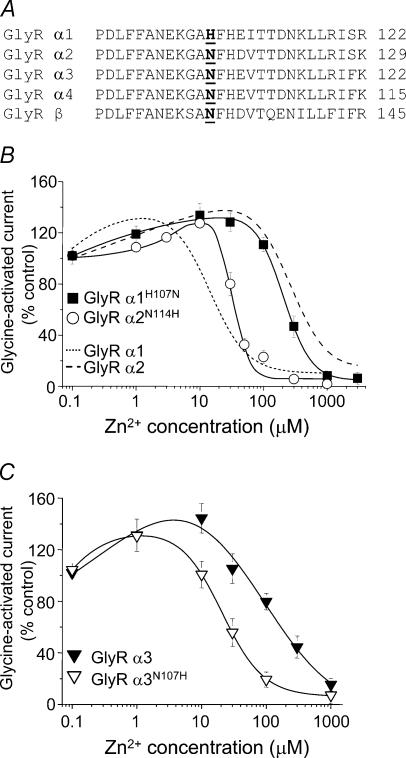
Comparing the N-terminal extracellular domains of the GlyRs reveals that GlyR α2, like all other α variants except GlyR α1, retains an asparagine (N114) residue at the homologous position to the putative Zn2+-binding residue, H107, in the GlyR α1 subunit (Fig. 2A). If H107 coordinates Zn2+, then the divergent N114 in GlyR α2 could be responsible for the differential sensitivity to Zn2+ as asparagines are poor coordinators for this cation (Auld, 2001). This was examined by exchanging residues between the GlyR α1 and α2 subunits at the equivalent positions of H107 (α1) and N114 (α2) to generate α1H107N and α2N114H. This exchange reversed the sensitivities of the GlyR α1 and α2 subunits with regard to Zn2+ inhibition, such that α1H107N (IC50, 230 ± 40 μm; n = 5) was now 8-fold less sensitive to Zn2+ compared to α2N114H (IC50, 29 ± 2 μm; n = 4; Fig. 2B; Table 1). Taken together this strongly suggests that H107 not only forms part of the inhibitory Zn2+-binding site but also is largely responsible for the different sensitivities of the GlyR α1 and GlyR α2 subunits to Zn2+ inhibition.
Figure 2. Reversal of GlyR α subunit sensitivities to Zn2+-mediated inhibition.
A, primary amino acid sequence alignment for a segment of the N-terminal domains highlighting the unique nature of H107 in the GlyR α1 subtype. B and C, Zn2+ concentration–response curves for the modulation of glycine (EC50)-activated currents for GlyR α1H107N and GlyR α2N114H (B), and GlyR α3 and GlyR α3N107H (C) wild-type and mutant subunits. Peak glycine (EC50)-evoked responses were measured in the absence and presence of varying concentrations of Zn2+ after 15 s pre-incubation with an equivalent Zn2+ concentration. The Zn2+ curves for wild-type GlyR α1 and GlyR α2 are included for comparison in (B) (taken from Fig. 1D). n = 3–5 for all experiments.
To further establish the significance of H107 in the subtype sensitivity to Zn2+ inhibition, GlyR α3 subunits, which possess an asparagine residue at the homologous position (N107 in α3), were also examined. This is of particular relevance as currently there are no selective pharmacological blockers to distinguish between the two adult GlyR subtypes, α1 and α3. Consistent with the data for GlyR α2, the potency of Zn2+ was also substantially reduced on GlyR α3 (IC50, 150 ± 10 μm; n = 4). In accord with the results for GlyR α2 subunits, replacing N107 with histidine in GlyR α3 subunits (α3N107H) substantially increased the potency of Zn2+ mediated inhibition (IC50, 26 ± 7; n = 3; P < 0.05; Fig. 2C).
Mechanism for Zn2+-mediated inhibition on GlyR α1 and GlyR α2 subtypes
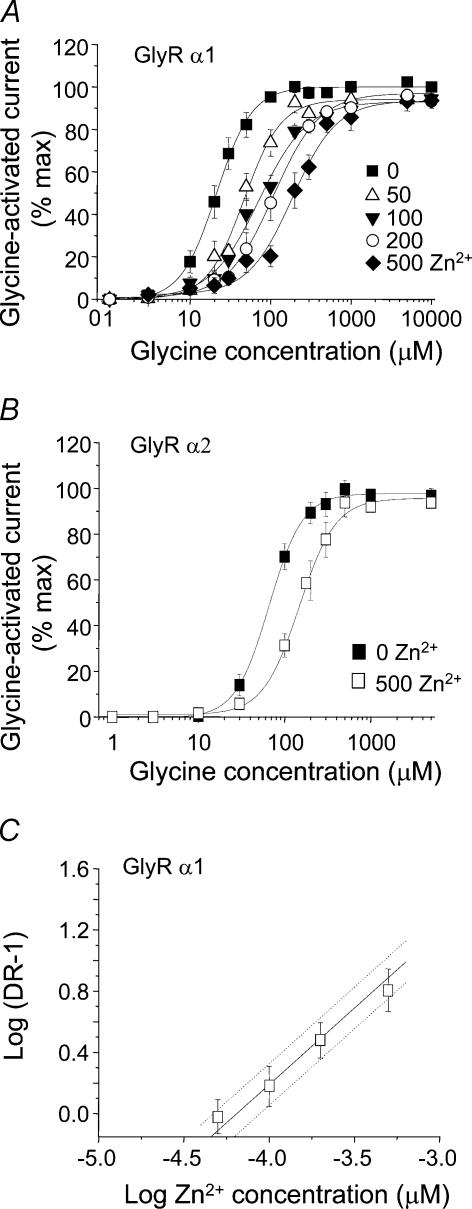
The proposed mechanism of Zn2+-mediated inhibition has not been fully investigated, although glycine concentration–response curves determined in the presence of a single concentration of Zn2+ exhibit the same maximum response (Laube et al. 1995; Han & Wu, 1999). The mode of Zn2+-mediated inhibition was characterized for both GlyR α1 and GlyR α2 subtypes by generating glycine concentration–response curves in the presence of several inhibitory concentrations of Zn2+ (Fig. 3A and B). For both GlyR α1 and GlyR α2, these curves were displaced by Zn2+ in a parallel manner without any significant reduction in the maximal current evoked by saturating concentrations of glycine, which is in accord with a competitive-type mechanism. In addition, due to the relatively high sensitivity of GlyR α1 to Zn2+-mediated inhibition it was possible to pre-incubate at several different inhibitory Zn2+ concentrations and achieve measurable separation between each of the glycine concentration–response curves. This was not possible for the less sensitive GlyR α2 subunit without incurring Zn2+ solubility problems. A Schild analysis was then used to determine a pA2 value for Zn2+ as an antagonist at the GlyR α1 subtype (Fig. 3C). The Schild plot slope was not significantly different from one, and the constrained slope provided a pA2 of 4.44 ± 0.14, yielding a dissociation constant for Zn2+ from the inhibitory site of 27.5 ± 0.87 μm (n = 7).
Figure 3. Zn2+ is a competitive inhibitor at GlyR α1 and GlyR α2.
Glycine concentration–response curves for both GlyR α1 (A) and GlyR α2 (B) are displaced in a competitive-type inhibitory manner by Zn2+. In all cases Zn2+ was co-applied with glycine (EC50) after a 15 s pre-incubation with Zn2+. Glycine curves for GlyR α1 were determined in the absence and presence of 50, 100, 200 and 500 μm Zn2+ and for GlyR α2, in the absence and presence of 500 μm Zn2+. C, Schild analysis generated from the GlyR α1 dose ratios taken from the curves in (A). The continuous line indicates the constrained unity slope with 95% confidence intervals assigned by dotted lines. All experiments were from 6 to 13 cells.
Rate of onset for Zn2+-mediated inhibition at GlyR α1 and GlyR α2
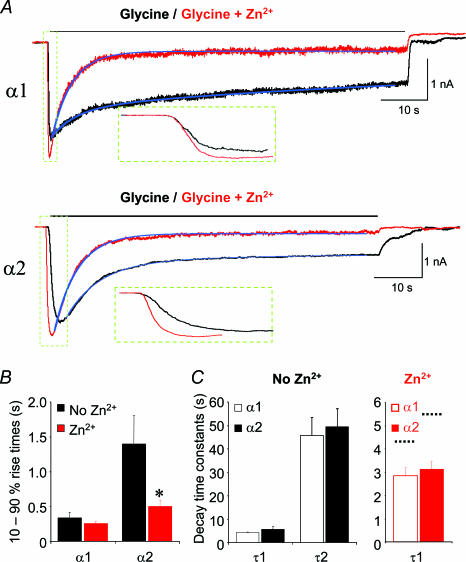
The mechanism of action for Zn2+-mediated inhibition was further investigated by analysing the rate of onset for the block by Zn2+ at GlyR α1 and GlyR α2. Using a pre-incubation protocol, 1000 μm Zn2+ caused substantial inhibition at both GlyR α1 (90 ± 2.4%; n = 6) and GlyR α2 (78 ± 4.4%; n = 11; Fig. 1D). During co-application, however, the level of inhibition induced by 1000 μm Zn2+ was dramatically different between the two GlyRs (compare current records in Fig. 1C). To assess the rates of onset for Zn2+-mediated inhibition at GlyR α1 and α2, potency matched concentrations of Zn2+ (40 and 1000 μm, respectively, corresponding to approximately 70% inhibition under pre-incubation conditions) were co-applied with half-maximally effective concentrations of glycine for a 60 s period to allow Zn2+-mediated inhibition to reach a steady state. A 60 s application of glycine alone revealed a biphasic desensitization profile for both GlyR α1 and α2, with initial fast desensitization time constants of 4.1 ± 0.2 s and 5.6 ± 1.3 s (n = 6), and slow time constants of 45.7 ± 8 s and 49.5 ± 8 s (n = 6), respectively (Fig. 4A and C). During the initial activation phase for glycine currents in the presence of Zn2+, an enhancement was observed for both GlyR α1 and α2 due to the rapid onset of Zn2+-dependent potentiation. In the case of the slowly activating GlyR α2 subtype (Mangin et al. 2003), the initial Zn2+-mediated enhancement caused a significant decrease in the 10–90% rise time to reach steady state from 1.4 ± 0.4 s in control, to 0.5 ± 0.1 s in the presence of Zn2+ (n = 5; P < 0.05; Fig. 4A and B). In contrast, the control activation rate for GlyR α1 is faster than that for GlyR α2, but this rate was not increased further by Zn2+ (340 ± 70 ms in control and 260 ± 30 ms in Zn2+; n = 6; P > 0.05; Fig. 4A and B).
Figure 4. The effect of co-applied Zn2+ on the rise and decay times for glycine-activated currents on GlyR α1 and α2.
A, glycine-activated currents during a prolonged 60 s application of half-maximally effective concentrations of glycine in the absence (black) and presence (red) of potency-matched doses of Zn2+ (40 and 1000 μm for GlyR α1 and GlyR α2, respectively). The blue lines indicate curve fits to determine the current decay times. B, 10–90% rise times in the absence and presence of the same Zn2+ concentrations, determined for glycine currents over the period highlighted and enlarged in the dashed green boxes in A. Note that the time for the slower activating macroscopic current of GlyR α2 to reach steady state is markedly reduced, whilst the time for α1 is unaffected, by Zn2+. C, bar graphs revealing that GlyR α1 and GlyR α2 have comparable desensitization time constants. In the absence of Zn2+ (black bars), glycine currents decay with two time constants, fast (τ1, 4–5 s) and slow (τ2, 40–50 s). In the presence of Zn2+ (red bars), glycine currents decay with only a fast time constant (approximately 3 s), dotted black lines denote the speed of the original fast decay time constants in the absence of Zn2+. n = 3–6. *P < 0.05 between the rise times for Gly α2.
Examination of the subsequent Zn2+-mediated inhibition revealed that the rate of onset was indistinguishable between the two receptor subtypes. In both cases, the slow desensitization phase was completely ablated leaving only a fast decaying component. This fast phase was accelerated by 1.5- to 2-fold compared to the previously identified fast desensitization phase in the absence of Zn2+. The decay time constants changed from 4.1 ± 0.2 s to 2.8 ± 0.4 s for GlyR α1 and from 5.6 ± 1.3 s to 3.1 ± 0.3 s for GlyR α2 (n = 6; Fig. 4A and C). The final levels of inhibition achieved after 60 s in 40 and 1000 μm Zn2+ were not significantly different for either receptor subtype, with 63 ± 7% inhibition achieved for GlyR α1 and 57 ± 13% for GlyR α2 (n = 6), indicating that the potencies for inhibition by Zn2+ were indeed accurately matched. The data therefore suggest that the substitution of GlyR α1 H107 with GlyR α2 N114 does not interfere with the rate at which Zn2+ can access the inhibitory site and cause inhibition.
Differential sensitivity to Zn2+-mediated inhibition is unaffected by the GlyR β subunit
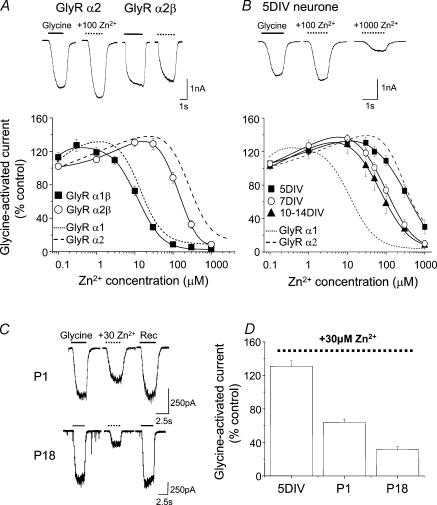
The 30-fold increased sensitivity of GlyR α1 over GlyR α2 to Zn2+-mediated inhibition makes this ion a potentially useful pharmacological tool. Whether this differential sensitivity is maintained at native neuronal GlyRs was assessed using recombinant GlyR αβ heteromers, which are a major physiological subtype (Pfeiffer et al. 1982). Successful co-assembly of GlyR α and β subunits was established by measuring a reduction in the sensitivity to the antagonist picrotoxin (by approximately 20-fold) compared to homomeric GlyR α subunits, as previously reported (data not shown; Pribilla et al. 1992; Handford et al. 1996). The GlyR α2β heteromers exhibited a subtle though consistent 2-fold increased sensitivity to Zn2+-mediated inhibition (IC50, 180 ± 30 μm; n = 13) compared to the GlyR α2 homomers (360 ± 40 μm; n = 11; P < 0.05; Table 1). Consistent with the previous findings using the GlyR homomers, the GlyR α2β sensitivity to Zn2+ remained substantially lower when compared with GlyR α1β, which retained a comparable sensitivity to the GlyR α1 homomer (Fig. 5A, Table 1).
Figure 5. Differential sensitivity to inhibitory Zn2+ in recombinant heteromeric GlyRs and neuronal GlyRs.
A, Zn2+ concentration–response curves for the modulation of glycine (EC50) responses on GlyR α1β and GlyR α2β heteromers in the presence of varying concentrations of Zn2+ after initial Zn2+ pre-incubation. For comparison, the Zn2+ sensitivities of GlyR α1 and GlyR α2 homomers are shown (taken from the curve fits in Fig. 1D). The inset shows typical currents activated by EC50 values of glycine on GlyR α2 and GlyR α2β, respectively, in the absence and presence of 100 μm Zn2+. B, Zn2+ concentration–response curves for native GlyRs determined on rat spinal cord cultures at 5, 7 and 10–14 DIV. The inset currents illustrate the lower sensitivity of glycine (EC50) responses to Zn2+-mediated inhibition in a 5 DIV spinal cord neurone. C, glycine-activated currents in the absence and presence of 30 μm Zn2+ and following recovery after 10 min for a neonatal (P1) and juvenile (P18) neurone. D, bar graph illustrating the effect of 30 μm pre-applied Zn2+ on glycine (EC50)-activated currents recorded from neurones at 5 DIV in culture and in spinal cord slices at P1 and P18. In all experiments n = 3–19.
Spinal cord GlyRs increase their sensitivity to Zn2+-mediated inhibition during development
To evaluate whether a differential sensitivity to Zn2+-mediated inhibition was apparent in a native environment, the Zn2+ sensitivity profiles of neuronal GlyRs were assessed in embryonic spinal cord cultures and in neonatal and juvenile rat acute spinal cord slices. During early embryonic development and in dissociated culture, the GlyR α2 subunit appears to be the predominant α subtype, with a limited up-regulation of GlyR α1 during the course of culture maturation (Hoch et al. 1989, 1992). Increased expression of the α1 subunit becomes more evident in vivo during late embryonic to early postnatal developmental stages (Becker et al. 1988; Takahashi et al. 1992; Watanabe & Akagi, 1995).
Whole-cell recordings from 90% of rat spinal cord cultured neurones responded to exogenously applied glycine and multipolar neurones at 7 days in vitro (DIV) or older, displayed robust synaptic activity (data not shown). Neurones at 5, 7 and 10–14 DIV, all showed an overall low sensitivity to Zn2+. The 5 DIV neuronal cultures especially exhibited a pharmacological profile consistent with the low Zn2+ sensitivity associated with the GlyR α2 subtype (Fig. 5B; Table 1). To follow the period during which GlyR expression changes, electrophysiological recordings were made from neurones in acute spinal cord slices at P0–1 and P17–18. Whole-cell recordings using pre-incubation with 30 μm Zn2+ exhibited only a partial inhibition of the response to 30 μm glycine at P0–1 (36 ± 4%; n = 19). This is consistent with the presence of a mixed population of GlyR α1 and GlyR α2. However, a profound inhibition (69 ± 4%; n = 13; Fig. 5C and D) was exhibited at P17–18, in accord with a progressive developmental transition to the adult, high Zn2+ sensitivity, GlyR α1 subtype (Fig. 5C and D).
Identification of the structural elements required for Zn2+ inhibition
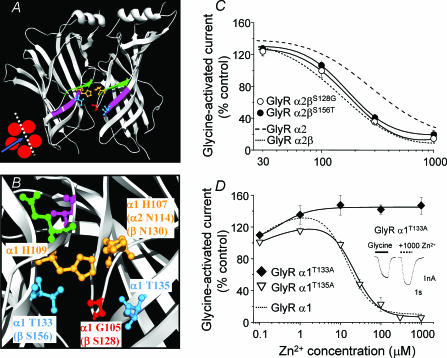
Inspecting the aligned primary sequences for GlyR α and β subunits reveals that the GlyR β subunit, like the α2 subunit, also retains the low affinity asparagine residue at the homologous position to the Zn2+-binding GlyR α1 subunit H107 (Fig. 2A). This was of interest as co-expression of the β subunit with the GlyR α2 subunit in this study caused a modest increase in sensitivity to Zn2+ inhibition, suggesting that the β subunit may actually contribute to the Zn2+-binding site. Such a β subunit-dependent increase in sensitivity was not observed for the α1β heteromer, possibly because the α1 subunit already possesses a higher sensitivity to Zn2+ realized through the presence of its unique residue, H107.
A directed comparative scan was therefore made of the α2 and β subunit primary amino acid sequences guided by modelling the GlyR N-terminal domains on the crystal structure of the homologous acetylcholine binding protein (AChBP; Brejc et al. 2001). This approach identified key differences in amino acids in the predicted vicinity of the GlyR α1 H107- and H109-based Zn2+ inhibitory binding site. Of these, only two residues differed that might impact on Zn2+ coordination at this site. In GlyR α2 subunits, G112 (α1 G105) and T140 (α1 T133) are replaced in the β subunit by S128 and S156, respectively (Fig. 6A and B). In view of their proximity to the Zn2+ inhibitory site and to elucidate the cause of the β subunit-induced increase in GlyR α2 sensitivity to Zn2+ inhibition, the β subunit residues were switched for their α2 subunit counterparts forming the mutants, GlyR βS128G and βS156T. However, neither GlyR βS128G nor βS156T affected the ability of the β subunit to increase GlyR α2 sensitivity to Zn2+ (Fig. 6C and Table 1).
Figure 6. Evaluation of potential binding residues in the immediate vicinity of the inhibitory Zn2+ site.
A, two neighbouring GlyR α1 subunits modelled on the AChBP and viewed from the inside face (see inset); expanded in B, to highlight inhibitory Zn2+-binding site residues, H107 and H109 (orange) and two newly identified potential Zn2+-binding residues, T133 and T135 (blue). An additional variant residue in the β subunit, S128, is also shown (red). The β-strand 5 (green) containing H107 and H109 is presented directly above β-strand 6 (pink) containing T133 and T135. B, homologous residues are shown in parentheses. For reference, GlyR α1 E110 (green) and T112 (pink), previously shown (Laube et al. 2000) to influence Zn2+-mediated inhibition, are included and are both predicted to face away from the site, suggesting the side chains of these residues are unlikely to directly coordinate Zn2+. C, assessment of β subunit residues that may be responsible for the increased sensitivity to Zn2+-mediated inhibition on the GlyR α2 subtype by measuring Zn2+ modulation of half-maximally effective glycine responses (using the pre-incubation protocol) on GlyR α2βS128G and GlyR α2βS156T heteromers. The curves for GlyR α2 and GlyR α2β are shown for comparison (taken from Figs 1D and 5A). D, Zn2+ concentration–response curves for modulation of half-maximal responses to glycine at GlyR α1T133A and GlyR α1T135A with the wild-type GlyR α1 curve for comparison (from Fig. 1D). Inset shows glycine currents demonstrating the resistance of GlyR α1T133A to inhibition by 1000 μm Zn2+. n = 3–6.
Using the homology model for the GlyR, no other suitable β subunit residues capable of interacting with the Zn2+ inhibitory site were identified. The effect of the β subunit on GlyR α2 was presumed to reflect an indirect structural effect akin to the role suggested for T112 in the GlyR α1 subunit (Nevin et al. 2003), which also affected the sensitivity to Zn2+-mediated inhibition (Laube et al. 2000). However, by conducting similar structural comparisons for the GlyR α1 subunits revealed that a T133 residue, located on β strand 6 (according to the nomenclature of Brejc et al. 2001), was predicted to reside directly below H109 on β strand 5. Moreover, another threonine in GlyR α1, T135, is also located on β strand 6 directly below H107 (Fig. 6A and B). As GlyR α1 T133 and T135 residues are ideally located as potential coordinating ligands for Zn2+, they were assessed for their role in Zn2+-mediated inhibition by individual mutation to alanines forming GlyR α1T133A and α1T135A. Although GlyR α1T135A exhibited a comparable Zn2+ IC50 (29 ± 2 μm; n = 3) to the wild-type GlyR α1 subunit, the GlyR α1T133A mutation ablated Zn2+-mediated inhibition up to 1 mm (Fig. 6D and Table 1).
Using the GlyR β subunit to investigate functional asymmetry at the inhibitory Zn2+-binding site
Although a role for the β subunit in coordinating Zn2+ has not been favoured previously (Bloomenthal et al. 1994; Nevin et al. 2003) our data suggested that this subunit subtly influenced the potency of Zn2+ at GlyR α2β heteromers. Using a different strategy, we assessed the ability of the β subunit to contribute directly to the Zn2+-binding site by examining whether it could, if appropriately mutated at homologous positions, compensate for mutated GlyR α1 subunits with reduced Zn2+ sensitivities. For example, to compensate for the mutation, GlyR α1H107N, with regard to the sidedness of the intersubunit Zn2+-binding site, would require co-expression of the β subunit carrying the mutation, βN130H (Fig. 7, lower schematic diagram). Similarly, the mutant GlyR α1H109F would require pairing with a wild-type β subunit, as this subunit already possesses H132 in the homologous position to H109 in the α subunit (Fig. 7, middle schematic diagram). Finally, the novel GlyR α1T133A mutant could be compensated by the βS156T mutation (Fig. 7, upper schematic diagram). GlyR α1 E110 and T112, previously found to influence Zn2+-mediated inhibition (Laube et al. 2000), were not investigated in this study as GlyR α1E110A exerted only a modest 5-fold increase in the Zn2+ IC50 (67 ± 4 μm; n = 3) and is predicted to face away from the H107/H109-based Zn2+-binding site (Fig. 6B). In addition, T112 (also facing away from the inhibitory site, Fig. 6B) has only an indirect effect on Zn2+ binding (Nevin et al. 2003) and previously, substitutions of this residue have been shown to influence the relative efficacy of partial agonists (Schmieden et al. 1999). This suggests that T112 may have a general role in multiple aspects of GlyR function rather than a specific role dedicated to Zn2+ coordination.
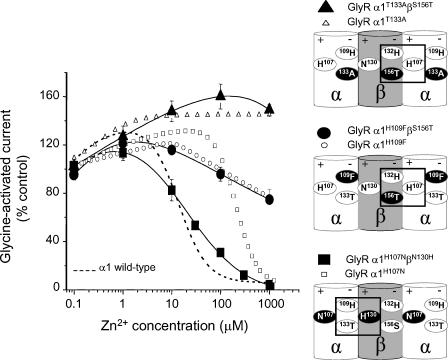
Figure 7. Functional asymmetry at the GlyR α subunit Zn2+-binding site.
Zn2+ concentration–response curves for the modulation of glycine (EC50)-activated currents. Although the co-expressed heteromeric GlyR α1β receptors are in each case predicted to have all the necessary components for the Zn2+-binding site reconstituted at the αβ subunit interface (see schematic diagrams), only when βN130H compensates for α1H107N (lower schematic diagram) is the sensitivity to Zn2+-mediated inhibition recovered. The Zn2+ concentration –response curves for GlyR α1H107N, α1H109F and α1T133A subunits are included for comparison (data for α1H107N and α1T133A are taken from Figs 2B and 5D, respectively; n = 3–6). The schematic side-view diagrams show part of the GlyR depicting the three examples where a compensatory β subunit (wild-type or mutant) is co-expressed with a GlyR α1 subunit lacking one component of the inhibitory Zn2+-binding site (H107, H109 or T133). The mutated residues around the Zn2+-binding site are shown in white on a black background. The corresponding wild-type residues are shown in black on a white background. The rectangles delineate the Zn2+-binding site where all the components under examination have been restored, which occurs at the αβ subunit interfaces labelled + and – (see text). These schematic diagrams assume that the GlyR is a pentamer where the β subunits are not juxtaposed; however, the predicted αβ interfaces containing all the components of the inhibitory Zn2+ site would still apply even for the unlikely scenario that the two β subunits are juxtaposed.
For the GlyR α1 subunit mutations, H109F and T133A, both of which reside on the same ‘−’ face (using the nomenclature from Fu & Sine, 1996; Fig. 7) of the inhibitory Zn2+-binding site, no recovery of Zn2+ potency was observed when either of these subunits were co-expressed with the compensating GlyR β subunits including either H132 or S156T, respectively (IC50 values, > 1 mm; n = 3; Fig. 7). Furthermore, we investigated this face of the Zn2+-binding site from the perspective of the GlyR α1 T133 residue and found that co-expression of the wild-type GlyR α1 subunit with a βS156A mutant also had no effect on the receptor sensitivity to Zn2+-mediated inhibition (IC50, 17 ± 7 μm; n = 3). These data, accrued from one face (−) of the Zn2+-binding site, concur with the previously reported lack of effect of co-expressing GlyR α1 with βH132A on Zn2+-mediated inhibition (Nevin et al. 2003).
In contrast, when the GlyR α1H107N mutant, which is present on the opposing ‘+’ face of the Zn2+-binding site to H109 and T133, was co-expressed with the GlyR βN130H subunit, a dramatic recovery in Zn2+-mediated inhibition was observed to almost wild-type GlyR α1β levels of sensitivity (IC50, 24 ± 3 μm; n = 5; Fig. 7). To ensure this mutation was not due to the de novo construction of an intrasubunit Zn2+-binding site in the β subunit, as this mutant βN130H subunit alone now contained two juxtaposed histidine residues (H130 and H132), we co-expressed the βN130H subunit with a GlyR α1H107N, H109F double mutant and this exhibited no recovery to Zn2+-mediated inhibition (IC50, > 1 mm; n = 4). This strongly suggested that the β subunit is indeed participating in an intersubunit Zn2+-binding site and that this contribution by the β subunit can only occur from the + face of the subunit.
Discussion
This study has identified a distinct difference in the potency of Zn2+-mediated inhibition between the GlyR α1 and GlyR α2 subunits, which is also seen with the corresponding αβ subunit heteromers. Comparison of the primary amino acid sequences around the previously deduced inhibitory Zn2+-binding site (Harvey et al. 1999) revealed that a single residue, H107 in the α1 subunit, was likely to be responsible for the different potencies of Zn2+. The absence of this residue in the α2 and α3 subunits substantially accounts for its reduced Zn2+ sensitivity, further supporting the assertion that this location on the GlyR is forming a binding site for Zn2+-induced inhibition (Harvey et al. 1999; Nevin et al. 2003). A previous study comparing Zn2+ potency at GlyR α1 and GlyR α2 subunits, in Xenopus oocytes, did not report any differences in sensitivity between these subtypes (Laube et al. 1995). This could reflect the different expression system, variation in Zn2+ application protocols, or possibly the glycine concentrations chosen for modulation by Zn2+, which is acting as a competitive antagonist. However, the data here are supported by the identification of a molecular basis for the differential sensitivity. Moreover, the previously demonstrated increased expression of GlyR α1 over the ‘embryonic’α2 during spinal cord development (Hoch et al. 1989, 1992), is in accord with the increased sensitivity to Zn2+-mediated inhibition that we observed in older acute spinal cord slices (Becker et al. 1988; Takahashi et al. 1992; Watanabe & Akagi, 1995). This suggests that the physiological significance of Zn2+-mediated inhibition is unlikely to be relevant at embryonic stages of development as native embryonic GlyRs require more than 50 μm Zn2+ before glycine currents are inhibited. This concurs with a previous report (Laube, 2002) where 50 μm Zn2+ induced only modest inhibition of glycinergic IPSCs in mature spinal cord cultures.
Despite the different sensitivities to inhibitory Zn2+, glycine concentration–response curves for both GlyR α1 and GlyR α2 were displaced in a parallel, competitive-type manner; however, this does not necessarily imply that Zn2+ is directly competing for the glycine recognition site, as Zn2+ could interact with the receptor in a mutually exclusive allosteric fashion reducing the ability of glycine to bind to its entirely non-overlapping site and vice versa. Indeed, this explanation is consistent with the current views on the discrete locations of the inhibitory Zn2+- and glycine-binding sites (Laube et al. 2002). Prior evidence also suggested that Zn2+-mediated inhibition of GlyRs is largely caused by a reduction in the agonist efficacy (E; Laube et al. 2000). The values of E reported for glycine at GlyR α1 vary from between 10 and 20 (Laube et al. 2000) and 16 (Lewis et al. 2003), to 40 (Beato et al. 2004) for the higher-liganded GlyR states. By assuming that a sequential ligand-binding site model is sufficient to explain GlyR activation and using this to simulate glycine dose–response curves, a reduction in E alone will not produce parallel displacements in the glycine concentration–response curves of the magnitude observed in our study without significant reductions in the maximum response. For example, for GlyR α1, E would need to be reduced from 40 to 0.19 to increase the glycine EC50 from 24 to 214 μm; however, this would cause the channel open probability (Po) to be reduced from 0.97 to 0.15, a substantial reduction in the maximum response. Thus it is highly likely that Zn2+ is also affecting the affinity of glycine for its recognition site. The Schild analysis here provides the first definitive measurement of Zn2+ affinity for the GlyR α1 (pA2 of 4.44) and indicates that Zn2+ has a much lower potency (329-fold) at the glycine receptor compared to the classical competitive GlyR antagonist, strychnine (pA2, 7.08; KB, 83.2 nm; Saitoh et al. 1994).
Previously, the GlyR β subunit has not been shown to exert any detectable influence on Zn2+-mediated inhibition at GlyR α subunits (Bloomenthal et al. 1994; Laube et al. 1995; Nevin et al. 2003). However, this report demonstrates that in the unique instance of the α2 subtype, co-expression with the β subunit increased the sensitivity to Zn2+ inhibition. Although we were unable to attribute this effect to a specific residue in the vicinity of the putative inhibitory Zn2+-binding site, as a consequence we identified GlyR α1 T133 as a vital component for Zn2+ inhibition. When considering the location of the intersubunit Zn2+-binding site, in accordance with the three-dimensional GlyR α1 model based on the AChBP, T133 is predicted to reside beneath H109, which is ideal for participating in the putative inhibitory Zn2+-binding site.
Co-expression of GlyR α subunits with complementary β subunits, designed to compensate for α subunits lacking specific components of the inhibitory Zn2+-binding site, revealed an asymmetry of function between the opposing faces of the intersubunit binding site. Effectively ‘knocking out’ either GlyR α1 H109 or T133, both predicted to be on the same – face of the subunit could not be compensated by β subunits mutated to contain equivalent α subunit residues. This demonstrates that the α subunit H107 + face and the mutant β subunit H132/S156T – face, cannot form a functional Zn2+ inhibitory site alone, when the α subunit – face has been disrupted by mutation. In contrast, following knock-out of the GlyR α1 H107 on the opposing + face, the attenuated sensitivity to Zn2+-mediated inhibition was almost fully restored by co-expression with βN130H subunits. The restoration of Zn2+-mediated inhibition for the GlyR α1H107N mutant implies that such inhibition could be mediated from either the GlyR α1 H109/T133 – face or the β N130H + face, or both acting together. As the wild-type GlyR α1 subunit is able to mediate Zn2+-mediated inhibition regardless of the removal of any inhibitory components in the β subunit this means that the H109 and T133 – face of the binding site must be responsible for connecting Zn2+ binding to an effect on receptor function (transduction). Furthermore, this transduction must be driven through the GlyR α1 subunit. In comparison, H107 can instead be regarded as a pure binding residue, as this can be donated from a neighbouring subunit that lacks its own Zn2+ transduction apparatus i.e. the β subunit.
In the GABAA receptor the interfacial nature of the high-sensitivity Zn2+-inhibition site along with pharmacological studies has led to the proposal that Zn2+ acts to stabilize the closed state of the receptor by stabilizing the interaction between the interfaces (Smart, 1992; Hosie et al. 2003). This has also been proposed for the GlyR (Lynch, 2004) and is supported here by the greater potency of inhibitory Zn2+ under pre-incubation conditions; that is, Zn2+ can access the closed GlyR state and stabilize it before agonist application. Under co-application, glycine activates the receptors before Zn2+ can bind, so it must wait until the receptor closes before it can access and stabilize the closed state to induce inhibition. This was manifest in the macroscopic currents by the delayed onset of inhibition. Single-channel studies of the activation mechanism for the GlyR α1 receptor suggest that the higher liganded and open channel state(s) have a higher agonist affinity (Beato et al. 2004). Zinc ions might then appear as competitive inhibitors because they stabilize the closed channel state(s) which also has a lower affinity for the agonist. Conversely, agonist activation stabilizes the open channel state(s), where Zn2+ cannot induce inhibition by stabilizing the subunit interfaces, so supporting a mutually exclusive, apparently competitive allostery between the agonist and Zn2+-binding sites.
How is this communication transmitted between two quite discrete binding sites? Current structural data for the extracellular domain of the nACh receptor (Unwin et al. 2002) suggest that upon agonist activation the inner – faces undergoes significant movement, greater than that at the inner + interface. This conformational flexibility accords with the transduction asymmetry that we have attributed to the Zn2+-binding site, which favours a predominant role for the – face in transducing Zn2+ inhibition. From this we would presume that Zn2+ stabilizes the GlyR closed state by preventing movement of the – subunit interface relative to the + interface.
With regard to the stoichiometry of glycine and Zn2+ binding, the mutually exclusive mechanism suggests that potentially, each site has five copies, one per subunit. However, the number of occupied Zn2+ sites required to ensure the receptor remains closed may not necessarily be five. This depends on how the receptor is activated. If a concerted switch of all five subunit extracellular domains is required, then possibly only one Zn2+ may be required to stabilize a single subunit interface to prevent this movement. This would seem the most plausible scenario because the GlyRα1β heteromers retain the same sensitivity and efficacy for Zn2+-mediated inhibition as the α1 homomer, even though the β subunit lacks homologous Zn2+-binding residues within the inhibitory site. Thus two to three Zn2+-binding sites contributed by the α subunits appear to be sufficient, in agreement with a previous study using mixed homomeric α1 receptors with disrupted Zn2+-binding sites (Nevin et al. 2003).
Taken overall, this study identifies Zn2+ as a useful pharmacological tool to distinguish between GlyR α1 and α2 or α3 subunits. It also provides a rationale by which Zn2+ binding initiates the transduction of Zn2+-mediated inhibition resulting in an effect on glycine binding to the receptor. This appears to be propagated asymmetrically from the Zn2+-binding site being driven from the – face. This phenomenon of asymmetric propagation of signal transduction is quite likely to be a common feature characterizing the binding of ligands to many different sites on ligand-gated ion channels.
Acknowledgments
This work was supported by the MRC and the Wellcome Trust. P.S.M. has a Wellcome Trust 4-year PhD postgraduate studentship. We would also like to thank Alastair Hosie for helpful advice and comments and Paul Groot-Kormelink for providing the human glycine receptor β subunit expression construct.
References
- Aprison MH, Daly EC. Biochemical aspects of transmission at inhibitory synapses: the role of glycine. In: Agranoff BW, Aprisan MH, editors. Advances in Neurochemistry. Vol. 3. New York: Plenum Press; 1978. pp. 203–294. [Google Scholar]
- Arunlakshana O, Schild HO. Some quantitative uses of drug antagonists. Br J Pharmacol. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf SY, Chung SH. Release of endogenous Zn2+ from brain tissue during activity. Nature. 1984;308:734–736. doi: 10.1038/308734a0. [DOI] [PubMed] [Google Scholar]
- Auld DS. Zinc coordination sphere in biochemical zinc sites. Biometals. 2001;14:271–313. doi: 10.1023/a:1012976615056. [DOI] [PubMed] [Google Scholar]
- Beato M, Groot-Kormelink PJ, Colquhoun D, Sivilotti LG. The activation mechanism of alpha1 homomeric glycine receptors. J Neurosci. 2004;24:895–906. doi: 10.1523/JNEUROSCI.4420-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker CM, Hoch W, Betz H. Glycine receptor heterogeneity in rat spinal cord during postnatal development. EMBO J. 1988;7:3717–3726. doi: 10.1002/j.1460-2075.1988.tb03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomenthal AB, Goldwater E, Pritchett DB, Harrison NL. Biphasic modulation of the strychnine-sensitive glycine receptor by Zn2+ Mol Pharmacol. 1994;46:1156–1159. [PubMed] [Google Scholar]
- Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der OJ, Smit AB, Sixma TK. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269–276. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- Celentano JJ, Gibbs TT, Farb DH. Ethanol potentiates GABA- and glycine-induced chloride currents in chick spinal cord neurons. Brain Res. 1988;455:377–380. doi: 10.1016/0006-8993(88)90098-4. [DOI] [PubMed] [Google Scholar]
- Draguhn A, Verdoorn TA, Ewert M, Seeburg PH, Sakmann B. Functional and molecular distinction between recombinant rat GABAA receptor subtypes by Zn2+ Neuron. 1990;5:781–788. doi: 10.1016/0896-6273(90)90337-f. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, Suh SW, Silva D, Frederickson CJ, Thompson RB. Importance of zinc in the central nervous system: the zinc-containing neuron. J Nutr. 2000;130:1471S–1483S. doi: 10.1093/jn/130.5.1471S. [DOI] [PubMed] [Google Scholar]
- Fu DX, Sine SM. Asymmetric contribution of the conserved disulphide loop to subunit oligomerisation and assembly of the nicotinic acetylcholine receptor. J Biol Chem. 1996;271:31479–31484. doi: 10.1074/jbc.271.49.31479. [DOI] [PubMed] [Google Scholar]
- Grenningloh G, Gundelfinger E, Schmidt B, Betz H, Dorlison MG, Barnard EA, Schofield PR, Seeburg PH. Glucine vs GABA receptors. Nature. 1987;330:25–26. doi: 10.1038/330025b0. [DOI] [PubMed] [Google Scholar]
- Han Y, Wu SM. Modulation of glycine receptors in retinal ganglion cells by zinc. Proc Natl Acad Sci U S A. 1999;96:3234–3238. doi: 10.1073/pnas.96.6.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handford CA, Lynch JW, Baker E, Webb GC, Ford JH, Sutherland GR, Schofield PR. The human glycine receptor β subunit: primary structure, functional characterisation and chromosomal localisation of the human and murine genes. Brain Res Mol Brain Res. 1996;35:211–219. [PubMed] [Google Scholar]
- Harrison NL, Gibbons SJ. Zn2+: an endogenous modulator of ligand- and voltage-gated ion channels. Neuropharmacology. 1994;33:935–952. doi: 10.1016/0028-3908(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Harrison NL, Kugler JL, Jones MV, Greenblatt EP, Pritchett DB. Positive modulation of human ?-aminobutyric acid type A and glycine receptors by the inhalation anesthetic isoflurane. Mol Pharmacol. 1993;44:628–632. [PubMed] [Google Scholar]
- Harvey RJ, Thomas P, James CH, Wilderspin A, Smart TG. Identification of an inhibitory Zn2+ binding site on the human glycine receptor alpha1 subunit. J Physiol. 1999;520:53–64. doi: 10.1111/j.1469-7793.1999.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch W, Betz H, Becker CM. Primary cultures of mouse spinal cord express the neonatal isoform of the inhibitory glycine receptor. Neuron. 1989;3:339–348. doi: 10.1016/0896-6273(89)90258-4. [DOI] [PubMed] [Google Scholar]
- Hoch W, Betz H, Schramm M, Wolters I, Becker CM. Modulation by NMDA receptor antagonists of glycine receptor isoform expression in cultured spinal cord neurons. Eur J Neurosci. 1992;4:389–395. doi: 10.1111/j.1460-9568.1992.tb00887.x. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Dunne EL, Harvey RJ, Smart TG. Zinc-mediated inhibition of GABAA receptors: discrete binding sites underlie subtype specificity. Nat Neurosci. 2003;6:362–369. doi: 10.1038/nn1030. [DOI] [PubMed] [Google Scholar]
- Howell GA, Welch MG, Frederickson CJ. Stimulation-induced uptake and release of zinc in hippocampal slices. Nature. 1984;308:736–738. doi: 10.1038/308736a0. [DOI] [PubMed] [Google Scholar]
- Kay AR. Evidence for chelatable zinc in the extracellular space of the hippocampus, but little evidence for synaptic release of Zn2+ J Neurosci. 2003;23:6847–6855. doi: 10.1523/JNEUROSCI.23-17-06847.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube B. Potentiation of inhibitory glycinergic neurotransmission by Zn2+: a synergistic interplay between presynaptic P2X2 and postsynaptic glycine receptors. Eur J Neurosci. 2002;16:1025–1036. doi: 10.1046/j.1460-9568.2002.02170.x. [DOI] [PubMed] [Google Scholar]
- Laube B, Kuhse J, Betz H. Kinetic and mutational analysis of Zn2+ modulation of recombinant human inhibitory glycine receptors. J Physiol. 2000;522:215–230. doi: 10.1111/j.1469-7793.2000.t01-1-00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube B, Kuhse J, Rundstrom N, Kirsch J, Schmieden V, Betz H. Modulation by zinc ions of native rat and recombinant human inhibitory glycine receptors. J Physiol. 1995;483:613–619. doi: 10.1113/jphysiol.1995.sp020610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube B, Maksay G, Schemm R, Betz H. Modulation of glycine receptor function: a novel approach for therapeutic intervention at inhibitory synapses? Trends Pharmacol Sci. 2002;23:519–527. doi: 10.1016/s0165-6147(02)02138-7. [DOI] [PubMed] [Google Scholar]
- Lewis TM, Schofield RP, McClellan AM. Kinetic determinants of agonist action at the recombinant human glycine receptor. J Physiol. 2003;549:361–374. doi: 10.1113/jphysiol.2002.037796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JW. Molecular structure and function of the glycine receptor chloride channel. Physiol Rev. 2004;84:1051–1095. doi: 10.1152/physrev.00042.2003. [DOI] [PubMed] [Google Scholar]
- Mangin JM, Baloul M, Prado DC, Rogister B, Rigo JM, Legendre P. Kinetic properties of the α2 homo-oligomeric glycine receptor impairs a proper synaptic functioning. J Physiol. 2003;553:369–386. doi: 10.1113/jphysiol.2003.052142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller PS, Harvey RJ, Smart TG. Differential agonist sensitivity of glycine receptor α2 subunit splice variants. Br J Pharmacol. 2004;143:19–26. doi: 10.1038/sj.bjp.0705875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin ST, Cromer BA, Haddrill JL, Morton CJ, Parker MW, Lynch JW. Insights into the structural basis for zinc inhibition of the glycine receptor. J Biol Chem. 2003;278:28985–28992. doi: 10.1074/jbc.M300097200. [DOI] [PubMed] [Google Scholar]
- Pfeiffer F, Graham D, Betz H. Purification by affinity chromatography of the glycine receptor of rat spinal cord. J Biol Chem. 1982;257:9389–9393. [PubMed] [Google Scholar]
- Pribilla I, Takagi T, Langosch D, Bormann J, Betz H. The atypical M2 segment of the α subunit confers picrotoxinin resistance to inhibitory glycine receptor channels. EMBO J. 1992;11:4305–4311. doi: 10.1002/j.1460-2075.1992.tb05529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh T, Ishida M, Maruyama M, Shinozaki H. A novel antagonist, phenylbenzene omega-phosphono-alpha-amino acid, for strychnine-sensitive glycine receptors in the rat spinal cord. Br J Pharmacol. 1994;113:165–170. doi: 10.1111/j.1476-5381.1994.tb16189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieden V, Grenningloh G, Schofield PR, Betz H. Functional expression in Xenopus oocytes of the strychnine binding 48 kd subunit of the glycine receptor. EMBO J. 1989;8:695–700. doi: 10.1002/j.1460-2075.1989.tb03428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieden V, Kuhse J, Betz H. A novel domain of the inhibitory glycine receptor determining antagonist efficacies: further evidence for partial agonism resulting from self-inhibition. Mol Pharmacol. 1999;56:464–472. doi: 10.1124/mol.56.3.464. [DOI] [PubMed] [Google Scholar]
- Smart TG. A novel modulatory binding site for zinc on the GABAA receptor complex in cultured rat neurones. J Physiol. 1992;447:587–625. doi: 10.1113/jphysiol.1992.sp019020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart TG, Hosie AM, Miller PS. Zn2+ ions: modulators of excitatory and inhibitory synaptic activity. Neuroscientist. 2004;10:432–442. doi: 10.1177/1073858404263463. [DOI] [PubMed] [Google Scholar]
- Smart TG, Moss SJ, Xie X, Huganir RL. GABAA receptors are differentially sensitive to zinc: dependence on subunit composition. Br J Pharmacol. 1991;103:1837–1839. doi: 10.1111/j.1476-5381.1991.tb12337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart TG, Xie X, Krishek BJ. Modulation of inhibitory and excitatory amino acid receptor ion channels by zinc. Prog Neurobiol. 1994;42:393–341. doi: 10.1016/0301-0082(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Momiyama A, Hirai K, Hishinuma F, Akagi H. Functional correlation of fetal and adult forms of glycine receptors with developmental changes in inhibitory synaptic receptor channels. Neuron. 1992;9:1155–1161. doi: 10.1016/0896-6273(92)90073-m. [DOI] [PubMed] [Google Scholar]
- Unwin N, Miyazawa A, Li J, Fujiyoshi Y. Activation of the nicotinic acetylcholine receptor involves a switch in conformation of the alpha subunits. J Mol Biol. 2002;319:1165–1176. doi: 10.1016/S0022-2836(02)00381-9. [DOI] [PubMed] [Google Scholar]
- Watanabe E, Akagi H. Distribution patterns of mRNAs encoding glycine receptor channels in the developing rat spinal cord. Neurosci Res. 1995;23:377–382. doi: 10.1016/0168-0102(95)00972-V. [DOI] [PubMed] [Google Scholar]