Abstract
Based on a molecular neuroendocrine theory about cold environments, thyroid hormone levels, and liganded thyroid hormone receptor interference with estrogen receptor function, experiments were designed to test female mouse reproductive behaviors in the cold. Because natural seasonal temperature declines would usually be associated with decreased photoperiods and reduced food supplies, we combined cold temperatures with short days and metabolic challenge. The simplest hypothesis was that lordosis quotients would be significantly reduced as a result of cold temperatures. That hypothesis was denied. Instead, female approaches to the stud male declined. Because cold temperatures also led to significant reductions of activity in locomotor wheels, a straightforward reduction of activity could explain the female's behavior during mating tests. We suggest that cold temperatures accompanied by reduced photoperiod and reduced metabolic fuel can reduce overall activity in female mice, thus indirectly blocking untimely reproductive behaviors.
A combination of molecular biological and zoological findings stimulated the following theory: (i) that cold environmental temperatures, which could reduce the biological adaptiveness of reproduction in female mice, would increase thyroid hormone binding to thyroid hormone receptors in the brain; and (ii) that, as a result, liganded thyroid hormone receptors, through competitive DNA binding and other mechanisms, would interfere with estrogen receptor-facilitated transcription in hypothalamic neurons important for reproductive behavior (1). Molecular data (refs. 2 and 3; N. Vasudevan, C. J. Krebs, Y.-S. Zhu, S. Daniels, N. Koibuchi, W. Chin, and D.P., unpublished data) and behavioral data (4, 5) have supported some features of this theory. However direct observations of female mouse mating behavior while living in a cold environment have been lacking and were now required.
Classical literature shows depressed rates of reproduction in the winter (6–11). Cold environmental temperatures often delay the onset of breeding and lengthen the intervals between litters (12, 13). Bronson and Perrigo (14–17) have made the point that normal reproduction may continue in the cold if food supplies are not limited. Nor are photoperiod changes alone enough to limit reproduction in mice (18). Because of the data of Bronson and Perrigo, and because under many natural conditions food availability, photoperiod, and environmental temperature would all decline seasonally, we chose to investigate the challenges combined. The experiments of Wade and his colleagues have shown that decreased availability of metabolic fuel for the brain, sensed in the area postrema, can be achieved by injection of 2-deoxy-d-glucose (2-DG), and that this metabolic alteration is permissive for reproductive behavior (see, e.g., refs. 19–21). Therefore, our primary experimental condition uses 2-DG and reduced photoperiod, as well as environmental cold.
Methods
First Experiment.
Animals.
All experimental procedures were carried out under a protocol approved by The Rockefeller University Institutional Animal Care and Use Committee, overseen by the Laboratory Animal Research Center in a facility approved by the American Association of Laboratory Animal Care. Sixteen young adult Swiss–Webster female mice were ovariectomized by the supplier (Taconic Farms) and delivered to our laboratory. They were housed individually in standard mouse cages (17.7 cm × 27.9 cm × 11.4 cm) under our laboratory conditions for 2 mo before the experiment began. Three weeks before testing, mice were shaved to increase the impact of cold temperature. A reverse 12:12 light cycle (lights on 10 p.m.–10 a.m.) was used, and all of the behavioral measurements were carried out in the dark part of the cycle. Food and water were available ad libitum.
Experimental design and environmental conditions.
The following experimental design and series of measurements were arrived at as a result of pilot experiments in which simple exposure to cold temperature did not reliably reduce lordosis behavior itself. Therefore, this experiment built on the reports by Bronson and others (reviewed in ref. 15), and on the fact that seasonally low temperatures would be accompanied by short days and limited metabolic fuel availability. The experimental design used three groups. In the control group, females were maintained at a normal room temperature (23°C), on a regular 12:12-h light cycle, with normal ad libitum food availability and no injection of 2-deoxyglucose (group name, NORM). A second experimental group was exposed to a living environment with reduced temperature, 4°C, 52 h before experimental test and a reduced period of light in the 24-h cycle (lights on 3 a.m.–4 a.m.). This group is referred to as the cold temperature/short day group (COLD/SD). The third experimental group received the same exposure to cold temperature and the same short day condition as the second experimental group, and in addition, received a challenge to metabolic fuel availability. In addition to a 52-h exposure to 4°C and a shortened (1-h) exposure to light per day, these animals also received 2-DG (Sigma, No. D6134, 2-DG, 600 mg/kg mouse body weight, i.p., dissolved in saline; see ref. 22) 1 h before behavioral testing (Group name, COLD/SD/2DG).
All females received (i) 10 μg estradiol benzoate, s.c., in sesame oil 52 h before testing; (ii) 5 μg estradiol benzoate, s.c., 28 h before behavioral test; and then (iii) 500 μg progesterone, s.c., in sesame oil 4 h before behavioral testing.
Behavioral testing.
After 52 h of exposure to one of the three experimental conditions, animals were tested for reproductive behavior. Animals in the cold environment were removed to a normal temperature testing room (23°C) 10 min before testing to acclimatize, so that their body surface would not present an unusual somatosensory stimulus to the stud male. All females were given mating behavior tests lasting 15 mounts or 15 min, whichever happened first. They were tested in the home cage of the stud male (17.7 cm × 27.9 cm × 11.4 cm). All tests were videotaped. Because of pilot experiments in which simple behavioral measurements restricted to lordosis did not give statistically significant results, an expanded range of behavioral measures was taken from the video and rated without knowledge of experimental group. The following behavioral measures were scored. For female approaches to the male to be scored from the videotape, the female mouse had to locomote in a straight line toward the male, arriving at a location less than one half body length from the male. For this measure, the first minute of behavioral testing was not considered, because of nonspecific exploratory behaviors occurring at that time. To measure the attractivity of the female, we counted male approaches to the female. For this measure to be scored from the videotape, the same considerations as the female approaches were applied, and again, measurements were not taken from the first minute of testing because of nonspecific exploratory responses. For a mount to be scored, the male had to approach from the rear and put his paws on the flanks of the female in a proper mounting position. Thrusts were not required for a mount to be scored. For lordosis, the female had to maintain the standing posture during the male's mount, coupled with a detectable vertebral dorsiflexion. Lordosis strength (1–3) was scored according to the degree of vertebral dorsiflexion. Regarding other measures, see Results.
Statistics.
Because numerical results did not meet the criteria for a normal distribution, nonparametric statistics were used (23); the Mann–Whitney U test was applied.
Second Experiment.
This series of observations represented an attempt to replicate the first. The two experimental groups were as follows: normal temperature (23°C), normal light cycle (12:12 reversed), and no metabolic challenge (group name, NORM); the second experimental group had 52 h exposure to a 4°C environmental temperature, a short day (1 h of light, 3 a.m.–4 a.m.), and a metabolic challenge (600 mg/kg body weight of 2-DG, injected i.p., in saline 1 h before behavioral testing) (group name, COLD/SD/2DG). Twenty-four female mice were used, 12 per experimental group. Hormone injections (estradiol benzoate and progesterone) were identical to the first experimental series. Testing conditions were identical to the first series except, to allow larger numbers of behavioral responses, the tests were extended to 25 min or 15 mounts, whichever happened first. Statistical analyses were carried out in the same manner as the first series.
Third Experiment.
Sixteen ovariectomized Swiss–Webster young adult female mice were purchased from Taconic Farms (Germantown, NY). After acclimation to the lab (2 wk), they were placed in one of two experimental conditions. In the first, they were placed in home cages (Nalgene/Mini Mitter polycarbonate cages, model 660-1284) attached to running wheels (Nalgene/Mini Mitter model 640-0701, equipped with counters, model 130-0023-00) at normal room temperature (23°C). Total revolutions per day were measured for a period of 21 days. Then, without removing the animals from the cage, they were moved into a 4°C environment, and total revolutions per day were again recorded, for a period of 10 days. The second experimental group (n = 8) had the order reversed. The first 21 days, they were living in cages attached to running wheels in a 4°C environment, and then, the final 10 days of testing, were returned to a normal temperature (23°C). Photoperiod was normal (12:12 light:dark, lights on 10 p.m.–10 a.m.), and food and water were available ad libitum.
Statistics.
For a nonparametric analysis (23) of differences between related samples (each animal yielded results both at normal and cold temperatures), the Wilcoxon Matched Pairs-Signal Ranks test was applied.
Results
First Experiment.
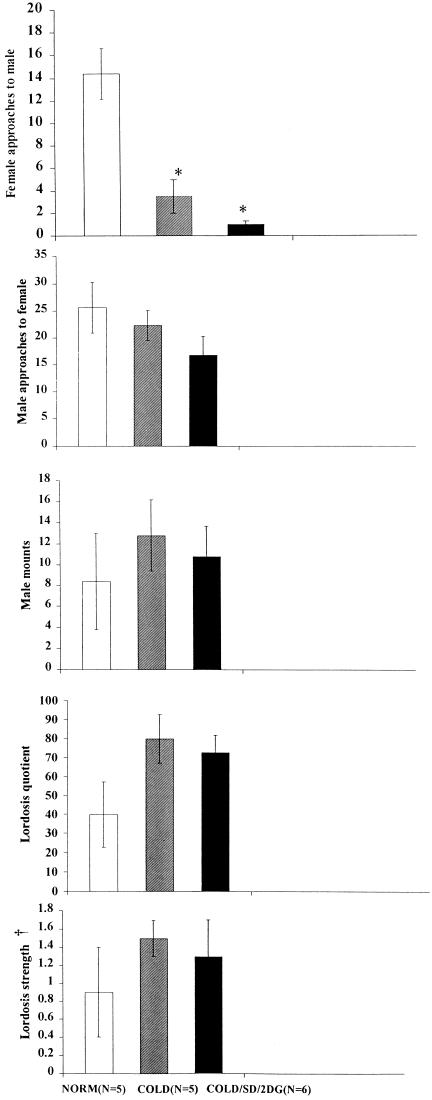
Total numbers of female approaches to the male were significantly less (P < 0.01) in the groups given cold environmental exposure (COLD/SD and COLD/SD/2DG), than in the controls (NORM) (Fig. 1). The difference in behavior of the females was evident despite the fact that the stud males were not approaching the three experimental groups differently, and in particular, were not approaching the normal-temperature group more frequently. Further, given that the males were able to attempt a mount, lordosis quotient and lordosis strength were not significantly different among groups. That is, the main experimental difference appeared to be in the behavior by the female initiated toward the male, rather than in the female's response to the male once a mount occurred.
Figure 1.
Total numbers of female approaches to the male were significantly less (*, P < 0.01) in both groups given cold environmental exposure than in the controls (NORM). †, In lordosis strength calculations, zeros were not included.
Second Experiment.
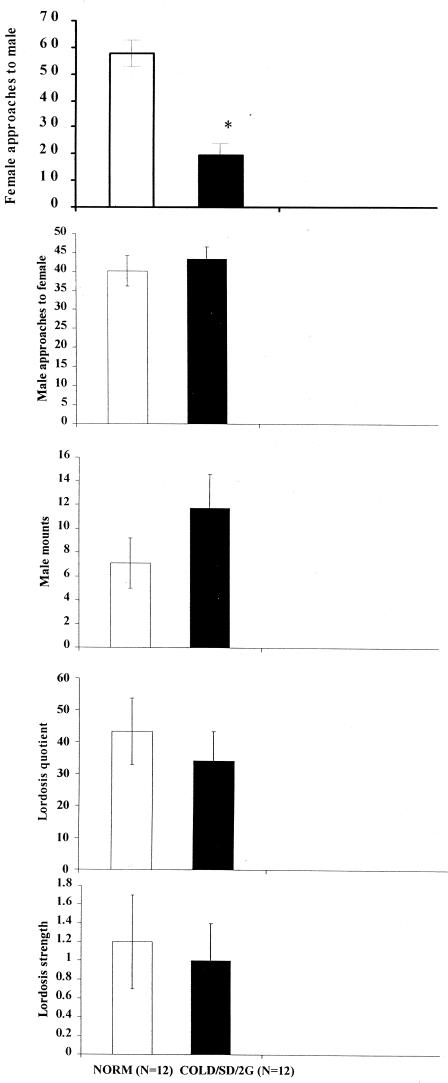
Once again, female approaches to the male occurred less frequently (P < 0.01) in the COLD/SD/2DG condition than in the NORM group (Fig. 2). This, despite the fact that there was not a reliable difference in lordosis quotient once the male actually mounted. Further, a large number of ancillary behaviors were measured. These behaviors included grooming, kicking, fighting, rolling under, boxing, other forms of rejection behavior, and transitional probabilities among all such behaviors. These other behaviors did not yield significant differences between groups.
Figure 2.
In the second experiment, female approaches to the male occurred significantly less frequently (*, P < 0.01) in the COLD/SD/2DG group than in the controls (NORM). In lordosis strength calculations, zeros were not included.
Third Experiment.
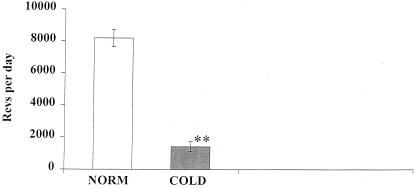
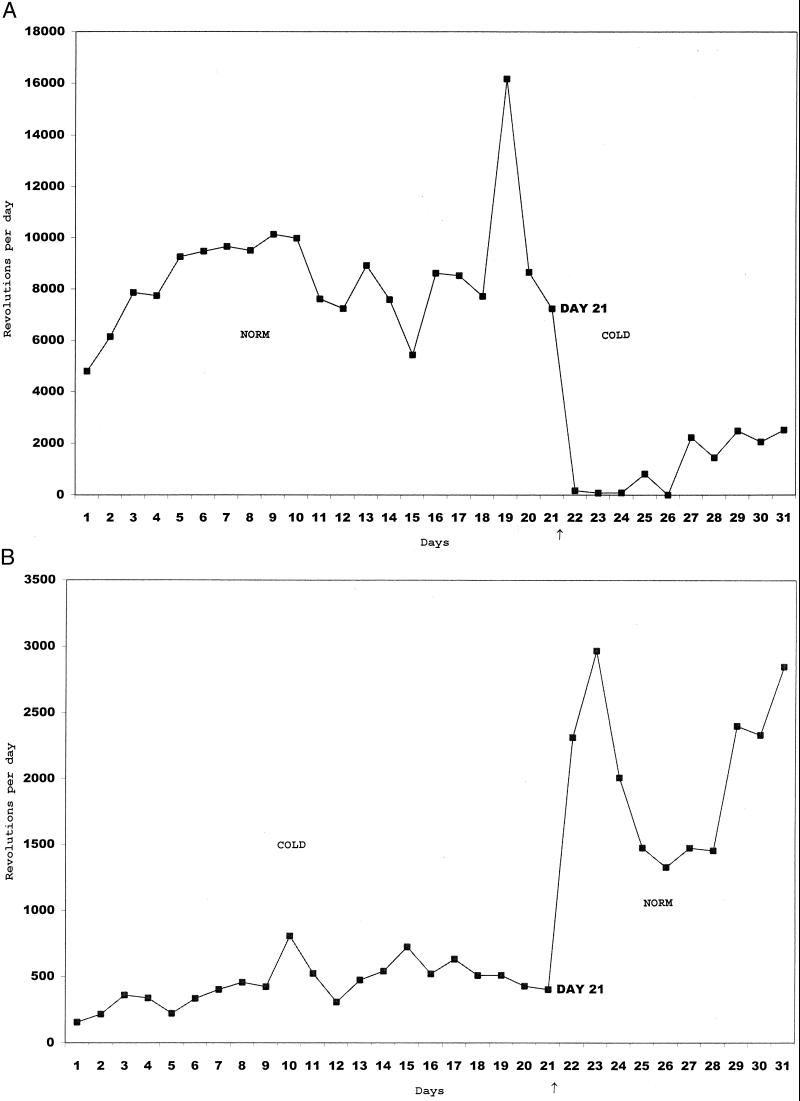
Because the main results from the first two experiments centered on the female's active approaches to the male, we carried out this experiment to see whether basic locomotor activity was greatly reduced under the same circumstances of cold environment. Dramatically, exposure to a cold environment significantly reduced (P < 0.005) locomotor activity in the wheel, regardless of the order of exposure (cold → normal or normal → cold) (Figs. 3 and 4).
Figure 3.
Locomotor activity in wheels attached to each mouse's home cage was significantly depressed (**, P < 0.005) when the animals were living in a cold environment.
Figure 4.
Revolutions per day in the locomotor wheels attached to the home cage, plotted here for two representative mice. (A) One started at normal room temperature (NORM) and was then switched to the cold (COLD) living environment (without being removed from her home cage). (B) The other was measured in the reverse order. Time of switching is shown by a small arrow below the abscissa.
Discussion
The theory that stimulated the present experiments centered on the potential role of environmental cold (see Introduction). Under a variety of experimental conditions, cold temperatures can lead to increased thyroid hormone release (24–26), because of increased thyroid stimulating hormone releasing hormone (TRH) release (27). As well, the deiodinase responsible for converting thyroxine (T4) into the active metabolite triiodothyronine (T3) can be stimulated by cold temperatures (28). Increased thyroid hormone activity is important for thermogenesis, crucial for survival in a small mammal whose large ratio of body surface to body volume would expose it to considerable heat loss.
The notion that, under environmentally challenging circumstances, reproductive behavior would be reduced seemed obvious. The simplest prediction was for reduced lordosis quotient. That prediction was not confirmed. Instead, overall locomotor activity was reduced during exposure to cold temperatures. We infer that, as a result, approaches by the female to the male were significantly less frequent. Such approaches are important components of the overall sequence of reproductive behaviors in female rodents, controlled by hormones in the basal forebrain where hormone-sensitive neurons send efferents back to the midbrain (29, 30). Estrogenic influences on preoptic neurons (31), perhaps including the preoptic locomotor region (32), appear to be crucial for such proceptive behaviors by the female. Therefore, cold temperatures, perhaps mediated by thyroid-related mechanisms, could interfere with active approaches by the female, which depend on locomotion.
Our experimental results appear to fit well into a zoological literature that describes torpor as well as reduced reproductive efficiency (33, 34) in certain mammals exposed to harsh environmental conditions. Such conditions frequently show interactions between cold temperatures and reduced photoperiods† but also can involve interactions between cold temperatures and reduced glucose availability (35–39). Thus, a larger perspective would state that such daily torpor would be associated with reduced motor activity and, as a result, a reduced tendency of the female to seek out the male.
Acknowledgments
We thank Professor John Axelson (Holy Cross College) for thoughtful comments on an early draft of the manuscript. This work was supported by HD-05751 from the National Institutes of Health.
Abbreviation
- 2-DG
2-deoxy-d-glucose
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.021554798.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.021554798
Kriegsfeld, L., Ranalli, N., Bober, M. & Nelson, R. (1999) Soc. Neurosci. Abstr. 25, abstr. 479.13.
References
- 1.Dellovade T, Zhu Y, Pfaff D W. J Steroid Biochem Mol Biol. 1995;53:27–31. doi: 10.1016/0960-0760(95)00037-z. [DOI] [PubMed] [Google Scholar]
- 2.Zhu Y-S, Dellovade T, Pfaff D W. J Neuroendocrinol. 1997;9:395–403. doi: 10.1046/j.1365-2826.1997.00590.x. [DOI] [PubMed] [Google Scholar]
- 3.Zhu Y-S, Chin W, Pfaff D W. Proc Natl Acad Sci USA. 1996;93:12587–12592. doi: 10.1073/pnas.93.22.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dellovade T, Zhu Y, Krey L, Pfaff D W. Proc Natl Acad Sci USA. 1996;93:12581–12586. doi: 10.1073/pnas.93.22.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan M, Dellovade T, Pfaff D W. Horm Behav. 2000;37:15–22. doi: 10.1006/hbeh.1999.1553. [DOI] [PubMed] [Google Scholar]
- 6.Baker J. Ecol Monogr. 1946;16:393–408. [Google Scholar]
- 7.King O. Trans Kan Acad Sci. 1950;53:500–528. [Google Scholar]
- 8.Parkes A. Br J Exp Biol. 1924;2:21–31. [Google Scholar]
- 9.Southern H, Laurie E. J Anim Ecol. 1946;15:134–149. [Google Scholar]
- 10.Southwick C. Ecology. 1955;36:212–225. [Google Scholar]
- 11.Strecker R, Emlen J. Ecology. 1953;34:375–385. [Google Scholar]
- 12.Barnett S. Biol Rev. 1973;48:477–508. doi: 10.1111/j.1469-185x.1973.tb01567.x. [DOI] [PubMed] [Google Scholar]
- 13.Barnett S, Dickson R. Biol Rev. 1989;64:317–340. doi: 10.1111/j.1469-185x.1989.tb00679.x. [DOI] [PubMed] [Google Scholar]
- 14.Bronson F. Mammalian Reproductive Biology. Chicago: Univ. Chicago Press; 1989. [Google Scholar]
- 15.Perrigo G, Bronson F. Biol Reprod. 1983;29:455–463. doi: 10.1095/biolreprod29.2.455. [DOI] [PubMed] [Google Scholar]
- 16.Perrigo G, Bronson F. Physiol Behav. 1985;34:437–440. doi: 10.1016/0031-9384(85)90208-2. [DOI] [PubMed] [Google Scholar]
- 17.Perrigo G, Bronson F. Behav Ecol Sociobiol. 1985;17:297–302. [Google Scholar]
- 18.Nelson R J. Physiol Behav. 1990;48:403–408. doi: 10.1016/0031-9384(90)90335-2. [DOI] [PubMed] [Google Scholar]
- 19.Li H-Y, Wade G, Blaustein J. Endocrinology. 1994;135:240–247. doi: 10.1210/endo.135.1.8013358. [DOI] [PubMed] [Google Scholar]
- 20.Schneider J, Wade G. Science. 1989;246:1326–1329. doi: 10.1126/science.2734610. [DOI] [PubMed] [Google Scholar]
- 21.Wade G, Schneider J, Li H-Y. Am J Physiol. 1996;270:E1–E19. doi: 10.1152/ajpendo.1996.270.1.E1. [DOI] [PubMed] [Google Scholar]
- 22.Czech D A. Physiol Behav. 1988;43:765–769. doi: 10.1016/0031-9384(88)90374-5. [DOI] [PubMed] [Google Scholar]
- 23.Siegel S. Nonparametric Statistics. New York: McGraw–Hill; 1956. [Google Scholar]
- 24.Fregly M. In: Thermoregulation: Physiology and Biochemistry. Schonbaum E, Lomax P, editors. New York: Pergamon; 1990. pp. 437–494. [Google Scholar]
- 25.Hefco E, Krulich L, Illner P, Larsen P. Endocrinology. 1975;97:1185–1195. doi: 10.1210/endo-97-5-1185. [DOI] [PubMed] [Google Scholar]
- 26.Rothwell N. In: Thermoregulation Physiology and Chemistry. Schonbaum E, Lomax P, editors. New York: Pergamon; 1990. pp. 316–319. [Google Scholar]
- 27.Arancibia S, Tapia-Arancibia L, Assenmacher I, Astier H. Neuroendocrinology. 1983;37:225–228. doi: 10.1159/000123547. [DOI] [PubMed] [Google Scholar]
- 28.Gabaldon A, Florez-Duquet M, Hamilton J, McDonald R, Horwitz B. Am J Physiol Regulatory Integrative Comp Physiol. 1995;268:R931–R941. doi: 10.1152/ajpregu.1995.268.4.R931. [DOI] [PubMed] [Google Scholar]
- 29.Clark A, Pfeiffle J, Edwards D. Physiol Behav. 1981;27:597–602. doi: 10.1016/0031-9384(81)90228-6. [DOI] [PubMed] [Google Scholar]
- 30.Edwards D, Pfeiffle J. Physiol Behav. 1981;26:1061–1067. doi: 10.1016/0031-9384(81)90210-9. [DOI] [PubMed] [Google Scholar]
- 31.Sakuma Y. Horm Behav. 1994;28:438–444. doi: 10.1006/hbeh.1994.1041. [DOI] [PubMed] [Google Scholar]
- 32.Sinnamon H. Neuroscience. 1992;50:197–207. doi: 10.1016/0306-4522(92)90392-f. [DOI] [PubMed] [Google Scholar]
- 33.Marsteller F A, Lynch C B. Biol Reprod. 1987;37:838–843. doi: 10.1095/biolreprod37.4.838. [DOI] [PubMed] [Google Scholar]
- 34.Marsteller F A, Lynch C B. Biol Reprod. 1987;37:844–850. doi: 10.1095/biolreprod37.4.844. [DOI] [PubMed] [Google Scholar]
- 35.Barnes B, Licht P, Zucker I. Can J Zool. 1987;65:3020–3023. [Google Scholar]
- 36.Dark J, Miller D, Licht P, Zucker I. Am J Physiol Regulatory Integrative Comp Physiol. 1996;270:R398–R403. doi: 10.1152/ajpregu.1996.270.2.R398. [DOI] [PubMed] [Google Scholar]
- 37.Dark J, Miller D, Zucker I. Am J Physiol Regulatory Integrative Comp Physiol. 1994;267:R496–R501. doi: 10.1152/ajpregu.1994.267.2.R496. [DOI] [PubMed] [Google Scholar]
- 38.Gavrilova O, Leon L, Marcus-Samuels B, Mason M, Castle A, Refetoff S, Vinson C, Reitman M. Proc Natl Acad Sci USA. 1999;96:14623–14628. doi: 10.1073/pnas.96.25.14623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stamper J, Zucker I, Lewis D, Dark J. Am J Physiol Regulatory Integrative Comp Physiol. 1998;43:R46–R51. doi: 10.1152/ajpregu.1998.274.1.R46. [DOI] [PubMed] [Google Scholar]