Abstract
Regional cerebral blood flow (BF) was examined in the human medial prefrontal cortex (MPFC) with positron emission tomography during anticipatory anxiety. Transient anxiety was induced in normal subjects by having them anticipate a painful shock to the fingers of one hand. BF was decreased during anticipatory anxiety, relative to an eyes-closed resting condition, in two regions of the MPFC (Brodmann Areas 10/32 and 24/25). BF decreases in these areas were inversely correlated with anxiety self rating, such that the least anxious subjects exhibited the largest BF reductions, whereas the most anxious subjects showed no significant BF reduction or a slight increase. BF changes in MPFC and in the midbrain were correlated with each other and with anxiety self rating. These results are consistent with the hypothesis that BF reductions in MPFC, previously observed in cognitive tasks, reflect a dynamic balance between focused attention and subject anxiety and may occur from a functionally active baseline or default state. The characterization of such relationships within the human brain enables new insights into the integration of cognition and emotion.
Previous functional brain imaging studies in humans, with positron emission tomography (PET) and functional MRI (fMRI), have demonstrated consistent reductions in medial prefrontal cortex (MPFC) blood flow (BF) during the performance of a wide range of cognitive tasks (e.g., see refs. 1–3), which may, in part, represent an attenuation or inactivation of elements of a functionally active, baseline, or default state of brain activity (4). Because of the large body of data implicating these areas, especially those within ventral MPFC, and their connections in emotional processing within the brain (e.g., see refs. 5–9), it has been hypothesized that the observed changes reflect a dynamic interplay between ongoing cognitive processes and the emotional state of the subject (10).
In pursuit of this hypothesis, we examined in an accompanying paper (11) the relationship between attention-demanding cognitive performance and activity in MPFC in the task of generating aloud verbs for visually presented nouns (12). As subjects initially performed the task, the expected decreases in BF in MPFC were observed with PET but, surprisingly, were no greater than those seen with the much less demanding task of word reading. Practice of the task significantly improved performance as we have previously shown (12). Somewhat more surprisingly, the practiced–induced performance improvement, as measured by voice-onset latency, was associated with further BF reductions in MPFC. The greater the improvement in performance, the greater the regional reductions in BF in selected regions of ventral MPFC. Furthermore, these reductions were accompanied by changes in hypothalamic BF (11).
These imaging results suggested a possible relationship between performance-induced anxiety (high initially but reduced with practice) and the BF changes we observed. To test this hypothesis, a parallel behavioral study of this cognitive task, without imaging, was performed, in which physiological as well as self-report measures of anxiety were obtained (11). Consistent with our hypothesis, anxiety was significantly increased during the initial performance of the task but decreased to baseline levels with practice as performance improved. Anxiety returned with the introduction of a novel word list.
The above data provided strong inferential evidence that changes observed in MPFC reflected a dynamic interplay between focused attention, elicited in this instance by a demanding cognitive task, and performance anxiety. Lacking were measurements of MPFC BF changes during more controlled manipulations of emotion, in this case anxiety. Therefore, the goal of the current experiment was to examine BF in the MPFC in normal subjects experiencing anxiety in the absence of having to perform an explicit cognitive task.
A preliminary report of this work has been presented in abstract form (13).
Materials and Methods
Subjects.
Sixteen normal volunteers were recruited from the local population of students and staff at Washington University and paid for their participation. They were divided into two groups, according to which hand-stimulating electrodes were placed (see below). There were 8 subjects in the right-hand group (2 females; mean age 22 ± 2, range 19–25) and 8 subjects in the left-hand group (2 females; mean age 25 ± 5, range 20–35). All subjects were strongly right handed, as measured by the Edinburgh handedness inventory (14), and had no significant neurological history. Informed consent was obtained before participation following guidelines approved by the Human Studies Committee and the Radioactive Drug Research Committee of Washington University.
Experimental Paradigm.
Subjects wore stimulator coils connected to a stimulator (model S8, Grass Instruments, West Warwick, RI) on the finger pads of the index and middle fingers. Subject preparation included delivery of one threshold and one suprathreshold electrical stimulus at least 25 min before scanning. Subjects were informed that this identified the voltage necessary to elicit pain. Stimulus voltage was in the range of 1–10 volts.
In the first scan, no stimulus was expected or received, and the subject rested with eyes closed. In the second scan, subjects were instructed that during the scan they would be shocked at approximately 10 times the intensity of the threshold stimulus, and that the longer the delay from the onset of the scan, the more intense the shock would be. Subjects were told that this level of electrical stimulus would be sufficient to cause pain but would not cause burning or other injury. No stimulus was in fact delivered during the scan. Rather, a stimulus of moderate intensity was delivered immediately after scan data acquisition. In the third scan, subjects were instructed that no stimulus would be delivered and were scanned again in the resting state.
PET Scanning Procedures.
Scans were performed by using a Siemens CTI 953B/31 PET camera in the two-dimensional mode with septa inserted. Brain BF was measured by using H215O (15, 16) and PET scanning methodology developed at Washington University (17–20).
Behavioral Measures.
Immediately after each of the three scans, subjects rated their anxiety level during the scan by using a 10-point visual analog scale (VAS) and the state portion of the Spielberger State–Trait Anxiety Inventory (STAI) (21). Heart rate was monitored throughout the experiment by using a portable electrocardiogram device (Lifepak 7, Physio-Control, Redmond, WA).
Several months after completion of the PET study, an attempt was made to contact all 16 participants in the study for the purpose of administering a personality questionnaire, the Temperament and Character Inventory [TCI (22, 23)]. We wished to determine whether any group differences existed as a function of the hand on which the stimulating electrodes had been placed. Twelve subjects were located, and they successfully completed the TCI. These included five of the eight who had had electrodes on the right hand (one female) and seven of the eight who had had electrodes on the left hand (two females).
Data Analysis.
Peak changes in MPFC BF were identified with an automated search algorithm (20) in the averaged subtraction image. Spherical regions of interest (ROI) were defined around those peaks nearest the coordinates of MPFC BF decreases listed in Table 1 of the metaanalysis reported by Shulman et al. [(1); focus 10 or Brodmann Area (BA) 8/9 and focus 12 or BA 32, which includes some of BA 25 as well]. The ROIs were used to quantify BF in the individual activation (anxiety) minus control (resting) scan pairs for each subject. One-sample t tests were used to determine the significance of BF changes.
Table 1.
BF decreases in MPFC
| Region | Brodmann's Area | x | y | z | P value |
|---|---|---|---|---|---|
| A. Ant. MPFC | 10/32 | 1 | 41 | −8 | <0.01 |
| B. SGPFC | 24/25 | −1 | 17 | −8 | <0.0001 |
Centers of mass are in the coordinates of Talairach and Tournoux (22). Ant., anterior; SGPFC, subgenual prefrontal cortex.
Pearson correlations were performed on the regional BF changes (i.e., value for anxious scan minus value for resting scan) for each subject with the behavioral and physiological measurements described above. Fisher's r to z test (statview, Abacus Concepts, Berkeley, CA) was used to assess the significance of Pearson r values.
To determine whether other brain areas were correlated with subjective anxiety, a covariance image was generated by using the VAS rating during the anxiety scan for each subject as the dependent variable. The formula for covariance,
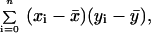 |
where xi is the VAS rating for subject i, yi is the value at a given voxel for that subject, and n is the number of subjects, was applied to generate this image. Regions of interest were defined on candidate regions of correlation in the image. Pearson correlations were then performed to assess significance.
Results
Self Report and Heart Rate.
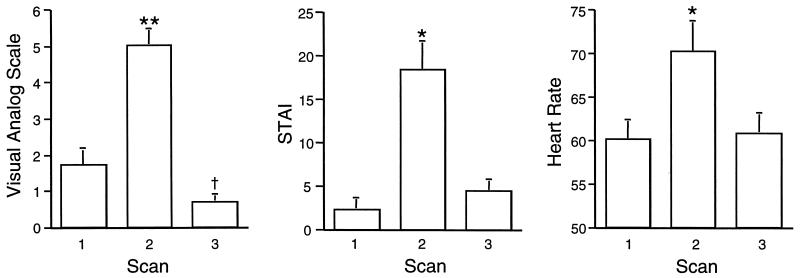
VAS, STAI, and heart rate measurements for the three scans are shown in Fig. 1. Repeated measures ANOVA (Super ANOVA, Abacus) revealed a significant effect of scan condition for VAS [F (3,15) = 56.6, P = 0.0001), STAI (F = 27.4, P = 0.0001)], and heart rate (F = 21.8, P = 0.0001) measurements. Post hoc comparisons (paired one-tailed t tests) indicated that VAS in the anxiety condition was significantly higher than in the first or second resting condition (P < 0.000001). This was also true for STAI (P < 0.0001) and heart rate (P < 0.0001). Post hoc comparisons of the two resting scans (paired two-tailed t test) indicated that VAS scores for the first resting scan (scan one) were significantly higher than those for the second resting scan (scan three) (P < 0.05). STAI scores and heart rate were not significantly different for scans one and three. Because VAS was lower for the second resting scan, this scan was used for image subtraction to avoid any potential confounding because of anxiety in the initial resting scan. Heart rate was significantly correlated with VAS rating (r = 0.57, P = 0.02). The STAI score was not significantly correlated with heart rate (r = 0.40, P > 0.1) or VAS (r = 0.41 P > 0.1).
Figure 1.
Behavioral measurements (mean + SEM) obtained from 16 normal subjects during PET BF scans obtained before (Scan 1), during (Scan 2), and after (Scan 3) the anticipation of a painful shock to the hand. STAI refers to the STAI (21). During Scan 3 (anticipatory anxiety), all three measurements differed significantly (**, P < 0.000001; *, P < 0.0001) from baseline measurements (Scans 1 and 3). For the VAS, Scan 3 was significantly lower than Scan 1 (†, P < 0.05).
The VAS scores were significantly higher for the subjects wearing electrodes on the fingers of the right hand (i.e., they were more anxious) than those wearing electrodes on the fingers of the left hand (6.00 ± 1.5 and 4.06 ± 1.7, respectively; two-tailed unpaired t test; P = 0.03).
PET.
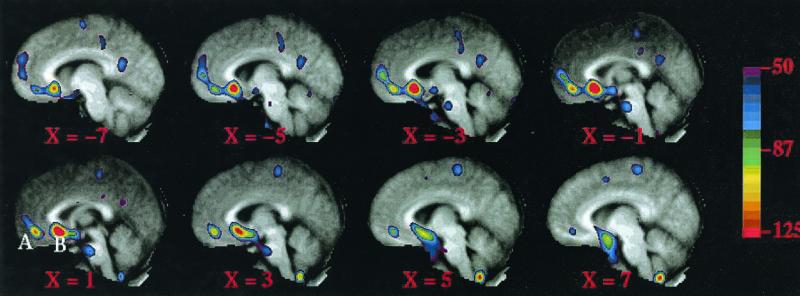
Overall for the group of 16 subjects, BF was not increased in the MPFC in the anxiety-minus-rest comparison. However, there were two prominent BF decreases (Fig. 2, Table 1). In the posterior region, which corresponds to the subgenual prefrontal cortex (SGPFC), 14 of 16 subjects exhibited a decrease, whereas in the anterior MPFC region, 12 of 16 subjects exhibited a decrease. In the tables and figures, the anterior MPFC region is referred to as region A and the SGPFC region as region B.
Figure 2.
An averaged PET subtraction image overlaid on an averaged anatomical MRI image showing significant decreases in two MPFC regions, A and B. Left, anterior. x coordinates indicate distance from the midline in millimeters.
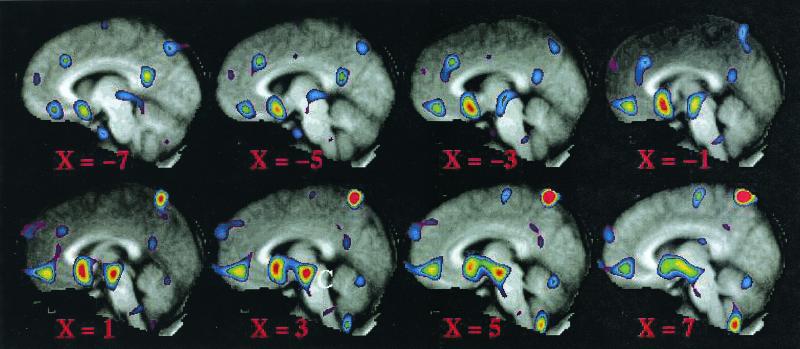
In the covariance image, in which we used the VAS rating for each subject as the dependent variable, we identified an additional region in or near the hypothalamus or midbrain (Fig. 3, region C) in addition to regions A and B (compare Figs. 2 and 3). A region of interest was defined for this hypothalamic/midbrain region (center of mass 3, −19, −20) and was applied to the original anxiety minus rest scans. We then assessed the correlations between changes in PET-measured activity induced by anticipatory anxiety in all three regions and our behavioral measurements.
Figure 3.
A covariance image indicating regions that varied with subject VAS ratings. The midbrain region referred to in the text is labeled C on slice x = 3. Regions A and B, seen in the averaged subtraction image (Fig. 1) are also seen in this covariance image.
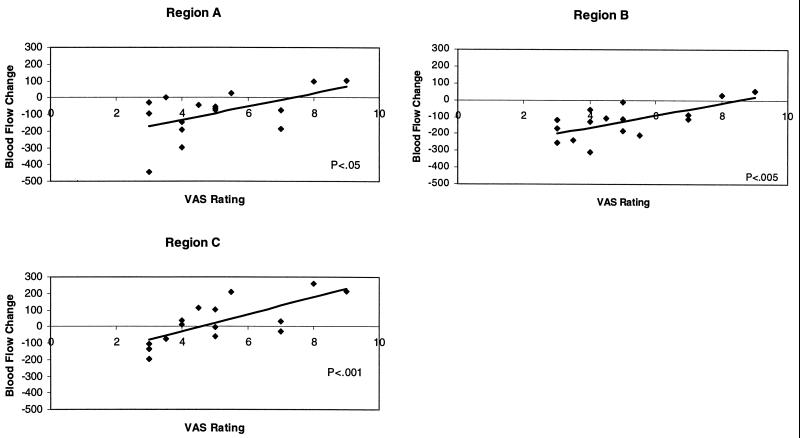
The anxiety-induced BF changes in MPFC regions A and B showed significant inverse correlations with the VAS rating during the anxiety scan (Fig. 4). Thus, subjects with the highest reported anxiety exhibited the least change from the resting baseline state, whereas those reporting the least anxiety exhibited the greatest decrease from the resting baseline. Heart rate was significantly correlated with BF change only in region A (r = 0.51; P < 0.05). There were no significant correlations with STAI.
Figure 4.
Scatter plots of VAS used for the assessment of anxiety during the anticipation of an electric shock on the hand vs. BF change in the regions identified in the averaged subtraction (regions A and B, Fig. 2.) and covariance (region C, Fig. 3) images. The Blood Flow Change scale is a linear scale of normalized radioactive count differences.
The reduction in BF in region B was greater in the eight subjects wearing the electrodes on the fingers of the left hand than in the eight subjects who had the electrodes placed on the fingers of the right hand (two-tailed unpaired t test; P = 0.059). This is consistent with the observation that the subjects with the electrodes on the fingers of the right hand were significantly more anxious, as measured by VAS, than the subjects with the electrodes on the fingers of the left hand (see above). Despite these differences in behavior and scan results, no significant differences were noted between the two groups along any of the seven trait dimensions measured by the Temperament and Character Inventory (22, 23) personality questionnaire (two-tailed unpaired t test; P = 0.575 to 0.996). We note, however, that our sample size was small, and data were missing on three subjects (see Materials and Methods).
Although BF change in region C (midbrain/hypothalamus; Fig. 3) exhibited the same significant (r = 0.73; P < 0.001) inverse correlation with the VAS as did regions A and B (Fig. 4), the changes appeared about equally distributed above and below the baseline.
Discussion
In our accompanying paper (11), we provided evidence supporting the hypothesis that the degree to which reductions in activity occur in regions of the MPFC while performing a cognitive task reflects a combined effect of the attentional demands of the task, causing reductions and accompanying performance anxiety that attenuate those reductions. Reductions were greatest when attentional demands were high and performance anxiety was minimal. Reductions were least when attentional demands and performance anxiety were both, either high or low.
In the present experiment, we eliminated an explicit attention-demanding cognitive task and deliberately introduced a significant degree of anxiety. The “task” became solely one of anticipating a painful shock to the fingers of one hand. We hoped thereby to obtain a more complete understanding of the relationship between anxiety itself and BF changes in MPFC. One might have anticipated that, whereas an attention-demanding cognitive task alone would depress activity in MPFC, anxiety alone would be associated with an increase in activity. Such a prediction would receive some support from extant studies in the literature (for review, see ref. 10).
Despite the prediction that anxiety alone would be associated with an increase in activity in the MPFC, the changes we observed there during anticipatory anxiety were more complex. In fact, decreases were observed (Fig. 2) and were inversely correlated with the anxiety level experienced by our subjects during the PET scan (Figs. 3 and 4). Relative to a baseline nonanxious resting condition, BF in the ventral MPFC regions was decreased most in those subjects with the lowest levels of anxiety. Subjects experiencing higher levels of anxiety exhibited much smaller decreases (Fig. 4). The two subjects with the highest levels of anxiety actually had slight increases in BF.
BF changes in the midbrain/hypothalamus (region C, Fig. 3) correlated in the same manner as did the regions in MPFC with the level of subject anxiety. However, the midbrain/hypothalamus BF changes were almost equally distributed between decreases and increases from the baseline resting level of activity (Fig. 4). As with the changes in the MPFC regions, the decreases below baseline were associated with lower levels of anxiety, whereas the increases above baseline were associated with higher levels of anxiety. Because these changes were almost equally distributed above and below the baseline level of activity in region C, the simple difference image comparing anxiety and rest (Fig. 2) does not show a change in this area, whereas the correlation image readily identifies the anxiety-correlated changes.
Correlated changes of this sort we have observed, of course, do not establish a causal relationship between the level of anxiety experienced by our subjects and the BF changes we observe in MPFC and the hypothalamus/brain stem. Other factors not immediately apparent to us might also be playing a role. However, the results presented in our accompanying paper (11) provide support for a causal relationship. There we varied anxiety within each subject producing deviations in MPFC BF directionally consistent with the results of the present experiment.
The cortical decreases we observed occurred in two ventral regions of the MPFC. The more posterior region (region B) lies at approximately the junction of areas 24a, 25, and 32m in what is more generally referred to as the subgenual prefrontal cortex. The more anterior region (region A) lies approximately in area 10r. The cytoarchitectonics of the ventral MPFC within which these areas lie, along with the orbital prefrontal cortex, have been mapped in detail in nonhuman primates (24–26). Recently these maps have been extended to the human cerebral cortex (27). The organization of the ventral medial and orbital prefrontal cortices reflected in these maps is relevant to an interpretation of our results.
In general, areas on the orbital surface of the prefrontal cortex indirectly receive sensory information from the external environment as well as the internal milieu (28). This sensory information is relayed to the ventral MPFC through a complex set of interconnections. Areas within the ventral MPFC are heavily interconnected with the hypothalamus (29), the amygdala, and the periaquaductal gray matter of the brain stem (6, 30). Such anatomical relationships suggest a role for these medial areas in the integration of the visceral motor aspects of emotion with information gathered from the internal and external environment. These connections would be consistent with the changes we observed in the hypothalamus (11) and midbrain (region C).
A conceptually important feature of our data is the fact that BF in regions of the ventral MPFC shows little difference between a nonanxious resting state (e.g., awake but resting quietly with eyes closed) and significant levels of anxiety. Although BF in these regions is positively correlated with the level of anxiety in anticipation of an aversive stimulus, those experiencing the most intense anxiety exhibit the smallest difference from the nonanxious baseline state in which no apparent threat of an anxiety-provoking event exists. Thus, a correlation with anxiety exists only in an anxiety-provoking state. This observation is of both practical and theoretical importance.
From a practical point of view, these results emphasize the importance of carefully understanding the reference state to which results are to be compared. Comparing anxiety levels among subjects anticipating an aversive stimulus might lead one to hypothesize that activity levels in regions of ventral MPFC directly reflect anxiety levels experienced by normal subjects, rising as levels of anxiety increase. However, such an interpretation is inconsistent with our observations that (i) those most anxious exhibited BF little different from our control state (i.e., eyes closed rest without the threat of an aversive event); and (ii) those least anxious in the face of a threatening aversive event showed the greatest difference (i.e., a decrease) from this control state. Clearly the baseline against which results are compared alters the perspective on which interpretations are based.
From a theoretical perspective, these relationships between anxiety and changes in MPFC BF revealed by our data have challenged us to rethink the functional significance of so-called baseline activity in areas like MPFC where reductions in activity are more commonly seen than increases or so-called “activations” (1). We suggest that our data are consistent with a hypothesis developed in detail in our accompanying paper (4) that within the MPFC as well as elsewhere in the cerebral cortex reside areas whose functionally active state is present as a default option rather than necessarily triggered by transiently occurring events. Further, we would like to suggest that this functionally active default state within regions of the ventral MPFC is necessary for the ongoing detection and evaluation of environmental and internal stimuli of relevance to the motivational state of the individual (4).
If elements of the MPFC are involved in ongoing monitoring of environmental and internal stimuli for their motivational significance, subjective feelings of anxiety may require an active state within elements of ventral MPFC, explaining the small or nonexistent decreases in MPFC BF in the most anxious subjects. Similarly, if cognitive or other coping strategies that reduce or suppress subjective anxiety are used, MPFC BF may be reduced in a manner analogous to the reductions seen in more “purely” cognitive experimental paradigms (1). Thus in the anticipatory anxiety paradigm, an anxiety-provoking thought (i.e., “I will receive a painful shock”) produces subjective anxiety unless the anxiety-provoking features of this thought are minimized by a coping strategy reflected in the changes we observe in the MPFC. We hypothesize that in the presence of such a strategy, ventral MPFC BF is lower, activation of the midbrain and elevation of the heart rate are attenuated, and subjective feelings of anxiety are minimized.
Interestingly, such a formulation parallels the relationship between attention and the subjectively evaluated threat value of a stimulus or situation described in the cognitive–motivational model of anxiety put forth by Mogg and Bradley (31). In this model, a situation that is evaluated as mildly threatening triggers the direction of attentional resources away from the stimulus, i.e., avoidance. As the evaluated threat value of the stimulus increases, attention is directed back toward the stimulus, in preparation for dealing with the threat. We suggest that a similar relationship between attention and threat might be instantiated in the brain, at least in part, within elements of the ventral MPFC.
Our observations have an interesting and potentially informative parallel in the rodent aversive conditioning literature. Lesions in the MPFC of rats have been shown to affect the acquisition and extinction of conditioned fear (32). Of interest is the fact that aversive conditioning can be associated with a significant reduction in unit activity in the prefrontal cortex of the rat (e.g., see ref. 33). Garcia et al. (33) were able to show a highly significant correlation between the freezing behavior of their rats and the suppression of unit activity in the prefrontal cortex. The greatest suppression of neuronal activity was associated with the most freezing behavior. Thomas and Yadin (34) suggested that freezing behavior might represent a mechanism for fear management (i.e., a coping strategy in anticipation of an aversive event). In the rat, better coping would then be equated with a greater tendency to freeze and a greater reduction in prefrontal unit activity. Although vast differences obviously exist between rats and humans, we venture to suggest that our observations would be consistent with such an interpretation.
Combining our observations with those from the rat aversive conditioning literature provides an opportunity to explore how the reductions in activity in the MPFC might be achieved neurobiologically. The connections between the MPFC and the amygdala are well established (6). Stimulation of the amygdala produces inhibition of unit activity in the MPFC of rats (35), yet direct projections from the amygdala to the MPFC are excitatory. Although inhibition could be achieved through the excitation of inhibitory interneurons, this seems an unlikely explanation of imaging findings. If this were the mechanism, functional brain imaging with either PET or fMRI would record an increase in activity rather than a decrease, as inhibitory activity of this type is associated with increased energy consumption equal to that of excitatory activity (2, 36).
Alternatively, the reductions in unit activity observed in rodents and the reductions in activity observed with PET and fMRI might both be mediated by the release of dopamine in the MPFC. Dopamine has been shown to exert an inhibitory effect on neuronal activity in the MPFC (37, 38). A reduction in activity mediated directly by dopamine might more likely result in reduced energy demands of the tissue and hence might be seen by imaging devices like PET and fMRI as a reduction in activity. Furthermore, it has been shown that the amygdala plays an important role in the coordination of dopamine release in response to psychological stress (39). Our data provide little additional insight into the role of the amygdala in such a process. In the present experiment, we observed no change in the amygdala, although a change in one of its component nuclei might well have been missed because of the limited resolution of PET. Future research may benefit from increased communication between aversive conditioning studies in laboratory animals and behavioral imaging work in humans. These two lines of investigation appear to offer very complementary insights.
Finally, we note the unexpected finding that subjects anticipating a shock on the right hand were, as a group, more anxious than those anticipating a shock on the left hand. Simple explanations for this effect of electrode placement seem unlikely. All subjects were strongly right handed. Furthermore, our retrospective assessment of their personalities, although missing a few subjects in both groups, revealed no obvious differences in our limited sample. Finally, such components as age and gender appear equally balanced between the two groups.
As we have previously reported (40), the anticipation of a tactile stimulus to the surface of the body resulted in significant reductions in those areas of the somatosensory cortex outside of those areas where the anticipated stimulus would be processed. Our interpretation of this finding was that anticipation at a specific location led to a filtering or suppression of information from nonattended locations, the implication being enhanced signal detection. Among the experimental groups included in that report (40) were the eight subjects from the present study with electrode placement on the right hand (the eight subjects with electrode placement on the left hand had not been studied at the time of that report). Of additional interest was the fact that the degree of suppression at the nonattended locations was directly related to the level of anxiety experienced by the subjects (i.e., the greater the anxiety, the greater the suppression). No suppression of activity in MPFC was noted. The other two groups included in that report (40) involved the anticipation of nonaversive stimuli to the right and left great toe.
The addition of subjects with electrode placement on the left hand brought to the study, as we have noted, subjects with a significantly lower group average level of anticipatory anxiety. This lower level of anticipatory anxiety was accompanied by significant reductions in activity within ventral MPFC not seen in the original eight subjects with electrode placement on the right hand (40) and no evidence of activity reductions in somatosensory areas outside of those areas where the anticipated stimulus would be processed.
Several questions come to mind. Are there both qualitative and quantitative differences in the neural instantiation of a response to threat that are reflective of the level of anxiety experienced by individual subjects? Do subjects who exhibit minimal anxiety preferentially use elements of the ventral MPFC and their connections to attenuate the visceral motor aspects of anxiety, whereas those who exhibit intense anxiety preferentially use a filtering strategy that focuses attention on the expected stimulus? Are these strategies mutually exclusive? Is the hemisphere in which the expected aversive stimulus is to be processed a factor in the strategy used? Our data are currently insufficient to answer such questions. Only further experimentation, which must include attention to personality differences, will suffice.
Acknowledgments
This work was supported by National Institutes of Health Grants NS06833, DC000093, DA07261, and NS10196, and by the Charles A. Dana Foundation.
Abbreviations
- PET
positron emission tomography
- fMRI
functional MRI
- MPFC
medial prefrontal cortex
- BF
blood flow
- VAS
visual analog scale
- STAI
Spielberger State–Trait Anxiety Inventory
References
- 1.Shulman G L, Fiez J A, Corbetta M, Buckner R L, Miezin F M, Raichle M E, Petersen S E. J Cognit Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 2.Raichle M E. Proc Natl Acad Sci USA. 1998;95:765–772. doi: 10.1073/pnas.95.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hutchinson M, Schiffer W, Joseffer S, Liu A, Schlosser R, Dikshit S, Goldberg E, Brodie J D. Magn Reson Imaging. 1999;17:1427–1436. doi: 10.1016/s0730-725x(99)00093-4. [DOI] [PubMed] [Google Scholar]
- 4.Raichle M E, MacLeod A M, Snyder A Z, Powers W J, Gusnard D A, Shulman G L. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eslinger P J, Damasio A R. Neurology. 1985;35:1731–1741. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- 6.Carmichael S T, Price J L. J Comp Neurol. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- 7.Barbas H. Neurosci Biobehav Rev. 1995;19:499–510. doi: 10.1016/0149-7634(94)00053-4. [DOI] [PubMed] [Google Scholar]
- 8.Shallice T, Burgess P W. Brain. 1991;114:727–741. doi: 10.1093/brain/114.2.727. [DOI] [PubMed] [Google Scholar]
- 9.Rolls E T, Hornak J, Wade D, McGrath J. J Neurol Neurosurg Psychiatry. 1994;57:1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drevets W C, Raichle M E. Cognit Emot. 1998;12:353–385. [Google Scholar]
- 11.Simpson J R, Jr, Snyder A Z, Gusnard D A, Raichle M E. Proc Natl Acad Sci USA. 2001;98:683–687. doi: 10.1073/pnas.98.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raichle M E, Fiez J A, Videen T O, Macleod A K, Pardo J V, Fox P T, Petersen S E. Cereb Cortex. 1994;4:8–26. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- 13.Simpson J R, Jr, MacLeod A K, Fiez J A, Drevets W C, Raichle M E. Soc Neurosci Abstr. 1997;23:1317. [Google Scholar]
- 14.Raczkowski D, Kalat J W, Nebes R. Neuropsychology. 1974;6:43–47. doi: 10.1016/0028-3932(74)90025-6. [DOI] [PubMed] [Google Scholar]
- 15.Herscovitch P, Markham J, Raichle M E. J Nucl Med. 1983;24:782–789. [PubMed] [Google Scholar]
- 16.Raichle M E, Martin W R W, Herscovitch P, Mintun M, Markham J. J Nucl Med. 1983;24:790–798. [PubMed] [Google Scholar]
- 17.Fox P T, Perlmutter J S, Raichle M E. J Comput Assist Tomogr. 1985;9:141–149. doi: 10.1097/00004728-198501000-00025. [DOI] [PubMed] [Google Scholar]
- 18.Fox P T, Mintun M A, Rieman E M, Raichle M E. J Cereb Blood Flow Metab. 1988;8:642–653. doi: 10.1038/jcbfm.1988.111. [DOI] [PubMed] [Google Scholar]
- 19.Fox P T, Mintun M A. J Nucl Med. 1989;30:141–149. [PubMed] [Google Scholar]
- 20.Mintun M A, Fox P T, Raichle M E. J Cereb Blood Flow Metab. 1989;9:96–103. doi: 10.1038/jcbfm.1989.13. [DOI] [PubMed] [Google Scholar]
- 21.Spielberger C D, Gorsuch R L, Lushene R E. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 22.Cloninger C R, Przybeck T R, Svrakic D M, Wetzel R D. Temperament and Character Inventory (TCI): A Guide to Its Development and Use. Washington Univ., St. Louis: Center for Psychobiology of Personality; 1994. [Google Scholar]
- 23.Cloninger C R, Svrakic D M, Prozybeck T R. Arch Gen Psychiatry. 1993;50:975–990. doi: 10.1001/archpsyc.1993.01820240059008. [DOI] [PubMed] [Google Scholar]
- 24.Barbas H. Neuroscience. 1993;56:841–864. doi: 10.1016/0306-4522(93)90132-y. [DOI] [PubMed] [Google Scholar]
- 25.Carmichael S T, Price J L. J Comp Neurol. 1994;346:366–402. doi: 10.1002/cne.903460305. [DOI] [PubMed] [Google Scholar]
- 26.Carmichael S T, Price J L. J Comp Neurol. 1996;371:179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 27.Ongur D, Price J L. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 28.Carmichael S T, Price J L. J Comp Neurol. 1995;363:642–664. doi: 10.1002/cne.903630409. [DOI] [PubMed] [Google Scholar]
- 29.Öngür D, An X, Price J L. J Comp Neurol. 1998;401:480–505. [PubMed] [Google Scholar]
- 30.An X, Bandler R, Öngür D, Price J L. J Comp Neurol. 1998;401:455–479. [PubMed] [Google Scholar]
- 31.Mogg K, Bradley B P. Behav Res Ther. 1998;36:809–848. doi: 10.1016/s0005-7967(98)00063-1. [DOI] [PubMed] [Google Scholar]
- 32.Morgan M A, LeDoux J E. Behav Neurosc. 1995;109:681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- 33.Garcia R, Vouimba R-M, Baudry M, Thompson R F. Nature (London) 1999;402:294–296. doi: 10.1038/46286. [DOI] [PubMed] [Google Scholar]
- 34.Thomas E, Yadin E. Exp Neurol. 1980;69:50–60. doi: 10.1016/0014-4886(80)90142-9. [DOI] [PubMed] [Google Scholar]
- 35.Perez-Jaranay J M, Vives F. Brain Res. 1991;564:97–101. doi: 10.1016/0006-8993(91)91357-7. [DOI] [PubMed] [Google Scholar]
- 36.Ackerman R F, Finch D M, Babb T L, Engel J J. J Neurosci. 1984;4:251–264. doi: 10.1523/JNEUROSCI.04-01-00251.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferron A, Thierry A M, Le Douarin C, Glowinski J. Brain Res. 1984;302:257–265. doi: 10.1016/0006-8993(84)90238-5. [DOI] [PubMed] [Google Scholar]
- 38.Mantz J, Milla C, Glowinski J, Thierry A M. Neuroscience. 1988;27:517–526. doi: 10.1016/0306-4522(88)90285-0. [DOI] [PubMed] [Google Scholar]
- 39.Goldstein L E, Rasmusson A M, Bunney B S, Roth R H. J Neurosci. 1996;16:4787–4798. doi: 10.1523/JNEUROSCI.16-15-04787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drevets W C, Burton H, Videen T O, Snyder A Z, Simpson J R, Jr, Raichle M E. Nature (London) 1995;373:249–252. doi: 10.1038/373249a0. [DOI] [PubMed] [Google Scholar]