Abstract
Regional cerebral blood flow (BF) was examined in regions of the medial prefrontal cortex (MPFC) with positron-emission tomography while subjects performed two cognitive tasks, reading nouns aloud and generating appropriate verbs for the same nouns. The control task was passive viewing of the same words. BF was reduced in regions of the MPFC during word reading and naïve verb generation, relative to a control state in which the subjects passively viewed nouns. Practicing verb generation produced improved performance, as measured by response time, which was strongly correlated with further reductions in MPFC and hypothalamic BF. After practice, when verb generation was performed on a novel list of words, reaction times slowed and the pattern of MPFC BF reverted to that seen in the word reading and naïve conditions. A separate behavioral study of the verb-generation task indicated that anxiety, high during naïve use-generation as measured by heart rate and self-report, decreased with practice on the task but returned with the introduction of a novel list of words. Taken together, these results suggest that the MPFC is part of a network, including the hypothalamus and brainstem, whose activity reflects a dynamic interplay between cognitive task performance and emotion.
It has long been recognized that cognitive and emotional processes are closely intertwined. Cognitive activity can attenuate emotional states and conversely, emotional and motivational factors can significantly affect cognitive performance. Although the existence of an interrelationship between cognition and emotion is intuitively appealing, because it seems to commonly manifest itself in human behavior, few empirical data exist to characterize its neural basis.
Traditionally, data have been sought in patients with brain injury, beginning with the landmark case of Phineas Gage who suffered a personality-changing injury to the orbital and medial prefrontal cortex (PFC) (1–4). Work on Gage and similar patients (5–7) has reenforced the belief that orbital and medial prefrontal cortices may play an important role in the interaction between cognition and emotion in the normal human brain.
Anatomically, the ventral medial prefrontal cortex (MPFC) is composed of cytoarchitectonically discrete areas that receive a wide range of sensory information from the body and the external environment via the orbital PFC (8–10) and are heavily interconnected with limbic structures such as the amygdala, ventral striatum, hypothalamus, midbrain periaquaductal gray region, and brainstem autonomic nuclei (11–17). It has been proposed that the MPFC plays a role in the integration of emotional and cognitive processes by incorporating emotional biasing signals or markers into decision-making processes (3, 18).
In contradistinction to the ventral MPFC and its association with emotional processing, the dorsal MPFC, composed of the anterior cingulate cortex (ACC) and adjacent areas, has been identified primarily with cognitive activity (19–23). However, a clean separation of functions relating to cognition (dorsal MPFC) and emotion (ventral MPFC) is probably an oversimplification (24). Rather, there likely exists a dynamic interplay between cognition and emotion within the MPFC as posited by us (25) and others (26, 27). This hypothesis receives support from the observation that in areas implicated in emotional processing, such as the amygdala and a variety of areas within ventral MPFC, blood flow (BF) decreases during the performance of attentionally demanding cognitive tasks. This was dramatically revealed in a large meta analysis of nine different cognitive tasks performed by Shulman and colleagues (28). In many of these same MPFC areas, BF may increase when experimental manipulations explicitly elicit emotional responses in subjects (29–34).
Conversely, in more dorsal areas of the PFC that appear to serve cognitive functions, such as the anterior cingulate and the dorsolateral PFC, BF increases while performing attentionally demanding cognitive tasks, but may decrease during some experimentally induced and pathological emotional states (25, 35).
One of the studies in the meta analysis mentioned above (28), generating verbs aloud for visually presented nouns (19, 36, 37), included a practice component. Familiarization with a difficult task likely reduces anxiety associated with task performance. Thus, this experiment (37) afforded us the potential opportunity to manipulate through practice both the attentional demands of the cognitive task and the emotional state within the same subject. Because the original positron-emission tomography (PET) experiment did not directly address the question of performance anxiety, an additional behavioral study, not associated with imaging, was performed in which subject anxiety was examined via self-report and physiological monitoring.
A preliminary report of the PET data has been presented in abstract form.‖ The PET results, not including the analysis described here, have been published (37).
Materials and Methods
PET Experiment.
Subjects.
Twelve (seven female, five male, mean age 24 ± 3.5 years) normal volunteers were recruited from the local population of undergraduate and graduate students and staff at Washington University and paid for their participation. All subjects were strongly right-handed as measured by the Edinburgh handedness inventory (39) and had no significant neurological history. Informed consent was obtained before participation following guidelines approved by the Human Studies Committee (Institutional Review Board) and the Radioactive Drug Research Committee of Washington University.
PET scanning procedures.
Brain BF was measured by using the PETT VI system in low-resolution mode (40), H215O (41, 42), and PET scanning methodology developed at Washington University (43–46). The scanning procedure for these subjects has been described in detail (37).
Task description and monitoring.
A Macintosh IIx computer system was used to control stimulus display and data acquisition. During all scans, subjects were instructed to fixate on a small crosshair presented in the center of a computer monitor suspended about 30 cm from the subject's head. For each scan, the stimuli consisted of words from one of two lists of 40 high-frequency concrete English words (more than five occurrences per million words; ref. 47) that were displayed just below the fixation point and subtended a visual angle of ≈0.8° per letter. The words were presented in random order at a rate of one every 1.5 s and were displayed for 150 ms.
An electronic circuit (a voice key) gated by the amplitude of microphone input was used to measure voice onset times for verbally produced responses. Responses were also recorded onto one track of a cassette tape, and a brief tone (inaudible to the subjects) synchronized to the onset of stimulus presentation was recorded on the second track of the cassette tape. In a few instances in which the response was not loud enough to trigger the voice key, reaction times were obtained by digitizing the taped responses and calculating the latencies between the tone and response onsets by using commercially available digitizing software (macrecorder, Farallon Computing, San Leandro, CA).
A total of eight PET scans were performed on each subject. A complete description of the scanning protocol has been published (37). During all scans, subjects were instructed to fixate on the crosshair. In the first scan, subjects viewed the nouns on one of the two lists passively. In the second scan, subjects read the same nouns aloud. During the third scan (naïve verb generate), subjects were instructed to produce an action word that described what the presented noun might do, or what it might be used for.
After the third scan, subjects performed the verb generate task for eight more blocks (a block equals one complete presentation of a 40-word list) with the same set of nouns, for a total of 11 blocks of the same list (i.e., one passive, one repeat, and nine verb generate blocks). During these eight additional practice blocks, the instructions were repeated between each block and the importance of speed stressed (subjects were told to come up with whatever verb response was fastest and easiest for them). The subject remained in position in the scanner during the practice period (10–15 min).
After the practice blocks, subjects were scanned a fourth time. In this fourth scan, the same list of words was presented and subjects again produced appropriate verbs (practiced verb generate). In the fifth scan, subjects again read nouns from the same list and in the sixth scan, the list was presented again passively. For the seventh scan, a novel list was introduced, and subjects again generated appropriate verbs for the presented nouns. In the eighth and final scan, subjects read the words of this novel list aloud.
The following comparisons were analyzed: (i) reading nouns minus passive viewing; (ii) naïve verb generate minus passive viewing; (iii) practiced verb generate minus passive viewing; (iv) novel verb generate minus passive viewing; (v) practiced minus naïve verb generate; and (vi) practiced minus novel verb generate.
Data analysis: Performance.
Median response times were calculated for each trial of the verb generation and noun-repetition tasks. Repeated-measures ANOVA were performed to assess significance across blocks (SuperANOVA, Abacus Concepts, Berkeley, CA). Post hoc comparisons were performed by using paired t tests.
Data analysis: PET.
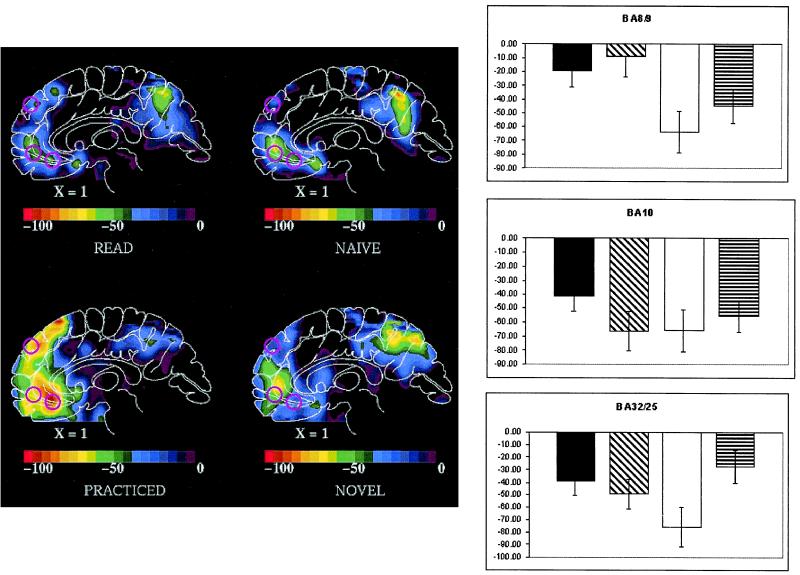
Spherical regions of interest (ROI) were defined by using the coordinates of three MPFC BF decreases listed in Table 1 of the meta analysis reported by Shulman and colleagues (28). These ROIs will be referred to by the Brodmann area (BA) originally assigned to them by Shulman and colleagues: BA 8/9 located at x = 5, y = 49, z = 36; BA 10 located at x = −1, y = 47, z = −4; and BA 32 located at x = 3, y = 31, z = −10. The locations of these ROIs are shown in Fig. 1. We used these ROIs to assess BF change for each of the comparisons mentioned above. BA 32 undoubtedly includes elements of BA 24 and 25, reflecting the very approximate nature of these original designations.
Table 1.
Regional correlations within MPFC and hypothalamus during naïve verb generation
| Region, BA | Coordinates, x, y, z | Peak Pearson r | P value |
|---|---|---|---|
| BA 9/10 | 7, 51, 26 | 0.75 | 0.005 |
| BA 32 | −5, 39, 22 | 0.69 | 0.01 |
| Hypothalamus | 3, −11, −8 | 0.62 | 0.03 |
Coordinates are in the space of Talairach and Tournoux (48). The coordinates of the Brodmann area used as a basis for this correlation analysis were 3, 31, −10 and come from the work of Shulman and colleagues (28) (see text and Fig. 1 for further details). BA refers to the approximate Brodmann area.
Figure 1.
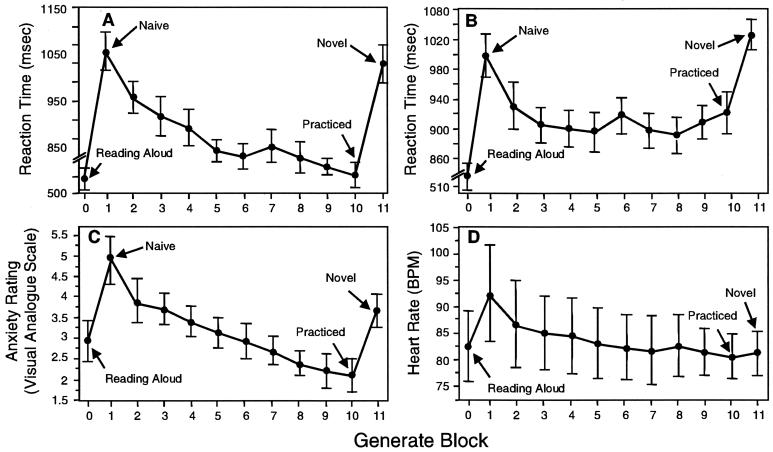
(A) Response times (means ± SEM) across verb generation trials in 12 subjects generating appropriate verbs while in a PET scanner. PET scans were performed during the naïve, practiced, and novel blocks. (B) Response times (n = 15); (C) visual analog scale rating of anxiety (n = 15); and (D) heart rate (n = 7) measurements for the behavioral study of the verb generation task. All measurements are means ± SEM. Block 0 is reading nouns, block 1 is the first (naïve) verb generation block, and block 11 is verb generation with a novel list of nouns.
Repeated-measures ANOVA were performed to assess the significance of changes across the active conditions (reading plus naïve, practiced, and novel verb generate) relative to the baseline of passive viewing of nouns. Post hoc comparisons were performed by using paired t tests.
To determine whether BF in other brain regions was correlated with changes in MPFC, a voxel-by-voxel correlation approach was used.
Behavioral Experiment.
Fifteen volunteers (four female; mean age 25.3 years) performed the verb-generate task, by using the same lists and stimulus presentation parameters as described above, in a behavioral testing environment outside of the PET scanner. Subjects sat in a chair facing a computer monitor and read 40 words aloud, then performed the verb generate task on the same list 10 times. Finally, they performed the verb generate task on a novel list of words. The words in both lists were the same as those used in the PET experiment. Reaction time was recorded as described above. For seven subjects (two female), heart rate was monitored throughout task performance by using a portable electrocardiogram device (LifePak 7, Physio-Control, Redmond, WA). At the conclusion of each block, subjects rated their level of anxiety during the task by using a 10-point visual analog scale, where zero indicated no anxiety or discomfort and 10 indicated extreme anxiety or discomfort. Fractional values were determined to the nearest one-quarter point. Heart rate in beats/min was determined over six 10-s bins for each block by counting the number of QRS complexes recorded on the electrocardiogram paper strip. Because no significant differences in heart rate within 1-min blocks were detected, heart rate was averaged across the six 10-s bins for each block. Because of technical problems, no reaction time data were recorded for one subject, and two subjects did not complete a novel verb generate block.
Results
PET Experiment.
Task performance.
Comparison of median response times (i.e., voice onset latencies) for the naïve, practiced, and novel blocks showed a significant effect of practice [F (2, 11) = 31.62 and P < 0.0001] (Fig. 1A). Specific comparisons showed a significant difference between the naïve and practiced conditions (t = 7.34, d.f. = 11, and P < 0.0001) and between the practiced and novel conditions (t = 6.32 and P < 0.001) but not between the naïve and novel conditions (t = 1.03 and P > 0.3).
Brain BF.
All three regions under consideration exhibited a main effect of task (i.e., verb generation compared with passive noun viewing). A significant effect of condition, however, was only observed for BA 8/9 [F (3,11) = 5.79 and P < 0.005] and BA 32 (F = 6.32 and P < 0.005) ROIs. Post hoc comparisons indicated that in BA R8/9, the practiced condition produced a significantly larger BF decrease than the read (t = 4.53 and P < 0.001), naïve (t = 3.69 and P < 0.005), or novel (t = 3.06 and P = 0.01) conditions. For BA 32, the practiced condition produced a significantly larger BF decrease than the read (t = 3.00 and P = 0.01) and novel (t = 3.45 and P = 0.005) conditions and a marginal decrease over the naïve condition (t = 1.98 and P = 0.057). The BF changes in the three regions are summarized in Fig. 2.
Figure 2.
(Left) Averaged subtraction PET images in atlas space (48) for each of the conditions examined. Upper Left, reading nouns aloud; Upper Right, naïve verb generation; Lower Left, practiced verb generation; and Lower Right, verb generation on a novel list of nouns. All comparisons are to passive viewing of nouns. Each image is a sagittal section taken 1 mm to the right of the midsaggital plane. Anterior is to the left. The color scale is a linear scale of normalized radioactive counts. The locations of the three MPFC regions studied are indicated with pink circles on each image. The ROIs are referred to by the BA originally assigned to them by Shulman and colleagues: BA 8/9 located at x = 5, y = 49, z = 36; BA 10 located at x = −1, y = 47, z = −4; and BA 32 located at x = 3, y = 31, z = −10. We note BA 32 most likely includes the adjacent BA 24 and 25 as well. (Right) Relative blood flow change (means ± SEM) in normalized counts within each region for each of the four comparisons: read = black bar; naïve = diagonal stripe bar; practiced = clear bar; and novel = horizontal stripe bar.
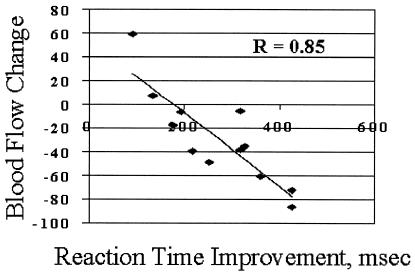
Because of the marginal difference in effect produced by the naïve and practiced conditions on area BA 32, we compared the difference between the practice and naïve condition for each subject as a function of their improvement in performance on the generate task. This comparison revealed that BF change for each subject in BA 32 was significantly correlated with improvement in performance on the verb generate task with practice, as measured by change in mean response time (r = −0.86 and P < 0.0005) (Fig. 3). No correlation between response time increase and BF increase with the introduction of a novel list of words was found. Nor was there any correlation between activity change and performance change in either BA 8/9 or BA 10 for the practiced relative to the naïve or novel conditions.
Figure 3.
Relative blood flow change in the BA 32 region (see Fig. 1) in the practiced minus the naïve verb generation condition compared with the response time improvement occurring as the result of practice on the task (r = −0.85 and P < 0.0005).
Because of the significant correlation between practice-induced performance change and BF in BA 32, a correlation analysis was performed by using the practiced minus naïve value in this region as the dependent variable. With this analysis, we hoped to determine whether BA 32 was unique in its relationship to behavior or was acting in concert with other areas. Two areas of significant correlation were found in the MPFC and one in the hypothalamus (Table 1). The two areas in the MPFC were within 1 and 2 cm (vector distance), respectively, of BA 8/9 used in our primary analysis (see Materials and Methods).
Finally, as originally reported by Shulman and colleagues (28), the naïve performance of the verb generate task resulted in a significant decrease in the activity of the right amygdala (stereotaxic coordinates: 21, −9, −18) when compared with passive viewing of words. A detectable but not significant decrease was also reported in the left amygdala (stereotaxic coordinates: −21, 9, −22). We examined the effect of practice on the activity in both amygdala regions. Both decreased further as the result of practice but this only achieved statistical significance on the left (paired t test; t = 2.515 and P = 0.0287). Thus, in the practiced state, activity in both amygdalae were reduced significantly below their baseline level.
Behavioral Study.
As originally reported (ref. 37; see also Fig. 1A), comparison of median response times for the naïve, practiced, and novel blocks showed a significant effect of practice [Fig. 1B, F (2,11) = 9.37 and P = 0.001]. Anxiety, as measured by self-ratings (Fig. 1C) and heart rate (Fig. 1D), was low during word reading, increased during the first verb generate block, and reduced after practice [visual analog scale, F (2,14) = 8.94 and P < 0.0001; heart rate, F (2,6) = 2.95 and P < 0.005]. Post hoc comparisons indicated that the visual analog scale rating was significantly higher for the first verb generation block than for reading (t = 3.37, d.f. = 13, and P < 0.005) or for the final practiced block (t = 3.92 and P < 0.001). The visual analog scale rating was also higher for the novel verb generation block than for the final practiced block (t = 3.41, d.f. = 11, and P < 0.005). Post hoc comparisons for heart rate indicated that heart rate was significantly higher for the first verb generation block than for reading (t = 2.34, d.f. = 6, and P = 0.03) with a marginally significant increase for the first verb generation block compared with the final practiced block (t = 1.87 and P < 0.056).
Discussion
Our functional imaging results are generally consistent with a large body of PET and functional MRI brain imaging data showing that MPFC activity is suppressed during attention-demanding cognitive task performance. However, our results simply do not follow from these earlier data (25). Thus, one might reasonably have expected that the naïve and novel verb generation conditions should have resulted in much larger reductions in MPFC than the read and practiced conditions. This prediction is based on the attentional demands (i.e., difficulty as reflected in the reaction time) of each task: least in word reading, most in naïve and novel verb generation, and intermediate in practiced verb generation. However, in the two areas in which change occurred as a function of task (BA 8/9 and BA 32), this was not observed. The changes were more nearly the same for the read, naïve, and novel conditions and less than those observed in the practiced condition. Our behavioral data provide a possible explanation.
Performance anxiety, documented by self-report and changes in heart rate, was elevated in the cognitively demanding parts of the experiment (i.e., naïve and novel verb generation) and much lower if present at all in word reading and practiced verb generation. Thus, practiced verb generation, and to a lesser extent, word reading, give the only unfettered view of attention-demanding cognitive activity on MPFC activity. This view is consistent with the hypothesis that the more difficult, attention-demanding cognitive activity of practiced verb generation, in the absence of anxiety, differentially suppresses activity in MPFC compared with word reading. Whereas naïve and novel verb generation differ even more strikingly from word reading in their attentional demands than does practiced verb generation, they are associated with performance anxiety whereas wording reading and practiced verb generation are not (Fig. 2). The prediction, based on our earlier review of the literature (25), is that, in isolation, performance anxiety might actually elevate activity in some of the limbic areas of MPFC frequently suppressed during cognitive activity.
In our results, the BF changes in BA 32, an area in ventral MPFC probably encompassing BA 24 and 25 (see Materials and Methods) and often referred to as the subgenual PFC, was of special interest for two reasons. First, the BF changes in this area were correlated with practiced-induced improvement in reaction time (i.e., voice onset latency). As our behavioral results indicate (Fig. 2), practice-induced improvement in response time was associated with a significant decline in the level of performance anxiety. Thus, by implication, this area may be associated with the level of performance anxiety experienced by our subjects. Second, the changes in BA 32 were correlated with three additional areas (Table 1), two in the more dorsal MPFC and one in the hypothalamus. Responses in this more dorsal, anterior region of MPFC have been specifically associated with the experiential aspects of emotion (31, 33, 34).
Correlated changes in the hypothalamus are consistent with anatomical studies in nonhuman primates (11, 12), suggesting that areas within the subgenual PFC integrate information from other areas of medial and orbital PFC with structures such as the amygdala, hypothalamus, and the midbrain periaqueductal gray. These observations strengthen our belief that the changes we observe may be related to the change in performance anxiety. Taken together, our imaging and behavioral results are consistent with the existence of a network of regions within the circuitry of the MPFC, possibly including the hypothalamus and brainstem (see accompanying paper; ref. 38), which reflects the level of anxiety in our normal subjects during the performance of an attention-demanding cognitive task.
From this analysis, we are led to the hypothesis that the changes in MPFC we observed represent an interaction between attention-demanding cognitive activity and performance anxiety. Lacking in this formulation, of course, is direct knowledge of exactly what changes in BF in MPFC might occur with anxiety. In the accompanying paper (38), we examine this question more directly.
It is noteworthy that few, if any, examples of the changes we observe have been reported in neurophysiological studies of awake behaving nonhuman primates. Our results, whereas not providing a definitive explanation for this apparent discrepancy, do offer a testable hypothesis. We suggest that the difference may lie in the level of attention usually required of awake, behaving, nonhuman primates during control states such as visual fixation. Only strict compliance is rewarded. As a result, one would anticipate a reduction in activity in MPFC during the control state. On the other hand, humans rest quietly with eyes closed or while maintaining visual fixation with little effort and certainly without the inducement of a reward for compliance or the fear of not receiving a reward for failure to comply. Not surprisingly to us, therefore, humans exhibit no reductions in activity in the MPFC or elsewhere during these control states.
Finally, it is good to keep in mind that performance anxiety is a likely accompaniment of most demanding cognitive tasks conducted in a laboratory or imaging setting no matter how emotionally impoverished they might superficially appear. This is particularly true, as our data show, when the tasks are performed without prior experience. As a consequence, areas of the brain concerned with emotion will change in concert with those more directly concerned with the cognitive aspects of the tasks.
Acknowledgments
This work was supported by National Institutes of Health Grants NS06833, DA07261, and NS10196, and the Charles A. Dana Foundation.
Abbreviations
- MPFC
medial prefrontal cortex
- BF
blood flow
- PET
positron-emission tomography
- ACC
anterior cingulate cortex
- ROI
region of interest
- BA
Brodmann area
- PFC
prefrontal cortex
Footnotes
Simpson, J. R., Jr., MacLeod, A. K., Fiez, J. A., Drevets, W. C. & Raichle, M. E. (1997) Soc. Neurosci. Abstr. 23, 1317 (abstr.).
References
- 1.Harlow J M. Boston Med Surg J. 1848;39:389–393. [Google Scholar]
- 2.Harlow J M. Pub MA Med Soc. 1868;2:327–347. [Google Scholar]
- 3.Damasio A R. Descartes' Error; Emotion, Reason, and the Human Brain. New York: Avon Books; 1994. [Google Scholar]
- 4.Bigelow H J. Am J Med Sci. 1850;39:2–23. [Google Scholar]
- 5.Eslinger P J, Damasio A R. Neurology. 1985;35:1731–1741. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- 6.Shallice T, Burgess P W. Brain. 1991;114:727–741. doi: 10.1093/brain/114.2.727. [DOI] [PubMed] [Google Scholar]
- 7.Rolls E T, Hornak J, Wade D, McGrath J. J Neurol Neurosurg Psychiatry. 1994;57:1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbas H. Neuroscience. 1993;56:841–864. doi: 10.1016/0306-4522(93)90132-y. [DOI] [PubMed] [Google Scholar]
- 9.Carmichael S T, Price J L. J Comp Neurol. 1995;363:642–664. doi: 10.1002/cne.903630409. [DOI] [PubMed] [Google Scholar]
- 10.Rolls E T, Baylis L L. J Neurosci. 1994;14:5437–5452. doi: 10.1523/JNEUROSCI.14-09-05437.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carmichael S T, Price J L. J Comp Neurol. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- 12.Barbas H. Neurosci Biobehav Rev. 1995;19:499–510. doi: 10.1016/0149-7634(94)00053-4. [DOI] [PubMed] [Google Scholar]
- 13.Haber S N, Kunishio K, Mizobuchi M, Lynd-Balta E. J Neurosci. 1995;15:4851–4867. doi: 10.1523/JNEUROSCI.15-07-04851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morecraft R J, Geula C, Mesulam M-M. J Comp Neurol. 1992;323:341–358. doi: 10.1002/cne.903230304. [DOI] [PubMed] [Google Scholar]
- 15.Neafsey E J, Terreberry R R, Hurley K M, Ruit K G, Frysztak R J. In: The Neurobiology of Cingulate Cortex and Limbic Thalamus. Vogt B A, Gabriel M, editors. Boston: Birkhauser; 1993. [Google Scholar]
- 16.Sesack S R, Deutch A Y, Roth R H, Bunney B S. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- 17.Bandler R, Shipley M T. Trends Neurosci. 1994;17:379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 18.Bechara A, Damasio A R, Damasio H, Anderson S W. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 19.Petersen S E, Fox P T, Posner M I, Mintun M A, Raichle M E. Nature (London) 1988;331:585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- 20.Vogt B A, Finch D M, Olson C R. Cerebral Cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- 21.Picard N, Strick P L. Cereb Cortex. 1996;6:342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- 22.Paus T, Koski L, Caramanos Z, Westbury C. NeuroReport. 1998;9:R37–R47. doi: 10.1097/00001756-199806220-00001. [DOI] [PubMed] [Google Scholar]
- 23.Carter C S, Braver T S, Barch D M, Botvinick M M, Noll D, Cohen J D. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 24.Teasdale J D, Howard R J, Cox S G, Ha Y, Brammer M J, Williams S C R, Checkley S A. Am J Psychiatry. 1999;156:209–215. doi: 10.1176/ajp.156.2.209. [DOI] [PubMed] [Google Scholar]
- 25.Drevets W C, Raichle M E. Cognit Emotion. 1998;12:353–385. [Google Scholar]
- 26.Bush G, Luu P, Posner M I. Trends Cognit Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 27.Mayberg H S. J Neuropsychiatry. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- 28.Shulman G L, Fiez J A, Corbetta M, Buckner R L, Miezin F M, Raichle M E, Petersen S E. J Cognit Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 29.Pardo J V, Pardo P J, Raichle M E. Am J Psychiatry. 1993;150:713–719. doi: 10.1176/ajp.150.5.713. [DOI] [PubMed] [Google Scholar]
- 30.George M S, Ketter T A, Parekh P I, Horwitz B, Herscovitch P, Post R M. Am J Psychiatry. 1995;152:341–351. doi: 10.1176/ajp.152.3.341. [DOI] [PubMed] [Google Scholar]
- 31.Lane R D, Fink G R, Chau P M-L, Dolan R J. NeuroReport. 1997;8:3969–3972. doi: 10.1097/00001756-199712220-00024. [DOI] [PubMed] [Google Scholar]
- 32.Whalen P J, Bush G, McNally R J, Wilhelm S, McInerney S C, Jenike M A, Rauch S L. Biol Psychiatry. 1998;44:1219–1228. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]
- 33.Chua P M-L, Krams M, Toni I, Passingham R, Dolan R J. NeuroImage. 1999;9:563–571. doi: 10.1006/nimg.1999.0407. [DOI] [PubMed] [Google Scholar]
- 34.Ploghaus A, Tracey I, Gati J S, Clare S, Menon R S, Matthews P M, Nicholas J, Rawlins P. Science. 1999;284:1979–1981. doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- 35.Mayberg H S, Liotti M, Brannan S K, McGinnis S, Mahurin R K, Jerabek P A, Silva J A, Tekell J L, Martin C C, Lancaster J L, et al. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 36.Petersen S E, Fox P T, Posner M I, Mintun M A, Raichle M E. J Cognit Neurosci. 1989;1:153–170. doi: 10.1162/jocn.1989.1.2.153. [DOI] [PubMed] [Google Scholar]
- 37.Raichle M E, Fiez J A, Videen T O, Macleod A K, Pardo J V, Fox P T, Petersen S E. Cereb Cortex. 1994;4:8–26. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- 38.Simpson J R, Jr, Drevets W C, Snyder A Z, Gusnard D A, Raichle M E. Proc Natl Acad Sci USA. 2001;98:688–693. doi: 10.1073/pnas.98.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raczkowski D, Kalat J W, Nebes R. Neuropsychology. 1974;6:43–47. doi: 10.1016/0028-3932(74)90025-6. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto M, Ficke D C, Ter-Pogossian M. IEEE Trans Nucl Sci. 1982;29:529–533. [Google Scholar]
- 41.Herscovitch P, Markham J, Raichle M E. J Nucl Med. 1983;24:782–789. [PubMed] [Google Scholar]
- 42.Raichle M E, Martin W R W, Herscovitch P, Mintun M, Markham J. J Nucl Med. 1983;24:790–798. [PubMed] [Google Scholar]
- 43.Fox P T, Perlmutter J S, Raichle M E. J Comput Assist Tomogr. 1985;9:141–149. doi: 10.1097/00004728-198501000-00025. [DOI] [PubMed] [Google Scholar]
- 44.Fox P T, Mintun M A, Rieman E M, Raichle M E. J Cereb Blood Flow Metab. 1988;8:642–653. doi: 10.1038/jcbfm.1988.111. [DOI] [PubMed] [Google Scholar]
- 45.Fox P T, Mintun M A. J Nucl Med. 1989;30:141–149. [PubMed] [Google Scholar]
- 46.Mintun M A, Fox P T, Raichle M E. J Cereb Blood Flow Metab. 1989;9:96–103. doi: 10.1038/jcbfm.1989.13. [DOI] [PubMed] [Google Scholar]
- 47.Kucera H, Francis W N. Computational Analysis of Present Day American English. Providence, RI: Brown Univ. Press; 1967. [Google Scholar]
- 48.Talairach J, Tournoux P. Co-Planar Stereotactic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]