Abstract
CUG-BP is the human homolog of the Xenopus EDEN-BP, which was shown previously to bind to mRNAs, such as c-mos, that exhibit rapid deadenylation following fertilization of the oocyte. While several studies have focused on roles of CUG-BP as a splicing or translation regulator in mammalian cells, its role in mRNA decay has not been examined in detail. Here, we have used an in vitro deadenylation assay to dissect the function of CUG-BP in the decay of two ARE-containing mRNAs: c-fos and TNFα. CUG-BP binds specifically to both of these RNAs and stimulates poly(A) shortening by PARN. Moreover, CUG-BP interacts with PARN in extracts by coimmunoprecipitation, and this interaction can be recapitulated using recombinant proteins. CUG-BP, therefore, is the first RNA-binding protein shown to directly recruit a deadenylase to an RNA substrate.
Keywords: deadenylation, mRNA stability, myotonic dystrophy, EDEN, AU-rich
INTRODUCTION
Deadenylation is a rate-limiting step in the turnover of most eukaryotic mRNAs. In addition, removal of the poly(A) tail is an effective means of inhibiting translation, as the poly(A) binding protein (PABP) is a translation initiation factor (Kahvejian et al. 2005). In Xenopus oocytes, poly(A) tail length is used extensively to control gene expression, as there is virtually no transcription ongoing during oocyte development (Richter 1999). The majority of maternal mRNAs undergo a default deadenylation during the maturation process, and this is executed by the PARN deadenylase (Korner et al. 1998). However, several mRNAs, including c-mos and x-myc II, become polyadenylated during maturation due to the presence of a cytoplasmic polyadenylation element (CPE). Immediately following fertilization, CPE-containing mRNAs are rapidly deadenylated, leading to translational silencing. In contrast to the default deadenylation pathway, post-fertilization deadenylation is dependent on the presence of cis-acting sequences: AU-rich elements (AREs) and/or EDEN (embryonic deadenylation element) found within the 3′UTR (Richter 1999).
AREs in Xenopus mRNAs appear similar to those in mammalian mRNAs that are able to modulate deadenylation rates. The AREs of either Xenopus x-myc II mRNA or human GM-CSF mRNAs mediate rapid deadenylation following fertilization (Voeltz and Steitz 1998). In mammalian cells, numerous ARE-binding proteins have been identified that either positively or negatively regulate decay and/or translation (Wilusz and Wilusz 2004), and several studies have shown interactions between ARE-binding proteins and the deadenylation machinery (Lai et al. 2003; Gherzi et al. 2004; Lykke-Andersen and Wagner 2005). However, there has been no direct evidence for an interaction between ARE-binding proteins and the deadenylation machinery that leads to modulation of this process.
The EDEN-binding protein, EDEN-BP, is necessary for the function of the EDEN sequence (Paillard et al. 1998). Subsequent studies have determined that EDEN-BP recognizes sequences that are rich in uridine/purine dinucleotides (Bonnet-Corven et al. 2002). Furthermore, EDEN-BP can also bind to AU-rich elements, such as that found in c-jun (Paillard et al. 2002). Interestingly, EDEN-dependent deadenylation appears to be facilitated by the presence of neighboring AU-rich sequences (Audic et al. 1998). As yet, the enzyme responsible for sequence-specific deadenylation following fertilization has not been identified, but it appears to be biochemically distinct from the enzyme that carries out the default deadenylation pathway (Paillard et al. 1996). Thus, it is not known how binding of EDEN-BP leads to activation of deadenylation.
EDEN-BP is conserved in higher eukaryotes; Bruno in Drosophila, etr-1 in Caenorhabditis elegans, and CUG-BP in human cells are all homologous to EDEN-BP. CUG-BP is of particular interest, as its overexpression results in a phenotype similar to myotonic dystrophy and is thought to be at least partially responsible for the muscle-specific effects of this disease (Ho et al. 2005). Previous studies of CUG-BP function have focused mainly on the roles of this protein in regulating alternative splicing (Philips et al. 1998) and also on its ability to modulate translation of several mRNAs (Mukhopadhyay et al. 2003; Timchenko et al. 2005). However, as CUG-BP is able to functionally substitute for EDEN-BP to induce deadenylation in Xenopus extracts (Paillard et al. 2003), it seems likely that it also plays a similar role in regulating poly(A) shortening in mammalian cells.
Our lab has previously developed an in vitro assay for assessing the mechanisms and regulation of mRNA decay (Ford et al. 1999). This assay shows accelerated turnover of ARE-containing mRNAs (Ford et al. 1999) as well as cap-dependent deadenylation by PARN (Gao et al. 2000), making it an appropriate system to examine the influence of CUG-BP on deadenylation. In this study, we demonstrate the binding of CUG-BP to two mammalian ARE-containing mRNAs in HeLa cell extracts. CUG-BP appears to recognize both the ARE and EDEN-like sequences in these RNAs. Furthermore, we show that CUG-BP can interact with the PARN deadenylase to promote deadenylation of these substrate RNAs. This is the first evidence for an RNA-binding protein directly recruiting an active deadenylase to its RNA substrate.
RESULTS
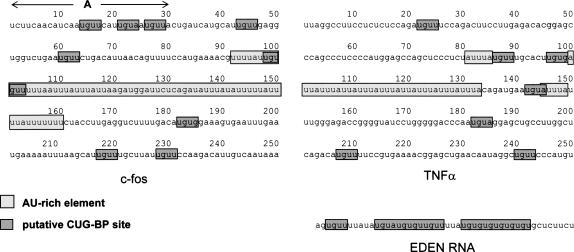
In order to study potential effects of CUG-BP on mRNA deadenylation it was first necessary to identify substrate RNAs that can be recognized by CUG-BP. Recent reports examined the binding specificity of CUG-BP and found that it exhibits a strong preference for UG repeats and UGUU sequences (Takahashi et al. 2000; Faustino and Cooper 2005). CUG-BP has also been shown to associate with CUG repeats (Lu et al. 1999) and AU-rich elements (Mukhopadhyay et al. 2003). We had previously shown that the c-fos and TNFα AU-rich elements can enhance mRNA decay in HeLa extracts (Ford et al. 1999), so these seemed appropriate candidates. We examined the regions flanking the AREs of these two RNAs for other sequences that might be recognized by CUG-BP based on the findings of the studies mentioned above. Those we found are highlighted in Figure 1. In addition to the ARE, both c-fos and TNFα RNAs contain multiple UGUA, UGUU, and UGUG sequences that could perhaps be recognized by CUG-BP. The occurrence of these sequences is significantly higher (nine in c-fos and seven in TNFα) than might be predicted at random in a 250 nt RNA (2.9 occurrences expected). The presence of potential CUG-BP binding sites and AU-rich elements in the same RNA is significant, as AU-rich sequences were shown to enhance the function of the EDEN element in the Xenopus system (Audic et al. 1998). Moreover, the mammalian c-jun ARE was shown to induce EDEN-dependent deadenylation in Xenopus embryos (Paillard et al. 2002).
FIGURE 1.
Mammalian ARE-containing mRNAs contain candidate binding sites for CUG-BP. The sequences of c-fos and TNFα RNA substrates are depicted, with AU-rich elements and putative CUG-BP binding sites highlighted. In addition, the region of the c-fos RNA with the best matches to CUG-BP consensus is labeled (A). The sequence of the EDEN region of c-mos, which is used as a competitor in Figure 3, is also shown.
CUG-BP binds to sequences from TNFα and c-fos mRNAs
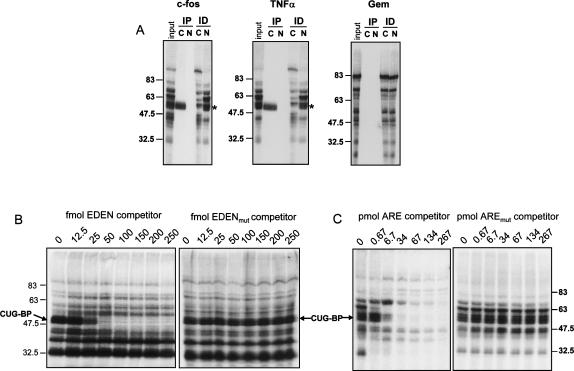
It was important to determine whether CUG-BP actually bound to the candidate substrate RNA in extracts. Radiolabeled TNFα, c-fos RNAs, and a control RNA(Gem) derived from the pGem4 polylinker were generated by in vitro transcription and incubated in HeLa cytoplasmic extracts. The Gem control RNA was chosen in part because it lacks any sequences with homology with known CUG-BP binding sites. UV-cross-linking was used to visualize proteins bound to each RNA, and the presence of CUG-BP was determined by immunoprecipitation of the cross-linked bands. As seen in Figure 2A, CUG-BP bound to both the TNFα RNA and the c-fos RNA substrates, as predicted, but not to the control RNA. Immunodepletion using CUG-BP-specific antiserum significantly reduced the level of CUG-BP in the extracts (Fig. 2A, ID lanes). Therefore, CUG-BP specifically binds the TNFα and c-fos RNA substrates.
FIGURE 2.
The c-fos and TNFα RNAs cross-link specifically to CUG-BP in vitro. (A) The indicated radiolabeled substrate RNA was incubated in extracts and irradiated with UV light to cross-link proteins to the RNA (input). A portion of the cross-linked proteins were subjected to immunoprecipitation (IP) with anti-CUG-BP antibody (C), or normal serum (N). In a separate experiment (ID), anti-CUG-BP antibody (C), or normal serum (N), were used to immunodeplete the extracts following cross-linking and the supernatants were analyzed. The products were separated by SDS-PAGE. The position of the cross-linked CUG-BP is indicated. (B) UV cross-linking to the c-fos RNA substrate was performed in the presence of increasing femtomolar amounts of EDEN RNA competitor or a control RNA (EDENmut), in which the CUG-BP binding sites had been mutated. The CUG-BP band is indicated. (C) UV cross-linking to the c-fos RNA substrate was performed in the presence of increasing picomolar amounts of TNFα ARE competitor or a control RNA in which the AUUUA pentamers had been mutated. The CUG-BP band is indicated, and molecular weight markers are shown.
We next examined the specificity of CUG-BP binding to c-fos RNA in more detail using unlabeled RNA competitors. As shown in Figure 2B, CUG-BP was efficiently competed by addition of 50 fmol or more of unlabeled EDEN RNA oligo, but not by a matched oligo in which the EDEN binding sites had been mutated. This is consistent with previous data showing that CUG-BP exhibits similar binding specificity to EDEN-BP. In addition, we were able to compete CUG-BP from the c-fos RNA using an RNA oligo containing just the 34-nt AU-rich sequence from TNFα (Fig. 2C). However, the TNFα ARE oligo was a weaker competitor than the EDEN oligo requiring between 6.7 and 34 pmol to completely compete the CUG-BP band. This suggests that CUG-BP has a higher affinity for uridine/purine-rich sequences than for AU-rich sequences.
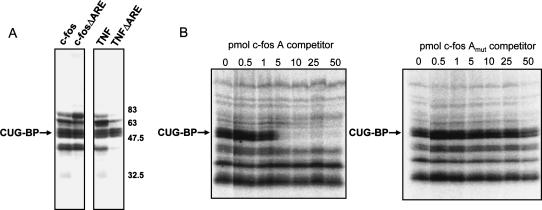
As both TNFα and c-fos RNA substrates contain AU-rich sequences as well as other potential CUG-BP binding sites, we wished to test whether CUG-BP bound to the ARE or elsewhere in the RNA. We deleted the ARE region from each RNA and repeated the UV cross-linking experiment. As seen in Figure 3A, deletion of the ARE had no discernable effect on CUG-BP cross-linking in either case. This strongly suggests that CUG-BP is binding to sequences other than the ARE, although it remains possible that the AREs are recognized as well. The best candidate EDEN-like sequence in c-fos lies at the 5′ end of the substrate RNA (labeled “A” in Fig. 1). This region has two UGUU sequences and a UGUA sequence in a 16-nt stretch. We therefore used an RNA oligo spanning this region as a cold competitor. As predicted, c-fos region A was able to compete CUG-BP effectively from the c-fos RNA substrate, but a mutant version in which the two UGUU sequences were changed to AGAA had no effect (Fig. 3B). Although c-fos region A is not as efficient a competitor as the EDEN RNA, this is not unexpected, as the EDEN RNA oligo has more and better matches to the optimal CUG-BP binding site. This result shows that CUG-BP is able to bind c-fos region A, probably by recognizing the UGUU sequences, but does not preclude that it may also bind to other regions of this RNA, including perhaps the ARE.
FIGURE 3.
CUG-BP binds to AREs and to sites outside those regions. (A) UV cross-linking was performed using c-fos and TNFα RNA substrates and variant RNAs that lacked the ARE. The CUG-BP band and molecular weight markers are indicated. (B) Increasing picomolar amounts of Region A of the c-fos RNA (see Fig. 1) or a mutant version were used as competitor RNAs in UV cross-linking reactions. The position of the CUG-BP band is indicated.
CUG-BP is required for rapid deadenylation
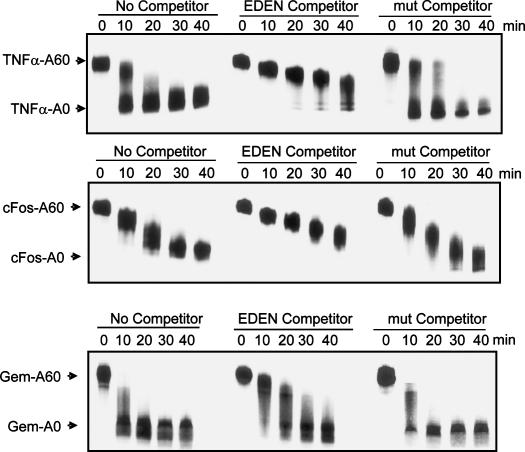
Having ascertained that CUG-BP could bind to the TNFα and c-fos substrate RNAs, we next wished to test whether CUG-BP binding influences the deadenylation of these RNAs. We again used the artificial Gem RNA as a control, as it did not cross-link to CUG-BP, but it still undergoes rapid deadenylation in our assay with a pattern similar to that of the TNFα RNA. Each RNA was transcribed with a 60-base poly(A) tail and incubated in our in vitro assay. As seen in Figure 4, during the time course of the reaction the poly(A) tail is shortened and a deadenylated intermediate accumulates. It is interesting to note that the TNFα and c-fos RNAs deadenylate with different patterns. TNFα exhibits processive deadenylation with completely deadenylated intermediates accumulating while fully adenylated RNA is still present (Fig. 4A). In contrast, the c-fos RNA substrate undergoes a more distributive poly(A) shortening, with the whole population undergoing deadenylation simultaneously. This reflects the patterns of deadenylation seen for these two mRNAs in vivo (Xu et al. 1997).
FIGURE 4.
Addition of excess EDEN RNA results in inhibition of deadenylation. Deadenylation assays were performed for the times indicated in the presence of no competitor (left), 250 fmol of EDEN RNA competitor (center), or 250 fmol of mutant EDEN competitor (right). Deadenylation of TNFα (upper panel), c-fos (middle panel), and Gem (lower panel) RNAs was visualized following separation on a 4% denaturing polyacrylamide gel. The migration positions of fully adenylated input RNA and deadenylated products are indicated on the left.
To determine the role of CUG-BP in the deadenylation of these RNAs, we added excess EDEN RNA oligo to compete off CUG-BP and performed the same deadenylation assay. This resulted in significant retardation of the deadenylation process for both the TNFα and c-fos RNA substrates such that both RNAs deadenylated in a much slower, distributive fashion. In contrast, the EDEN oligo had only a minimal effect on the deadenylation of the Gem control RNA (Fig. 4, lower panel). A mutant EDEN oligo had no effect on the deadenylation rate of any of the three RNA substrates. From this we can conclude that a protein binding to the EDEN sequence, probably CUG-BP, is required for deadenylation in these extracts. The slight effect of the EDEN oligo on the Gem control RNA may be due to sequestration of the PARN deadenylase by the oligo (see below; Figs. 5A, 6).
FIGURE 5.
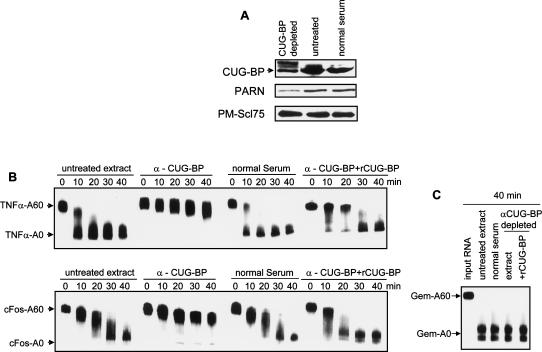
CUG-BP is required for deadenylation of TNFα and c-fos RNAs. (A) Extracts were depleted using anti-CUG-BP antibodies or normal serum, and CUG-BP levels were determined by Western blotting. The extent of depletion was confirmed by comparison with untreated extract (untreated lane). The band above the CUG-BP band is from immunoglobulin. The same extracts were assayed for levels of PARN before and after CUG-BP depletion by Western blotting using anti-PARN antibodies (middle panel). Blots were reprobed with anti-PMScl75 antibodies as a loading control (lower panel). (B) Extracts were either untreated, depleted for CUG-BP (α-CUG-BP), or mock-depleted (normal serum) and used in deadenylation assays over the time course indicated. Where indicated, 30 ng of recombinant CUG-BP (α-CUG-BP + rCUG-BP) was added back to the extract prior to the assay. Deadenylation for TNFα (upper panel) and c-fos (lower panel) RNAs was assessed over the time course indicated. The migration positions of the fully adenylated and deadenylated RNAs are shown. (C) Extracts were treated as in B and deadenylation of the Gem-A60 control RNA was assessed following incubation in the extracts for 40 min. The migration positions of the fully adenylated and deadenylated RNAs are shown.
FIGURE 6.
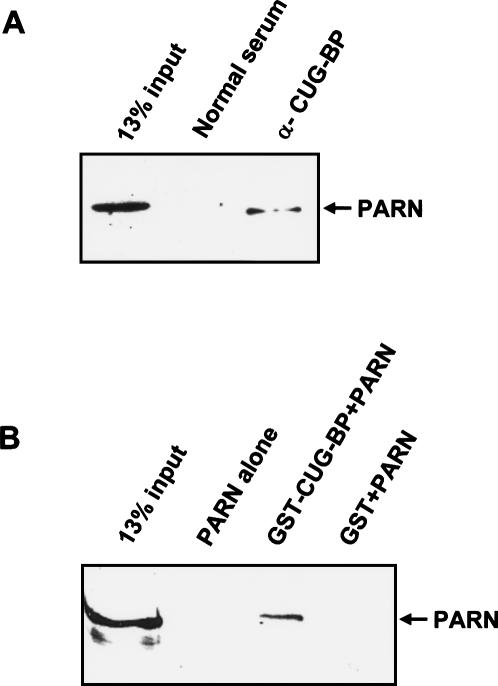
(A) CUG-BP and PARN coimmunoprecipitate. CUG-BP was immunoprecipitated from HeLa extracts with anti-CUG-BP antibodies and the immunoprecipitate was separated on a 10% gel containing SDS and tested for the presence of PARN by Western blotting. A mock immunoprecipitation was performed with normal serum as a control. (B) CUG-BP and PARN interact directly. Recombinant 6HisPARN was incubated in the presence of RNAse A with glutathione Sepharose beads (PARN alone) or with similar beads that had been prebound with GST-CUG-BP (GST-CUG-BP + PARN) or GST (GST + PARN). After extensive washing, the bound proteins were separated by SDS-PAGE and PARN was detected by Western blot.
In order to confirm that the effect of the EDEN oligo was due to sequestration of CUG-BP, we used antibodies to CUG-BP to deplete ∼70% of the protein from the extracts prior to performing the deadenylation assay (Fig. 5A). Immunodepletion of CUG-BP had an identical effect to addition of the EDEN oligo—deadenylation of both c-fos and TNFα RNAs was drastically slowed (Fig. 5B), but deadenylation of the Gem control RNA was unaffected (Fig. 5C). Mock depletion with normal serum had no effect. Importantly, we were able to reactivate deadenylation of the TNFα and c-fos RNAs by adding recombinant CUG-BP to the immunodepleted extracts (Fig. 5B). Taken together, these results suggest that CUG-BP is required to recruit the deadenylase to these RNA substrates.
CUG-BP interacts with the PARN deadenylase
The data described above show that CUG-BP enhances deadenylation of the RNA substrates that it binds to. One way that CUG-BP could achieve this is by directly interacting with the deadenylase and recruiting it to the RNA. In HeLa cytoplasmic extracts the major detectable deadenylase activity is PARN (Gao et al. 2000). We therefore surmised that CUG-BP and PARN may form a complex. This hypothesis is supported by the observation that PARN is codepleted from the extracts during CUG-BP immunodepletion (Fig. 5A, middle panel). We confirmed this interaction in the extracts by immunoprecipitating CUG-BP and looking for PARN in the immunoprecipitate by Western blotting. As seen in Figure 6A, PARN is clearly detectable in the anti-CUG-BP immunoprecipitate but not in that from normal serum. Therefore, PARN and CUG-BP do indeed form a complex in extracts. Moreover, this interaction must be mediated by proteins and not through interaction of both factors with the same RNA substrate as the extracts were treated with RNAse A prior to the immunoprecipitation.
The interaction between CUG-BP and PARN could be direct or mediated by other proteins. In order to distinguish these possibilities, we used recombinant GST-CUG-BP and 6His-PARN in a GST pull-down assay. 6His-PARN was incubated with glutathione Sepharose beads or with similar beads that had been prebound to GST or GST-CUG-BP. The PARN protein bound to the beads was then detected by Western blotting. As seen in Figure 6B, PARN bound only to the GST-CUG-BP and exhibited no nonspecific affinity for GST or the glutathione beads. As this experiment was performed in the presence of RNAse A, this result shows that the PARN and CUG-BP proteins interact directly.
DISCUSSION
Our results show that PARN can be recruited to an RNA substrate directly through interaction with CUG-BP bound to that RNA. Although other proteins have been suggested to recruit deadenylases to ARE-containing RNAs, namely tristetraprolin (TTP) (Lai et al. 2003; Lykke-Andersen and Wagner 2005) and KSRP (Gherzi et al. 2004), this is the first time that an interaction between an RNA binding protein and a deadenylase has been shown to affect deadenylation rates.
CUG-BP appears to bind to our two RNA substrates, c-fos and TNFα, through EDEN-like sequences that are scattered throughout the RNAs. However, binding of CUG-BP can be also be inhibited by addition of excess cold ARE-containing RNA, indicating that CUG-BP may also recognize the AREs in our substrate RNAs. It is possible that multiple CUG-BP monomers are binding cooperatively to several binding sites in each of these substrates to facilitate efficient recruitment of the deadenylase. In support of this hypothesis, EDEN-BP, the Xenopus homolog of CUG-BP, is reported to dimerize (Bonnet-Corven et al. 2002).
The effect of CUG-BP appears to be to increase the processivity of PARN as depletion or competition of CUG-BP results in a much slower, more synchronous deadenylation pattern (Fig. 4). This is particularly striking in the case of the TNFα RNA substrate, which usually undergoes highly asynchronous deadenylation with unadenylated RNA accumulating in the first time point while some fully adenylated RNA still remains. In contrast, upon depletion of CUG-BP, the deadenylation pattern changes drastically with the entire population of RNA now undergoing slow, synchronous poly(A) shortening. This change in pattern is not due to codepletion of PARN, as addition of recombinant PARN to the depleted extract has very little effect on the deadenylation pattern (data not shown). CUG-BP must be restored to the extract in order to induce rapid asynchronous poly(A) removal (Fig. 5B). Our results suggest that CUG-BP acts to anchor PARN to the RNA substrate, thus reducing the off-rate of the protein and increasing its processivity.
It is useful to draw some comparisons between the regulation of PARN by CUG-BP and the effects of EDEN-BP on deadenylation in Xenopus embryos. As mentioned above, during oocyte maturation, PARN is responsible for the default deadenylation of mRNAs. Following fertilization, several polyadenylated RNAs including c-mos and Eg2 undergo rapid deadenylation that requires EDEN-BP. Intriguingly, the default pathway, seen prior to fertilization, induces slow, synchronous poly(A) shortening (Legagneux et al. 1995), similar to that seen in our extracts following depletion of CUG-BP. In contrast, EDEN-dependent deadenylation is more rapid and asynchronous (Legagneux et al. 1995), similar to that of TNFα in our untreated extracts. Although the deadenylase responsible for EDEN-dependent deadenylation has not yet been identified, our data suggest that it may well be PARN. There appear to be significant changes in phosphorylation state of EDEN-BP during oocyte maturation and following fertilization (Detivaud et al. 2003). These may well correlate with its ability to interact with PARN and thereby influence deadenylation. There are already indications that CUG-BP phosphorylation state influences its subcellular localization (Roberts et al. 1997). In the future, it will be interesting to determine whether phosphorylation of CUG-BP affects its ability to interact with PARN.
CUG-BP was first identified as a protein able to bind to the CUG repeats found in the 3′UTR of the DMPK gene implicated in type I myotonic dystrophy (Timchenko et al. 1996). Overexpression of CUG-BP has since been shown to mimic several of the phenotypes of this debilitating disease in mice, including effects on muscle-specific splicing (Ho et al. 2005). Our results suggest that another mechanism should also be considered; reduced stability of specific mRNAs due to enhanced deadenylation. The implication of our data is that overexpression of CUG-BP could result in binding to inappropriate target mRNAs inducing their decay. Future experiments will test this hypothesis by assessing decay rates of mRNAs in cells overexpressing CUG-BP.
MATERIALS AND METHODS
Plasmid and DNA template constructions
The 250-nt fragments of c-fos and TNFα 3′UTRs were generated by RT-PCR using standard conditions and cloned between the EcoRI and PstI sites of pGem-4 (Promega). These plasmids were used as templates to generate the ΔARE mutants, which lacked the sequences indicated as ARE in Figure 1. A ligation-PCR approach was utilized to generate templates for RNAs bearing a 60-nt poly(A) tail (Ford et al. 1999).
Substrate RNAs and competitor RNAs
RNAs were transcribed in vitro using SP6 RNA polymerase and purified on denaturing acrylamide gels. Transcripts were capped cotranscriptionally by the addition of 5 mM 7meGpppG to the reaction.
Synthetic RNAs used in competition analyses were prepared by Dharmacon or IDT. The specific ARE competitor contained a 34-nt AU-rich sequence derived from TNFα 3′UTR (Fig. 1). The corresponding AREmut had the sequence 5′-GGAUUAACUAAUUGAUACCGCGUAUACACGCGG-3′. The sequence of the EDEN competitor RNA derived from c-mos is shown in Figure 1. The sequence of the EDENmut oligo was 5′- AGUCUUUUAUAUCUAUCUCUUCUUUUAUCUCUCUCUCUCUCCUCUUCU-3′. The sequence corresponding to c-fos region A is shown in Figure 1, and the mutant RNA had the UGUU sequences changed to AGAA.
Preparation of cell extracts and in vitro assay
HeLa S100 cytoplasmic extracts were prepared as described previously (Dignam et al. 1983; Ford and Wilusz 1999). Fifty (50) fmol of polyadenylated RNAs radiolabeled internally at U-residues were incubated in the extracts (∼40 μg) at 30°C in the presence of exogenous poly(A) as described previously (Ford et al. 1999). RNA competitors were added where indicated, and the reaction products were analyzed on 4% polyacrylamide gels containing 7 M urea.
UV cross-linking/immunoprecipitation/competition assays
UV cross-linking analyses were performed as described using 50 fmol substrate RNA (Wilusz and Shenk 1988). Cross-linking assays were done in the presence of 25 mM EDTA to inhibit-RNA turnover and allow for accurate comparisons between samples. After digestion with RNase A, cross-linked proteins were analyzed on 10% SDS acrylamide gels.
For immunoprecipitation analysis following UV cross-linking, 400 μL of Net2 buffer (50 mM Tris at pH 7.6, 75 mM NaCl, 0.05% NP-40) was added to samples, and they were incubated at 4°C with anti-CUG-BP antibodies for 3 h. Antigen–antibody complexes were collected on protein A-positive Staphylococcus aureus cells and washed five times with Net2 buffer. The immunoprecipitated cross-linked proteins were separated on a 10% SDS acrylamide gel.
The UV cross-linking/competition analyses were done as described above using the indicated amounts of specific or nonspecific cold competitor RNAs.
Immunodepletion and coimmunoprecipitation
HeLa cytoplasmic extracts were immunodepleted with either control rabbit serum or an anti-CUG-BP monoclonal antibody (Upstate Biotechnology). Briefly, immunodepletions were performed using 3 μL of antisera and 20 U of RNAsin. Immune complexes were precipitated using Protein A Sepharose, and depleted extracts were used in the in vitro mRNA decay system described above. In order to check the efficiency of the immunodepletion, a fraction of the immunodepleted extract was loaded on a 10% SDS polyacrylamide gel and subjected to Western blotting using, in order, rabbit anti-PARN antiserum (1:2000; supplied by M. Wormington), anti-CUG-BP (1:5000; Upstate Biotechnology), and anti-PMSCL 75 (1:10,000; Mukherjee et al. 2002).
For the coimmunoprecipitation of PARN with CUG-BP, 60 μL of HeLa cytoplasmic extract was first precleared by the incubation with 20 μL of a 50% slurry of Protein A Sepharose for 30 min at 4°C. The precleared extract was then incubated with α-CUG-BP α-Normal serum in 1 mL of buffer B (300 mM HEPES at pH 7.9/1.5 mM MgCl2/10 mM KCl) containing 0.5 mM DTT for 2 h at 4°C. The incubation was performed in the presence of 20 μg of RNase A. After this first incubation, 18 μL of a 50% slurry of Protein A Sepharose was added; the mixture was incubated for another hour at 4°C. The beads were then washed three times with NET 2 buffer (50 mM Tris at pH 7.6, 75 mM NaCl, 0.05% Nonidet P-40) for 5 min at 4°C, and bound proteins were eluted by addition of 2× SDS gel loading buffer and boiling for 5 min. Following centrifugation, the supernatant was collected and proteins separated on a 10% SDS polyacrylamide gel. PARN was detected by Western blotting using rabbit anti-PARN antiserum as described above.
Recombinant proteins and GST pull-down assay
CUG-BP was amplified by RT-PCR and cloned into pGEX-2T. GST-tagged CUG-BP was produced in BL21(DE3) cells and purified on glutathione Sepharose 4B. 6His-tagged PARN was expressed and purified as described previously (Gao et al. 2000).
For the GST pull-down assay, excess GST or GST-CUG-BP fusion protein were bound to 10 μL of a 50% slurry of glutathione Sepharose 4B beads in 600 μL of PBS for 2 h at 4°C. After removal of unbound GST proteins by washing with PBS, the beads were incubated with 200 ng of recombinant 6His-PARN in 600 μL of PBS buffer containing 0.2% Triton X-100 at 4°C for 1 h. This incubation was performed in the presence of 10 μg of RNAse A. The beads were then washed three times with PBS containing 0.2% Triton X-100 and eluted by addition of 2× SDS gel loading buffer and boiling for 5 min. Following centrifugation, the supernatant was collected and proteins separated on a 10% SDS polyacrylamide gel. PARN was detected by Western blotting using rabbit anti-PARN antiserum (a kind gift from M. Wormington).
ACKNOWLEDGMENTS
This research was supported by an award to J.W. from the NIH (GM063832). We thank Maurice Swanson and Michael Wormington for supplying antibodies to CUG-BP and PARN, respectively.
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.59606.
REFERENCES
- Audic Y., Omilli F., Osborne H.B. Embryo deadenylation element-dependent deadenylation is enhanced by a cis element containing AUU repeats. Mol. Cell. Biol. 1998;18:6879–6884. doi: 10.1128/mcb.18.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet-Corven S., Audic Y., Omilli F., Osborne H.B. An analysis of the sequence requirements of EDEN-BP for specific RNA binding. Nucleic Acids Res. 2002;30:4667–4674. doi: 10.1093/nar/gkf586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detivaud L., Pascreau G., Karaiskou A., Osborne H.B., Kubiak J.Z. Regulation of EDEN-dependent deadenylation of Aurora A/Eg2-derived mRNA via phosphorylation and dephosphorylation in Xenopus laevis egg extracts. J. Cell Sci. 2003;116:2697–2705. doi: 10.1242/jcs.00477. [DOI] [PubMed] [Google Scholar]
- Dignam J.D., Lebovitz R.M., Roeder R.G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustino N.A., Cooper T.A. Identification of putative new splicing targets for ETR-3 using sequences identified by systematic evolution of ligands by exponential enrichment. Mol. Cell. Biol. 2005;25:879–887. doi: 10.1128/MCB.25.3.879-887.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L.P., Wilusz J. An in vitro system using HeLa cytoplasmic extracts that reproduces regulated mRNA stability. Methods. 1999;17:21–27. doi: 10.1006/meth.1998.0703. [DOI] [PubMed] [Google Scholar]
- Ford L.P., Watson J., Keene J.D., Wilusz J. ELAV proteins stabilize deadenylated intermediates in a novel in vitro mRNA deadenylation/degradation system. Genes & Dev. 1999;13:188–201. doi: 10.1101/gad.13.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M., Fritz D.T., Ford L.P., Wilusz J. Interaction between a poly(A)-specific ribonuclease and the 5′ cap influences mRNA deadenylation rates in vitro. Mol. Cell. 2000;5:479–488. doi: 10.1016/s1097-2765(00)80442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherzi R., Lee K.Y., Briata P., Wegmuller D., Moroni C., Karin M., Chen C.Y. A KH domain RNA binding protein, KSRP, promotes ARE-directed mRNA turnover by recruiting the degradation machinery. Mol. Cell. 2004;14:571–583. doi: 10.1016/j.molcel.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Ho T.H., Bundman D., Armstrong D.L., Cooper T.A. Transgenic mice expressing CUG-BP1 reproduce splicing mis-regulation observed in myotonic dystrophy. Hum. Mol. Genet. 2005;14:1539–1547. doi: 10.1093/hmg/ddi162. [DOI] [PubMed] [Google Scholar]
- Kahvejian A., Svitkin Y.V., Sukarieh R., M'Boutchou M.N., Sonenberg N. Mammalian poly(A)-binding protein is a eukaryotic translation initiation factor, which acts via multiple mechanisms. Genes & Dev. 2005;19:104–113. doi: 10.1101/gad.1262905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner C.G., Wormington M., Muckenthaler M., Schneider S., Dehlin E., Wahle E. The deadenylating nuclease (DAN) is involved in poly(A) tail removal during the meiotic maturation of Xenopus oocytes. EMBO J. 1998;17:5427–5437. doi: 10.1093/emboj/17.18.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai W.S., Kennington E.A., Blackshear P.J. Tristetraprolin and its family members can promote the cell-free deadenylation of AU-rich element-containing mRNAs by poly(A) ribonuclease. Mol. Cell. Biol. 2003;23:3798–3812. doi: 10.1128/MCB.23.11.3798-3812.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legagneux V., Omilli F., Osborne H.B. Substrate-specific regulation of RNA deadenylation in Xenopus embryo and activated egg extracts. RNA. 1995;1:1001–1008. [PMC free article] [PubMed] [Google Scholar]
- Lu X., Timchenko N.A., Timchenko L.T. Cardiac elav-type RNA-binding protein (ETR-3) binds to RNA CUG repeats expanded in myotonic dystrophy. Hum. Mol. Genet. 1999;8:53–60. doi: 10.1093/hmg/8.1.53. [DOI] [PubMed] [Google Scholar]
- Lykke-Andersen J., Wagner E. Recruitment and activation of mRNA decay enzymes by two ARE-mediated decay activation domains in the proteins TTP and BRF-1. Genes & Dev. 2005;19:351–361. doi: 10.1101/gad.1282305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee D., Gao M., O'Connor J.P., Raijmakers R., Pruijn G., Lutz C.S., Wilusz J. The mammalian exosome mediates the efficient degradation of mRNAs that contain AU-rich elements. EMBO J. 2002;21:165–174. doi: 10.1093/emboj/21.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay D., Houchen C.W., Kennedy S., Dieckgraefe B.K., Anant S. Coupled mRNA stabilization and translational silencing of cyclooxygenase-2 by a novel RNA binding protein, CUGBP2. Mol. Cell. 2003;11:113–126. doi: 10.1016/s1097-2765(03)00012-1. [DOI] [PubMed] [Google Scholar]
- Paillard L., Legagneux V., Osborne H.B. Poly(A) metabolism in Xenopus laevis embryos: Substrate-specific and default poly(A) nuclease activities are mediated by two distinct complexes. Biochimie. 1996;78:399–407. doi: 10.1016/0300-9084(96)84746-8. [DOI] [PubMed] [Google Scholar]
- Paillard L., Omilli F., Legagneux V., Bassez T., Maniey D., Osborne H.B. EDEN and EDEN-BP, a cis element and an associated factor that mediate sequence-specific mRNA deadenylation in Xenopus embryos. EMBO J. 1998;17:278–287. doi: 10.1093/emboj/17.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillard L., Legagneux V., Maniey D., Osborne H.B. c-Jun ARE targets mRNA deadenylation by an EDEN-BP (embryo deadenylation element-binding protein)-dependent pathway. J. Biol.Chem. 2002;277:3232–3235. doi: 10.1074/jbc.M109362200. [DOI] [PubMed] [Google Scholar]
- Paillard L., Legagneux V., Beverley O.H. A functional deadenylation assay identifies human CUG-BP as a deadenylation factor. Biol. Cell. 2003;95:107–113. doi: 10.1016/s0248-4900(03)00010-8. [DOI] [PubMed] [Google Scholar]
- Philips A.V., Timchenko L.T., Cooper T.A. Disruption of splicing regulated by a CUG-binding protein in myotonic dystrophy. Science. 1998;280:737–741. doi: 10.1126/science.280.5364.737. [DOI] [PubMed] [Google Scholar]
- Richter J.D. Cytoplasmic polyadenylation in development and beyond. Microbiol. Mol. Biol. Rev. 1999;63:446–456. doi: 10.1128/mmbr.63.2.446-456.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R., Timchenko N.A., Miller J.W., Reddy S., Caskey C.T., Swanson M.S., Timchenko L.T. Altered phosphorylation and intracellular distribution of a (CUG)n triplet repeat RNA-binding protein in patients with myotonic dystrophy and in myotonin protein kinase knockout mice. Proc. Natl. Acad. Sci. 1997;94:13221–13226. doi: 10.1073/pnas.94.24.13221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Sasagawa N., Suzuki K., Ishiura S. The CUG-binding protein binds specifically to UG dinucleotide repeats in a yeast three-hybrid system. Biochem. Biophys. Res. Commun. 2000;277:518–523. doi: 10.1006/bbrc.2000.3694. [DOI] [PubMed] [Google Scholar]
- Timchenko L.T., Miller J.W., Timchenko N.A., DeVore D.R., Datar K.V., Lin L., Roberts R., Caskey C.T., Swanson M.S. Identification of a (CUG)n triplet repeat RNA-binding protein and its expression in myotonic dystrophy. Nucleic Acids Res. 1996;24:4407–4414. doi: 10.1093/nar/24.22.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timchenko N.A., Wang G.L., Timchenko L.T. RNA CUG-binding protein 1 increases translation of 20-kDa isoform of CCAAT/enhancer-binding protein β by interacting with the α and β subunits of eukaryotic initiation translation factor 2. J. Biol. Chem. 2005;280:20549–20557. doi: 10.1074/jbc.M409563200. [DOI] [PubMed] [Google Scholar]
- Voeltz G.K., Steitz J.A. AUUUA sequences direct mRNA deadenylation uncoupled from decay during Xenopus early development. Mol. Cell. Biol. 1998;18:7537–7545. doi: 10.1128/mcb.18.12.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilusz J., Shenk T. A 64 kd nuclear protein binds to RNA segments that include the AAUAAA polyadenylation motif. Cell. 1988;52:221–228. doi: 10.1016/0092-8674(88)90510-7. [DOI] [PubMed] [Google Scholar]
- Wilusz C.J., Wilusz J. Bringing the role of mRNA decay in the control of gene expression into focus. Trends Genet. 2004;20:491–497. doi: 10.1016/j.tig.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Xu N., Chen C.Y., Shyu A.B. Modulation of the fate of cytoplasmic mRNA by AU-rich elements: Key sequence features controlling mRNA deadenylation and decay. Mol. Cell. Biol. 1997;17:4611–4621. doi: 10.1128/mcb.17.8.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]