Abstract
The question of what differentiates physiological from pathological cardiac hypertrophy remains one of the most clinically relevant questions in basic cardiovascular research. The answer(s) to this question will have far-ranging importance in the fight against hypertrophic heart disease and failure. In this issue of the JCI, Perrino et al. have used a unique model system to mimic the pathophysiologic effects of an intermittent pressure overload on the heart — in effect, to examine the basic issue of what determines an in vivo pathogenic stimulus (see the related article beginning on page 1547). Their findings clearly show that it is the nature of the inciting stimulus, as opposed to chronicity, that establishes the initial pathogenic response and that a distinct disruption of the β-adrenergic system is centrally involved in the earliest alterations of myocellular physiology. These results suggest both a new paradigm for treatment options in hypertrophic cardiac disease and novel methodologies for further studies.
In order to maintain sufficient cardiac output over an expected lifespan of 70-plus years, the heart must respond to a myriad of physiologic and pathophysiologic stimuli. To meet these physiological demands on a day-to-day basis the heart relies on a phenomenon known as myocardial reserve, whereby it can reversibly alter cardiac output in response to a sudden increase in demand via a broad range of myocellular signaling pathways anchored, in part, by the β-adrenergic system. It has long been noted, however, that sustained or progressive demands on the heart can result in cardiac hypertrophy (1). Many common disease states lead to the prolonged increase in hemodynamic demand (either pressure- or volume-based) that causes the characteristic pathogenic myocellular hypertrophy including hypertension, valvular abnormalities, and post–myocardial infarction remodeling (2). In the pressure-overloaded state, the heart maintains cardiac output in the context of elevated afterload by increasing ventricular wall thickness as dictated by the Law of Laplace. The end result, however, is only a short-term solution. If the inciting pathogenic stimulus is not relieved, the “adaptive” increase in myocellular mass leads to impaired ventricular relaxation, filling, and, in many cases, eventual cardiac failure. Thus given the continuing high incidence of inciting disorders and the resultant profound degree of associated morbidity and mortality, understanding the pathogenesis of hypertrophic heart disease remains a central focus of cardiovascular research (3).
Cardiac hypertrophy can be adaptive or maladaptive
It has long been noted that ventricular enlargement does not necessarily lead to a decrease in cardiac performance. In fact, numerous studies dating back nearly 40 years have demonstrated that the mild to moderate left ventricular hypertrophy observed in highly trained athletes actually enhances cardiac performance via increases in stroke volume, contractility, and oxygen consumption with preserved relaxation (4, 5). This surprising observation, that similar degrees of left ventricular hypertrophy could exhibit strikingly different profiles of cardiovascular function, led to a series of animal studies that began to establish the link between whole-heart and myocellular physiology. Early results revealed significant differences in myosin isoform distribution, myofibrillar ATPase activity, and cardiac function between aortic-banded and exercise-trained rats, thus establishing the early groundwork for the molecular basis of primary cardiac disease (6). Fast-forward 30 years and the availability of elegantly designed cardiac-restricted transgenic and knockout mice, sophisticated in vivo physiologic measurements, and an extensive knowledge of myocellular signaling pathways have converged to finally begin to comprehensively delineate the basic mechanisms that differentiate physiologic from pathologic hypertrophy (7, 8).
The nature of the pathogenic stimulus determines the cardiac response — chronicity is not sufficient
In this issue of the JCI, Perrino et al. (9) use a unique model system to address some of the more intractable questions regarding the nature of pathogenic stimuli and the resultant cardiovascular responses that determine the clinical course of hypertrophic heart disease.
The first set of studies was designed to determine the influence of the nature and duration of the cardiovascular stimulus on the development of cardiac hypertrophy in mice using a unique modification of transverse aortic constriction (TAC), known as intermittent TAC (iTAC), that allows for the controlled and intermittent application of a pathogenic pressure overload. Interestingly, 4 weeks of iTAC performed in tandem with chronic pressure overload by chronic TAC (cTAC) and independent forms of exercise (i.e., forced swimming and voluntary wheel running) resulted in a distinct and somewhat surprising pattern of ventricular remodeling characterized by mild hypertrophy in the absence of fetal gene induction, preserved global LV function, and a marked decrease in capillary density. This seemingly intermediary phenotype (sharing components of the cTAC and exercise groups) at the whole-heart and tissue levels is in stark contrast to the cellular findings, in which isolated ventricular myocytes from iTAC mice demonstrated significantly impaired contractility and relaxation parameters both at baseline and in response to β-agonist exposure similar to those observed in cTAC myocytes.
These complex results highlight the profound specificity of the likely sarcolemmal “sensor(s)” that transduce the nature of the input stimulus to the cardiovascular response. Moreover, the fact that intermittent exposure to a pathogenic stimulus was sufficient to dissociate the global hypertrophic phenotype (mild) from the cellular phenotype (moderate to severe) in iTAC mice was striking; not only does this finding support several recent studies in which the pathogenic cardiovascular response was separated from myocellular growth, it extends our understanding of the central role of the nature of the inciting stimulus (10–12). Thus, these results definitively show that while the duration of the discrete pathogenic stimulus may dictate the magnitude of ventricular hypertrophy, chronicity is not sufficient to cause hypertrophic heart disease — the nature of the inciting stimulus must be pathogenic.
Early β-adrenergic receptor system dysfunction drives the pathogenic hypertrophic phenotype
Based on their initial findings (9), the authors were able to use the specificity of the iTAC stimulus to begin to address both the timing and the potential mechanisms of the pathogenic cardiovascular response to intermittent overload. As recently noted in an excellent review by Dorn and Force, one of the inherent difficulties in studying the primary mechanisms involved in the development of cardiac hypertrophy and heart failure lies in the complex interplay among the intersecting protein kinase cascades that regulate both physiologic and pathologic hypertrophy (13). This complexity limits the utility of studying late-stage cardiac tissue to determine cause and effect.
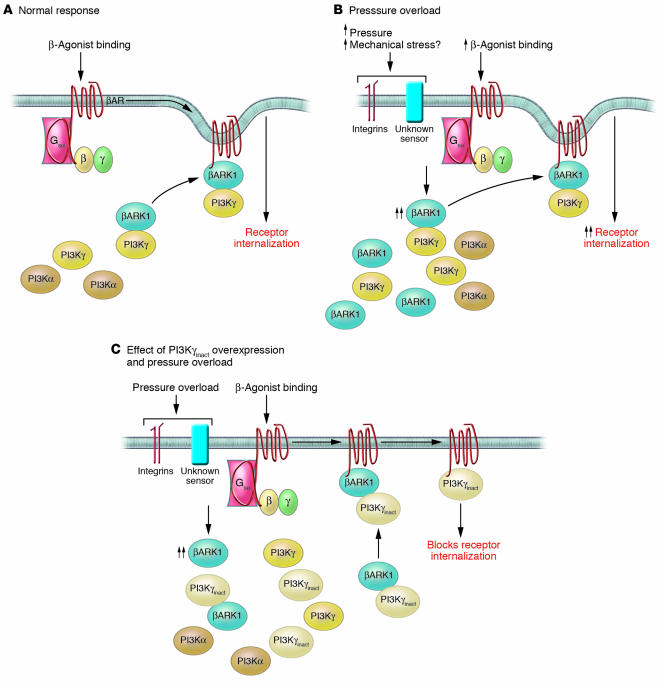
In their initial 4-week studies, Perrino et al. (9) showed that only the mouse hearts exposed to pressure overload (iTAC and cTAC) exhibited abnormalities in the β-adrenergic receptor (βAR) system (Figure 1). Specifically, they observed similar decreases in βAR density with a corresponding loss in isoproterenol-dependent (ISO-dependent) cAMP generation and a concomitant increase in βAR kinase 1 (βARK1) levels and βARK1-associated PI3Kγ. Previous work by this group and others had demonstrated that PI3Kγ decreases both cardiac contractility via inhibition of β2AR-associated adenylate cyclase and attenuation of β2AR internalization (14–16). Building on these observations, the authors then directly addressed the basic question of whether the observed derangements in βAR-mediated pathways were causally involved in iTAC pathology by examining the early pre-hypertrophic changes induced by 1 week of either pathologic (iTAC, cTAC) or physiologic stimuli (9). This elegant series of in vivo studies using a previously described transgenic mouse model (iTACγinact mice, which express a catalytically inactive form of PI3Kγ [PI3Kγinact] that displaces the endogenous PI3Kγ) revealed that the inhibition of βARK1-mediated PI3K recruitment to ligand-activated receptors (and thus the maintenance of βAR function) abrogated a significant subset of the prehypertrophic functional and structural changes induced by iTAC.
Figure 1. Potential mechanism for the role of early βAR dysfunction in the hypertrophy-independent pathological phenotype induced by iTAC.
(A) Role of PI3Kγ in modulating βAR internalization. Chronic β-agonist binding to βARs leads to an induction in myocellular βARK1 levels. βARK1 binds to PI3Kγ and facilitates its translocation to the receptor complex, where the subsequent generation of D-3 phosphoinositides leads to the recruitment of multiple adapter proteins and receptor internalization. (B) Intermittent pressure overload (iTAC) promotes early βAR dysfunction. Normal mice exposed to both 1 week and 4 weeks of iTAC exhibited significant βAR downregulation and desensitization. In the case of the 1-week iTAC mice, this effect was seen despite normal steady-state catecholamine levels and highlights the pathogenic nature of the pressure-overload stimulus. While the pressure-overload sensor mechanism is not fully understood, the participation of the molecules involved in mechanical stress (e.g., integrins) is likely. These 2 stimuli result in the observed pathogenic response, including a significant induction of βARK1 levels causing an increase in PI3Kγ translocation to the agonist-bound receptor complex and subsequent increase in receptor internalization. (C) Overexpression of PI3Kγinact in the heart reverses the downregulation of βARs and the early pathogenic changes in iTAC mice. Unlike normal mice, iTACγinact mice subjected to 1 week of iTAC exhibited normal βAR density and βAR/Gs coupling in the context of elevated βARK1. Overexpression of PI3Kγinact leads to a competitive displacement of all PI3K isoforms from the βARK1/PI3K complex. This displacement blocks the effects of the early induction of βARK1 caused by iTAC and effectively preserves βAR levels and function.
Of note, while treatment with the βAR-blocking agent metoprolol recapitulated many aspects of this apparent rescue via PI3Kγinact overexpression, including preserved βAR levels and coupling as well as a decrease in hypercontractility and in the slope of the end-diastolic pressure–volume relationship (EDPVR), no effect was seen on either vascular rarefaction or the decrease in the rate of isovolumic relaxation — further evidence that the complex early pathogenic response to pressure overload may involve additional G protein–independent signaling pathways. Finally, while acute plasma catecholamine levels from matched sets of swimming, iTAC, and iTACγinact mice were equivalent, only the iTAC mice exhibited evidence of “pure” βAR uncoupling to Gs subunits (e.g., in the context of normal receptor levels), a provocative result that suggests that the observed early βAR desensitization is not due solely to the marked increase in sympathetic activation, but is highly dependent on the nature of the pathogenic pressure overload.
A potentially broader paradigm to spur new approaches to an old problem
The compelling findings presented by Perrino et al. (9) regarding the ability of controlled intermittent pressure overload to finely dissociate multiple aspects of the regulation of pathogenic hypertrophy and the central role of early β-adrenergic dysfunction provide both a new framework for further investigation and a new in vivo methodology. Like all new answers to old problems, this work raises a host of interesting questions. First, it remains unclear what constitutes the pathogenic nature of the intermittent pressure overload. One possibility is that, unlike the increase in venous return and oxygen demand that occurs with exercise, the establishment of an acute pressure overload for even a short period of time cannot be overcome by any of the normal cardiovascular responses. The heart is, in effect, defenseless against such an assault. The nearly immediate uncoupling of the βAR, rapid development of significant vascular rarefaction, hypercontractility, and impaired relaxation in the iTAC mice is consistent with a “surrender” phenotype. It would be interesting to determine whether a different time course of applied intermittent pressure overload would alter the observed phenotype.
Another question involves the identity of the actual trigger, or sensor, of the pressure overload. The 7-day studies clearly establish that the pathogenic phenotype in iTAC can be fully dissociated from hypertrophic growth in the context of early βAR dysfunction and is not fully dependent on neurohormonal stimulation. In this context, the specificity of the response is striking. While the central role of integrins and stretch-activated channels in transducing mechanical stretch into the activation of downstream pathways and kinase cascades has been well established, the proteins or structures that actually sense stretch remain unknown. Activation of myocellular remodeling via alterations in biomechanical stretch (mechanotransduction) plays an important role in many cardiomyopathies including those caused by postinfarction remodeling and mutations in cytoskeletal and sarcomeric proteins (17). Indeed, animal models of both dilated and hypertrophic cardiomyopathies (representing intrinsic mechanical stress) have demonstrated a similar dissociation between myocellular pathology and growth, as seen in the iTAC mice (12, 18). Thus, both extrinsic and intrinsic pathologic stimuli may activate similar myocellular responses involving both G protein–dependent and –independent signaling pathways.
Finally, from the clinical standpoint the current studies (9) fully expose the inherent limitations in using cardiac hypertrophy as a noninvasive prognostic indicator. The importance of decreasing LV mass during antihypertensive therapy has been clearly shown to decrease the rate of clinical endpoints; indeed, based on the results in the current paper, the aggressive treatment of hypertension as a means of directly decreasing the pathogenic stimulus of the perceived pressure load is an important therapeutic goal (19). The markedly abnormal phenotype of the iTAC mice (after both 7 days and 4 weeks of exposure to the intermittent pressure load) in the absence of either the reactivation of the fetal gene program or a large increase in ventricular mass was surprising and, frankly, sobering from a clinical standpoint. It is reminiscent of the oft-noted clinical findings in hypertrophic cardiomyopathy wherein the degree of diastolic impairment is often described as “out of proportion” to the observed ventricular hypertrophy, thus suggesting a significant myocellular contribution to disease pathogenesis. It is interesting to speculate that the well-known salutary effects of postinfarction treatment with metoprolol may reflect the molecular findings in the current paper. Thus the results presented by Perrino et al. lay the groundwork for a robust 2-pronged therapeutic approach based on relief of the inciting pathogenic stimulus and direct treatment of the underlying molecular derangements, perhaps via novel compounds to displace active PI3K isoforms from the receptor complex.
Footnotes
Nonstandard abbreviations used: βAR, β-adrenergic receptor; βARK1, βAR kinase 1; cTAC, chronic TAC; EDPVR, end-diastolic pressure–volume relationship; ISO, isoproterenol; iTAC, intermittent TAC; iTACγinact, cardiac-specific overexpression of PI3Kγinact; PI3Kγinact, catalytically inactive PI3Kγ; TAC, transverse aortic constriction.
Conflict of interest: The author has declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 116:1467–1470 (2006). doi:10.1172/JCI28884.
See the related article beginning on page 1547.
References
- 1.Meerson F.Z. Compensatory hyperfunction of the heart and cardiac insufficiency. Circ. Res. 1962;10:250–258. doi: 10.1161/01.res.10.3.250. [DOI] [PubMed] [Google Scholar]
- 2.Lorell B.H., Apstein C.S., Weinberg E.O., Cunningham M.J. Diastolic function in left ventricular hypertrophy: clinical and experimental relationships. Eur. Heart J. 1990;11(Suppl. G):54–64. doi: 10.1093/eurheartj/11.suppl_g.54. [DOI] [PubMed] [Google Scholar]
- 3.Ho K.K., Pinsky J.L., Kannel W.B., Levy D. The epidemiology of heart failure: the Framingham Study. J. Am. Coll. Cardiol. 1993;22:6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 4.Raskoff W.J., Goldman S., Cohn K. The “athletic heart”. Prevalence and physiological significance of left ventricular enlargement in distance runners. Jama. 1976;236:158–162. doi: 10.1001/jama.236.2.158. [DOI] [PubMed] [Google Scholar]
- 5.Bevegard B.S., Shepherd J.T. Regulation of the circulation during exercise in man. Physiol. Rev. 1967;47:178–213. doi: 10.1152/physrev.1967.47.2.178. [DOI] [PubMed] [Google Scholar]
- 6.Giusti R., Bersohn M.M., Malhotra A., Scheuer J. Cardiac function and actomyosin ATPase activity in hearts of conditioned and deconditioned rats. J. Appl. Physiol. 1978;44:171–174. doi: 10.1152/jappl.1978.44.2.171. [DOI] [PubMed] [Google Scholar]
- 7.Wilkins B.J., et al. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ. Res. 2004;94:110–118. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- 8.Barki-Harrington L., Perrino C., Rockman H.A. Network integration of the adrenergic system in cardiac hypertrophy. Cardiovasc. Res. 2004;63:391–402. doi: 10.1016/j.cardiores.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Perrino C., et al. 2006Intermittent pressure overload triggers hypertrophy-independent cardiac dysfunction and vascular rarefactio n . J. Clin. Invest. 1161547–1560. 10.1172/JCI25397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shioi T., et al. The conserved phosphoinositide 3-kinase pathway determines heart size in mice. Embo J. 2000;19:2537–2548. doi: 10.1093/emboj/19.11.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frey N., et al. Mice lacking calsarcin-1 are sensitized to calcineurin signaling and show accelerated cardiomyopathy in response to pathological biomechanical stress. Nat. Med. 2004;10:1336–1343. doi: 10.1038/nm1132. [DOI] [PubMed] [Google Scholar]
- 12.Ertz-Berger B.R., et al. Changes in the chemical and dynamic properties of cardiac troponin T cause discrete cardiomyopathies in transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 2005;102:18219–18224. doi: 10.1073/pnas.0509181102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorn G.W., Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J. Clin. Invest. 2005;115:527–537. doi: 10.1172/JCI200524178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nienaber J.J., et al. Inhibition of receptor-localized PI3K preserves cardiac β-adrenergic receptor function and ameliorates pressure overload heart failure. J. Clin. Invest. 2003;112:1067–1079. doi: 10.1172/JCI200318213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naga Prasad S.V., Esposito G., Mao L., Koch W.J., Rockman H.A. Gbetagamma-dependent phosphoinositide 3-kinase activation in hearts with in vivo pressure overload hypertrophy. J. Biol. Chem. 2000;275:4693–4698. doi: 10.1074/jbc.275.7.4693. [DOI] [PubMed] [Google Scholar]
- 16.Crackower M.A., et al. Regulation of myocardial contractility and cell size by distinct PI3K-PTEN signaling pathways. Cell. 2002;110:737–749. doi: 10.1016/s0092-8674(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 17.Mann D.L. Left ventricular size and shape: determinants of mechanical signal transduction pathways. Heart Fail. Rev. 2005;10:95–100. doi: 10.1007/s10741-005-4636-y. [DOI] [PubMed] [Google Scholar]
- 18.Knoll R., et al. The cardiac mechanical stretch sensor machinery involves a Z disc complex that is defective in a subset of human dilated cardiomyopathy. Cell. 2002;111:943–955. doi: 10.1016/s0092-8674(02)01226-6. [DOI] [PubMed] [Google Scholar]
- 19.Devereux R.B., et al. Prognostic significance of left ventricular mass change during treatment of hypertension. Jama. 2004;292:2350–2356. doi: 10.1001/jama.292.19.2350. [DOI] [PubMed] [Google Scholar]