Abstract
A reduced sympathoadrenal response, induced by recent antecedent hypoglycemia, is the key feature of hypoglycemia-associated autonomic failure (HAAF) and, thus, the pathogenesis of iatrogenic hypoglycemia in diabetes. Understanding of the mechanism(s) of that reduced response awaits new insight into its basic molecular, cellular, organ, and whole-body physiology and pathophysiology in experimental models. In this issue of the JCI, McCrimmon and colleagues report that application of urocortin I (a corticotrophin-releasing factor receptor–2 agonist) to the ventromedial hypothalamus reduces the glucose counterregulatory response to hypoglycemia in rats (see the related article beginning on page 1723). Thus, hypothalamic urocortin I release during antecedent hypoglycemia is, among other possibilities, a potential mechanism of HAAF.
The clinical problem of iatrogenic hypoglycemia in people with diabetes mellitus (1) has stimulated bidirectional translational research. That included, initially, patient-oriented research based on the then-existing body of knowledge and, more recently, basic research into the molecular, cellular, organ, and whole-body physiology of glucose counterregulation — the mechanisms that normally prevent or rapidly correct hypoglycemia — and its pathophysiology in experimental models (2). The latter is exemplified by the report from McCrimmon and colleagues (3) in this issue of the JCI. In the aggregate, such basic research will undoubtedly lead to more informative studies in humans and, hopefully, the ultimate elimination of hypoglycemia from the lives of people with diabetes.
The clinical problem is clear to millions of people with diabetes and, increasingly, to their caregivers: iatrogenic hypoglycemia is the limiting factor in the glycemic management of diabetes (1, 2). It causes recurrent morbidity in most people with type 1 diabetes mellitus (T1DM) and many with T2DM and is sometimes fatal. The barrier of hypoglycemia precludes maintenance of euglycemia over a lifetime of diabetes and, thus, full realization of the now well-established vascular benefits of glycemic control (4, 5). Even asymptomatic episodes of hypoglycemia impair defenses against subsequent hypoglycemia by causing hypoglycemia-associated autonomic, specifically sympathoadrenal, failure and thus a vicious cycle of recurrent hypoglycemia.
Compromised defenses against hypoglycemia
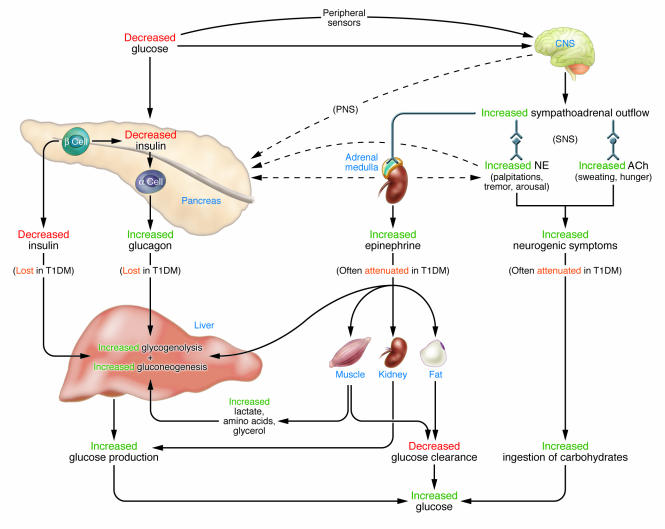
The physiological defenses against falling plasma glucose concentrations (Figure 1) include: (a) decreased pancreatic islet β cell insulin secretion; (b) increased pancreatic islet α cell glucagon secretion; and, absent the latter, (c) increased adrenomedullary epinephrine secretion (6). The behavioral form of defense (Figure 1) is the ingestion of food prompted by symptoms of hypoglycemia (6). All of these defenses are compromised (1, 2) in T1DM (7) and advanced T2DM (8). This involves both peripheral (pancreatic islet) and CNS alterations. Both decrements in insulin and increments in glucagon are lost. The former is the result of β cell failure. The latter is plausibly attributed to loss of the decrement in intra-islet insulin that normally signals increased glucagon secretion (9–11). In the setting of absent insulin and glucagon responses, attenuated epinephrine responses cause the clinical syndrome of defective glucose counterregulation that is associated with a 25-fold or greater increased risk of severe hypoglycemia (1, 2). Attenuated sympathoadrenal, largely sympathetic neural (12), responses also cause the clinical syndrome of hypoglycemia unawareness that, by compromising the behavioral defense, is also associated with an increased risk of severe hypoglycemia (1, 2). Thus, hypoglycemia is the result of the interplay of absolute or relative (to exogenous glucose delivery, endogenous glucose production, or insulin sensitivity) therapeutic insulin excess and compromised glucose counterregulation (1, 2, 13).
Figure 1. Physiological and behavioral defenses against hypoglycemia.
Decrements in insulin and increments in glucagon are lost and increments in epinephrine and neurogenic symptoms are often attenuated in insulin-deficient — T1DM and advanced T2DM — diabetes. SNS, sympathetic nervous system; PNS, parasympathetic nervous system; NE, norepinephrine; ACh, acetylcholine; α cell, pancreatic islet α cells; β cell, pancreatic islet β cells.
Hypoglycemia-associated autonomic failure
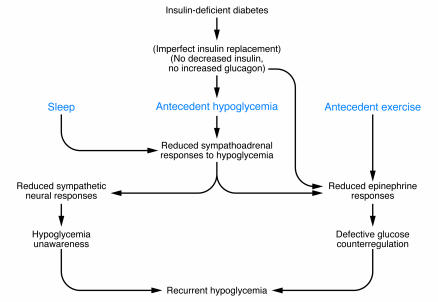
The concept of hypoglycemia-associated autonomic failure (HAAF) in T1DM (7) and advanced T2DM (8) (Figure 2) posits that recent antecedent iatrogenic hypoglycemia causes both defective glucose counterregulation and hypoglycemia unawareness and, thus, a vicious cycle of recurrent hypoglycemia (1, 2). It causes defective glucose counterregulation by reducing epinephrine responses to a given level of subsequent hypoglycemia in the setting of absent decrements in insulin and increments in glucagon. It causes hypoglycemia unawareness by reducing sympathoadrenal and the resulting neurogenic symptom responses to a given level of subsequent hypoglycemia. Sleep and antecedent exercise cause a similar phenomenon (1) (Figure 2). The clinical impact of HAAF, including the finding that hypoglycemia unawareness and the reduced epinephrine component of defective glucose counterregulation are reversed by as little as 2–3 weeks of scrupulous avoidance of hypoglycemia in most affected patients, is well established (1, 2). However, the mechanism(s) of the key feature of HAAF, the attenuated sympathoadrenal response to falling plasma glucose concentrations, are unknown (2).
Figure 2. HAAF in T1DM and advanced T2DM.
Figure modified from the New England Journal of Medicine, with permission from the Massachusetts Medical Society (1).
The shift of the glycemic thresholds for sympathoadrenal responses to lower plasma glucose concentrations caused by recent antecedent hypoglycemia (or by sleep or prior exercise) could be the result of alterations in the peripheral afferent or efferent components of the autonomic nervous system or within the CNS (2). Much of the recent research has focused on the latter, especially the hypothalamus (2, 3). However, hypoglycemia activates widespread brain regions including the medial prefrontal cortex (14).
The potential CNS mechanisms of the reduced sympathoadrenal response, and thus of HAAF, have been reviewed (2). In my view (2), the balance of evidence, including more recent evidence (15), weighs heavily against the systemic mediator (e.g., cortisol) hypothesis and the brain fuel (e.g., glucose) transport hypothesis, although increased transport of other fuels remains to be explored fully (16). Thus, assuming a primary CNS alteration, the global brain metabolism hypothesis is the most attractive. The array of potential mechanisms within the brain (reviewed in ref. 2) range from posthypoglycemic increased glucokinase activity in critical hypothalamic neurons (17) to posthypoglycemic brain glycogen supercompensation (18). In addition, alterations of ATP-sensitive K+ channel function, AMP-activated protein kinase activity, γ-amino butyric acid release, insulin signaling, and expression of angiotensinogen and related genes as well as of paraventricular nucleus activity, cerebral glucose metabolism, and cerebral blood flow have been proposed (2). The findings of McCrimmon and colleagues (3) raise another possibility. They report that ventromedial hypothalamus (VMH) application of urocortin I, a corticotrophin-releasing factor receptor–2 agonist, suppresses the counterregulatory response to hypoglycemia (an effect that lasted for at least 24 hours), whereas that of corticotrophin-releasing factor, a predominant corticotrophin-releasing factor receptor–1 agonist, amplifies that response in rats. Urocortin I was also shown to alter the glucose sensitivity of VMH glucose-sensing neurons in whole-cell current clamp experiments in brain slices. Thus, increased hypothalamic urocortin I release during antecedent hypoglycemia could explain a decreased sympathoadrenal response to subsequent hypoglycemia. However, that causal connection was not demonstrated and, therefore, remains a provocative theoretical possibility.
Ultimately, the problem of hypoglycemia (and that of hyperglycemia) in diabetes will likely be solved by the development of safe and effective methods that provide plasma glucose–regulated insulin replacement or secretion. That will probably involve closed-loop insulin replacement (online glucose sensor–computer interface–insulin infusion pump) until implantation of glucose-responsive, insulin-secreting cells becomes feasible for widespread use. Pending that, there is clear a need for new insight into the fundamental mechanisms of the physiology of glucose counterregulation and its pathophysiology in experimental models that leads to clinical strategies that are then shown to both reduce the risk of hypoglycemia and facilitate glycemic control in people with diabetes (i.e., bidirectional translational research).
Acknowledgments
The author’s cited work was supported, in part, by US Public Health Service/NIH grants R37 DK27085, MO1 RR00036, P60 DK20579, and T32 DK07120 and by a fellowship award from the American Diabetes Association. Janet Dedeke prepared this manuscript.
Footnotes
Nonstandard abbreviations used: HAAF, hypoglycemia-associated autonomic failure; T1DM, type 1 diabetes mellitus; VMH, ventromedial hypothalamus.
Conflict of interest: The author has served on advisory boards of Novo Nordisk Inc., Takeda Pharmaceuticals North America Inc., and MannKind Corp. in recent years.
Citation for this article: J. Clin. Invest. 116:1470–1473 (2006). doi:10.1172/JCI28735.
See the related article beginning on page 1723.
References
- 1.Cryer P.E. Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N. Engl. J. Med. 2004;350:2272–2279. doi: 10.1056/NEJMra031354. [DOI] [PubMed] [Google Scholar]
- 2.Cryer P.E. Mechanisms of hypoglycemia-associated autonomic failure and its component syndromes in diabetes. Diabetes. 2005;54:3592–3601. doi: 10.2337/diabetes.54.12.3592. [DOI] [PubMed] [Google Scholar]
- 3.McCrimmon R.J., et al. Corticotrophin-releasing factor receptors within the ventromedial hypothalamus regulate hypoglycemia-induced hormonal counterregulation. J. Clin. Invest. . 2006;116:1723–1730. . doi: 10.1172/JCI27775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 5.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. . Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. . N. Engl. J. Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cryer P.E. Handbook of physiology: a critical, comprehensive presentation of physiological knowledge and concepts. Section 7, The endocrine system. Volume 2, The endocrine pancreas and regulation of metabolism. L.S. Jefferson and A.D. Cherrington, editors. Oxford University Press.; New York, New York, USA.: 2001. The prevention and correction of hypoglycemia. pp. 1057–1092. [Google Scholar]
- 7.Dagogo-Jack S.E., Craft S., Cryer P.E. Hypoglycemia-associated autonomic failure in insulin-dependent diabetes mellitus: recent antecedent hypoglycemia reduces autonomic responses to, symptoms of, and defense against subsequent hypoglycemia. J. Clin. Invest. 1993;91:819–828. doi: 10.1172/JCI116302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Segel S.A., Paramore D.S., Cryer P.E. Hypoglycemia-associated autonomic failure in advanced type 2 diabetes. Diabetes. 2002;51:724–733. doi: 10.2337/diabetes.51.3.724. [DOI] [PubMed] [Google Scholar]
- 9.Raju B., Cryer P.E. Loss of the decrement in intraislet insulin plausibly explains loss of the glucagon response to hypoglycemia in insulin-deficient diabetes. Diabetes. 2005;54:757–764. doi: 10.2337/diabetes.54.3.757. [DOI] [PubMed] [Google Scholar]
- 10.Gosmanov N.R., et al. Role of the decrement in intraislet insulin for the glucagon response to hypoglycemia in humans. Diabetes Care. 2005;28:1124–1131. doi: 10.2337/diacare.28.5.1124. [DOI] [PubMed] [Google Scholar]
- 11.Israelian Z., et al. Increasing the decrement in insulin secretion improves glucagon responses to hypoglycemia in advanced type 2 diabetes. Diabetes Care. 2005;28:2691–2696. doi: 10.2337/diacare.28.11.2691. [DOI] [PubMed] [Google Scholar]
- 12.DeRosa M.A., Cryer P.E. Hypoglycemia and the sympathoadrenal system: neurogenic symptoms are largely the result of sympathetic neural, rather than adrenomedullary, activation. Am. J. Physiol. Endocrinol. Metab. 2004;287:E32–E41. doi: 10.1152/ajpendo.00539.2003. [DOI] [PubMed] [Google Scholar]
- 13.Cryer P.E., Davis S.N., Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003;26:1902–1912. doi: 10.2337/diacare.26.6.1902. [DOI] [PubMed] [Google Scholar]
- 14.Teves D., Videen T.O., Cryer P.E., Powers W.J. Activation of human medial prefrontal cortex during autonomic responses to hypoglycemia. Proc. Natl. Acad. Sci. U. S. A. 2004;101:6217–6221. doi: 10.1073/pnas.0307048101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldberg P.A., et al. Antecedent hypercortisolemia is not primarily responsible for generating hypoglycemia-associated autonomic failure. Diabetes. 2006;55:1121–1126. doi: 10.2337/diabetes.55.04.06.db05-1169. [DOI] [PubMed] [Google Scholar]
- 16.Mason G.F., Petersen K.F., Lebon V., Rothman D.L., Shulman G.I. Increased brain monocarboxylic acid transport and utilization in type 1 diabetes. Diabetes. 2006;55:929–934. doi: 10.2337/diabetes.55.04.06.db05-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang L., et al. Glucokinase is a critical regulator of ventromedial hypothalamic neuronal glucosensing. Diabetes. 2006;55:412–420. doi: 10.2337/diabetes.55.02.06.db05-1229. [DOI] [PubMed] [Google Scholar]
- 18.Choi I.-Y., Seaquist E.R., Gruetter R. Effect of hypoglycemia on brain glycogen metabolism in vivo. J. Neurosci. Res. 2003;72:25–32.. doi: 10.1002/jnr.10574. [DOI] [PMC free article] [PubMed] [Google Scholar]