Abstract
Cardiac afferents are sensory neurons that mediate angina, pain that occurs when the heart receives insufficient blood supply for its metabolic demand (ischemia). These neurons display enormous acid-evoked depolarizing currents, and they fire action potentials in response to extracellular acidification that accompanies myocardial ischemia. Here we show that acid-sensing ion channel 3 (ASIC3), but no other known acid-sensing ion channel, reproduces the functional features of the channel that underlies the large acid-evoked current in cardiac afferents. ASIC3 and the native channel are both especially sensitive to pH, interact similarly with Ca2+, and gate rapidly between closed, open, and desensitized states. Particularly important is the ability of ASIC3 and the native channel to open at pH 7, a value reached in the first few minutes of a heart attack. The steep activation curve suggests that the channel opens when four protons bind. We propose that ASIC3, a member of the degenerin channel (of Caenorhabditis elegans)/epithelial sodium channel family of ion channels, is the sensor of myocardial acidity that triggers cardiac pain, and that it might be a useful pharmaceutical target for treating angina.
Chest pain occurs when the heart receives insufficient oxygen because of coronary artery blockade or disease. Thomas Lewis proposed that the pain is caused by the buildup of compounds, released from metabolically stressed muscle, that then act on cardiac afferents, the sensory neurons that innervate the heart (1, 2). Lactic acid, a primary metabolite released from oxygen-deprived muscle, is among the candidate compounds, because adding strong pH buffer to the pericardial space suppresses firing of cardiac afferents in response to coronary artery occlusion, and lactic acid evokes pain behavior (3, 4). Myocardial extracellular pH drops to 6.7 during severe cardiac ischemia and is unlikely to drop further without being fatal (5, 6). On the other hand, pH 7.2 occurs in various kinds of systemic acidosis without causing chest pain. Thus, the acid sensor for cardiac pain must respond within this critical range of extracellular pH. The goal of the present work was to find the molecular identity of this sensor.
We previously showed that one population of sensory neurons that innervates the heart responds to acidity by generating extraordinarily large Na+ currents (7). These neurons, called sympathetic cardiac afferents, have cell bodies in the upper thoracic dorsal root ganglia (C8–T3) and axons that follow sympathetic nerve trunks to innervate the epicardium, the outer layer of the heart (8). Sympathetic afferents are distinct from parasympathetic cardiac afferents, which also innervate the heart but have cell bodies in the nodose ganglia and axons that follow the vagus nerve. Because surgical dissection of sympathetic cardiac afferents relieves angina, they are considered the mediators of cardiac pain (but see ref. 9 for evidence that parasympathetic afferents also contribute to pain). In addition to evoking pain, these neurons trigger a sympathetic reflex response to cardiac ischemia that is counterproductive because it causes stronger and faster contraction at a time when the heart is getting insufficient oxygen (10–12). Thus, suppression of sympathetic cardiac afferents might relieve both pain and a damaging reflex that accompanies it.
We showed that both sympathetic and parasympathetic cardiac afferents have acid-evoked currents, but the currents have dramatically (about 10-fold) greater amplitude in the sympathetics (7). The currents are blocked by amiloride and pass Na+ better than K+. This behavior of the currents identifies them as being carried by acid-sensing ion channels (ASICs) and distinguishes them from another type of proton-gated channel, vanilloid receptors. Krishtal's group identified ASICs as several kinetically distinct, Na+-selective, Ca2+-permeant currents in sensory neurons that are evoked by lowered pH and are blocked by amiloride (13–16). Lazdunski's group found that the currents are carried by a proton-sensitive subfamily of channels within the larger family of epithelial Na+ channels and degenerins of Caenorhabditis elegans (17). At present, the ASIC family has five members in rat: ASIC1a (17, 18), ASIC1b (19), ASIC2a (18, 20–22), ASIC2b (22), and ASIC3 (23) (nomenclature as in ref. 24). The proteins are small (≈500 aa) with two putative transmembrane domains, and several subunits are required to form functional channels (25).
Sensory ganglia are richly endowed with ASIC mRNAs. The mRNA for four of the five family members (all but ASIC2a) are detected in sensory ganglia (26), and two (1b and 3, also called ASIC-β and DRASIC, respectively) are only in sensory ganglia (19, 23). We sought to find which, if any, of these clones forms the ion channel responsible for the large acid-gated currents in sympathetic cardiac afferents. There is no pharmacological agent that distinguishes the different ASICs. Therefore, we measured eight different functional properties of the native cardiac afferent channel and compared these to each cloned ASIC expressed in COS-7 cells. ASIC3 matched the native currents in all parameters, many of which excluded the other ASIC subtypes.
Methods
Electrophysiology.
All experiments used the whole-cell patch–clamp method, except for measurements of activation rate, which used the outside-out patch method. Recordings were made with an EPC-9 amplifier (HEKA Electronics, Lambrecht, Germany). Extracellular solutions were changed within 5 ms, in patch recordings, or 20 ms, in whole-cell recordings, by using a computer-driven solenoid valve system (7). Recordings were made at −70 mV unless otherwise stated. Micropipettes were pulled from borosilicate glass (no. 7052; Garner Glass, Claremont, CA) to 1–5-MΩ resistance.
The standard internal solution contained (in mM) 100 KCl, 10 EGTA, 40 Hepes, 5 MgCl2, 2 Na2ATP, and 0.3 Na3GTP, adjusted to pH 7.4 with KOH. The standard external solution contained (in mM) 130 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 Hepes, 10 Mes, with the pH adjusted to 8.0, 7.4, 7.0, 6.8, 6.5, 6.0, 5.5, 5.0, or 4.0 with tetramethylammonium hydroxide and the osmolarity adjusted with tetramethylammonium chloride. In the Ca2+ block experiments, standard solution was used, except that both control (pH 8) and test (pH 6) solutions contained 0.5, 1, 2, or 10 mM CaCl2. In the Cs+ selectivity experiments, CsCl replaced NaCl in both control (pH 7.4) and test (pH 5) solutions.
For Ca2+ permeability experiments, the internal solution contained (in mM) 90 N-methyl glucamine, 10 NaCl, 2 Na2ATP, 0.3 Na3GTP, 10 EGTA, 5 MgCl2, 40 Hepes, with the pH adjusted to 7.4 with HCl. The external solution contained (in mM) 120 N-methyl glucamine, 10 Hepes, 10 Mes, and 10 CaCl2, adjusted to pH 7.4 or 6.0 with HCl. Voltage steps were made to −40, 0, 40, and 80 mV for 7 s, during which a 4-s pulse of pH 6.0 was applied. PNa/PCa was determined from the reversal potential, by using Erev = (RT/2F)ln{4PCa[Ca2+]o/PNa[Na+]i}. PNa/PK was determined in standard solutions (control pH 7.4, test pH 5.0) from the reversal potential, by using Erev = (RT/F)ln{(PNa[Na+]o + PK[K+]o)/(PNa[Na+]i + PK[K+]i)}. Time courses were fit with single exponentials, by using HEKA PulseFit software. Activation curves were fit with the Hill equation, Fraction open = [H+]n/([H+]n + K0.5n), by using nfit (University of Texas, Galveston, TX), where K0.5 is the proton concentration that causes half the channels to open. All data are reported as the average ± SEM.
Cell Culture and Transfection.
Cardiac sympathetic afferents were labeled in vivo and prepared as previously described (7). Briefly, about 4 weeks after 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine was placed in the pericardial space, dorsal root ganglia from the level of C8–T3 were dissociated with papain, collagenase, and dispase plated on laminin-coated plastic, and stored at room temperature in L15 medium supplemented with 50 nM nerve growth factor. The mechanosensor neurons were prepared from the mesencephalic nucleus of the trigeminal nerve as previously described (27). Briefly, cells were dissociated with papain, plated on a bed of glial cells, and stored at 37°C in F12 medium supplemented with 50 nM neurotrophin 3 and glial cell line-derived neurotrophic factor. Most recordings from neurons were made the day after dissociation, and none were made after 3 days; we saw no evident change in currents in this time.
All ASIC clones were kindly provided by R. Waldmann and M. Lazdunski (Institut de Pharmacologie Moléculaire et Cellulaire, CNRS Valbonne, France). Our sequence analysis of the ASIC1b clone used in this study differs from the GenBank ASIC-β sequence (accession no. AJ006519) at one residue: a threonine instead of a serine at position 82. ASIC clones were transfected into a line of COS-7 cells that we found had less than 100 pA of acid-evoked current at pH 5 and no transient acid-evoked current. The COS-7 cells were cultured in DMEM media with 10% heat-inactivated FCS (GIBCO) and 1% Pen/Strep (GIBCO). Cells at about 50% confluence were transfected by using lipofectin reagent (GIBCO/BRL no. 18292) with DNA for various ASICs and for the CD4 receptor in the pcDNA3 vector (Invitrogen). All recordings were made 24–72 h later; transfected cells were identified with CD4-coated microbeads (Dynal no. 111.05).
Results
Extreme Size and Sensitivity of Acid-Gated Currents in Cardiac Afferents.
We fluorescently labeled cardiac afferents in rats by placing a lipid-soluble dye (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine) in the pericardial space (methods described in ref. 7). The dye intercalates into membranes of nerve endings in the epicardium and becomes endocytosed, and the resulting fluorescent vesicles are transported to the neurons' cell bodies. Upper thoracic dorsal root ganglia are dissected and dissociated about 4 weeks after dye placement, and the sympathetic cardiac afferents are distinguished from other kinds of sensory neurons by fluorescence. To highlight the unique properties of the cardiac afferents, they are compared in Fig. 1 to nonnociceptive, low-threshold mechanosensors (sensors of either fine touch or muscle length) that are isolated from the mesencephalic nucleus of the trigeminal tract (methods previously described in ref. 27).
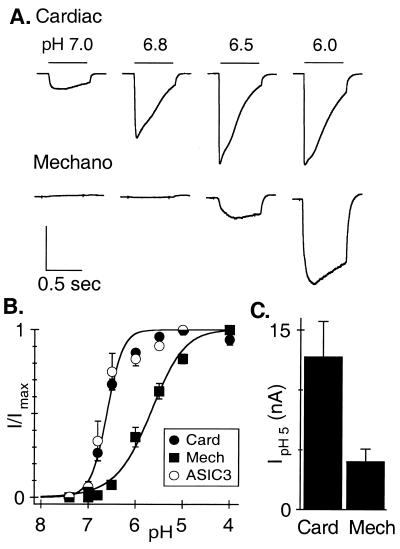
Figure 1.
Cardiac afferents have larger and more sensitive acid-evoked currents than do nonnociceptive mechanosensors. (A) Currents evoked from a cardiac afferent and a mechano-sensing afferent by pulses to the indicated pH from pH 8. Vertical scales: 8 nA (Upper), 1 nA (Lower). Horizontal scale: 500 ms. (B) Average (± SEM) fractional current vs. pH. Solid lines are fits of the Hill equation for cardiac afferents (filled circles, n = 14, normalized to current evoked by pH 5) and mechanosensors (squares, n = 7, normalized to current evoked by pH 4). COS-7 cells expressing ASIC3 show an identical dose-response curve to that of cardiac afferents (open circles, pH0.5 = 6.6, n = 11, normalized to current evoked by pH 5, curve fit not shown). (C) Average amplitude of currents evoked by pH 5 in cardiac afferents (12.8 nA) is over 3 times larger than in mechanosensors (4.04 nA). Data are from the same cells used in B.
The pH of solutions flowing onto individual dissociated neurons was changed from 8.0 to the indicated value (Fig. 1A) for 600 ms (20-ms delay time for solution exchange). The currents activated by these pH changes were dramatically larger in cardiac afferents than in mechanosensors (note the 8-fold difference in the vertical scales), and cardiac afferents responded at a 3-fold lower proton concentration. Normalized peak currents are plotted against the activating pH and fit with the Hill equation (Fig. 1B). Half-activation of the cardiac afferent channel occurs at about pH 6.6, and it clearly opens at pH 7, a value reached within the first few minutes of severe cardiac ischemia (3, 5, 6). The average maximal acid-sensing current in the cardiac afferents was 13 nA, over three times larger than in the mechanosensors (Fig. 1C). Acid-evoked currents in cardiac afferents routinely reach well over 20 nA, certainly the largest depolarizing current in rat sensory neurons and among the largest in the rat nervous system. Because the current has extreme amplitude in neurons specialized to detect cardiac ischemia and has the correct proton sensitivity to detect the pH changes that occur, it likely is the sensor that triggers acid-evoked cardiac pain.
Only ASIC3 Mimics the Acid-Evoked Current in Cardiac Afferents.
In considering whether a known ASIC clone might generate the current in cardiac afferents, we ruled out two subtypes on the basis of published data: ASIC2b does not form a functional channel as a homomer (22), mRNA for ASIC2a is reported to be absent from sensory ganglia (26), and we confirmed that ASIC2a channels require nonphysiological acidity (pH 5) to open. Therefore, we asked whether the characteristics of the native channel in cardiac afferents could be matched when ASIC1a, 1b, or 3 is expressed in COS-7 cells alone or in combination with ASIC2b.
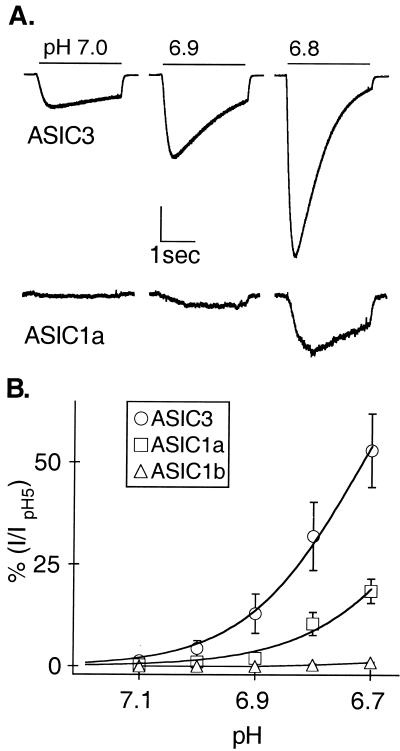
The sensitivity of ASIC3 to protons precisely mimics the cardiac afferent channel (Fig. 1B, open symbols). The other ASICs are either slightly less (ASIC1a) or much less (ASIC1b) sensitive to protons (Table 1). Because the critical range for cardiac pain is pH 7.1 to 6.7 (5, 6), Fig. 2 explores the sensitivity of the various clones in this range. ASIC3 is the most sensitive (pH0.5 = 6.7). Its activation curve is best fit with a Hill coefficient of 4.3, which suggests that at least four protons bind to the channel to open it. ASIC1a has similar steepness (Hill coefficient 3.9), but with slightly lower apparent proton binding affinity (pH0.5 = 6.4). ASIC1b does not respond in this physiological pH range (Fig. 2B and Table 1).
Table 1.
ASIC3 matches the native cardiac afferent channel in all functional properties, whereas the other ASIC subtypes do not
| Functional property | Cardiac | ASIC3 | ASIC1a | ASIC1b |
|---|---|---|---|---|
| pH0.5 activation | 6.6 | 6.7 | 6.4 | 5.9 (19) |
| pH0.5 desensitization | 7.2 (7) | 7.1 | 7.3 | |
| τ act. (msec, at pH 6) | <5 | <5 | 13.7 ± 3.5* | |
| τ densens. (sec, at pH 6) | 0.35 ± 0.04 | 0.32 ± 0.07 | 3.5 ± 0.39* | 1.7 ± 0.27* |
| τ recovery (sec, at pH 7.4) | 0.61 | 0.58 | 13 | 5.9 |
| % block by 10 mM Ca2+ | 44 ± 7.3 | 34 ± 9.2 | 82 ± 4.9* | 83 ± 2.5* |
| I30Ca/I10Ca | 3.1 ± 0.77 | 2.5 ± 0.42 | 0.99 ± 0.16* | |
| PNa/PK | 6.8 (7) | 4.5 | 5.5 | 2.6 (19) |
| IC50 amiloride, μM | 37 | 63 (23) | 10 (17) | 21 (19) |
Asterisks are means that differ from the cardiac afferent with greater than 99% certainty (two-tailed t-test, n = 3–6). Values without standard errors are derived from curve fits (n = 3–12). I30Ca/I10Ca is the ratio of peak amplitudes when Ca2+, at either 30 mM or 10 mM, is the only permeant ion. Numbers obtained from the literature are indicated by reference numbers in parentheses; we confirmed similar results from our clones. Activation rates were measured in outside-out patches; all others were made by using the whole-cell configuration. The different IC50s for amiloride likely reflect use of different stimulating pH in different studies; for example, the IC50s for the cardiac afferent channel are 40 μM and 10 μM when measured at pH 5 and pH 6.8, respectively. Because of the wide range in the measurements of PNa/PK, we do not consider the differences in averages to be real. PNa/PK ranged from 1.99 to 6.42 for ASIC3 (n = 6) and from 3.64 to 12.04 for ASIC1a (n = 6), all measured at pH 5.
Figure 2.
ASIC3 responds to protons in the neutral range. (A) Currents evoked from COS-7 cells expressing either ASIC3 or ASIC1a by steps to pH 7, 6.9, and 6.8. Vertical scale bar represents 10% of current evoked by pH 5 (2 nA ASIC3; 46 pA ASIC1a). Horizontal scale bar: 1 s. (B) Average currents of the indicated clones normalized to the value at pH 5.0 and fit with the Hill equation. Hill coefficients are 4.3 (ASIC3, pH0.5 6.7) and 3.9 (ASIC1a, pH0.5 6.4).
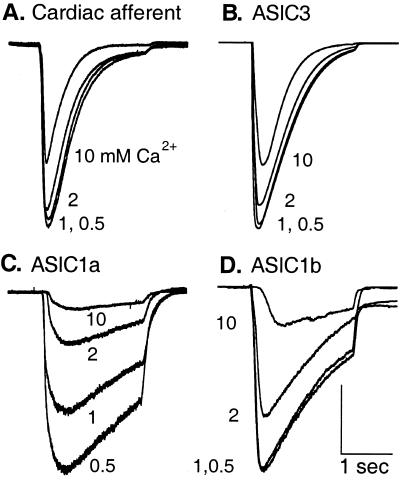
Fig. 3 compares the kinetics and calcium inhibition of current from cardiac afferents to those of cloned channels. Steps to pH 6.0 were made with solutions having the indicated Ca2+ concentrations. The native channel and ASIC3 share essentially the same time course, whereas the other channels open and desensitize more slowly (see Table 1 for time constants). Increasing Ca2+ concentration inhibits all of the currents, but the native channel and ASIC3 are relatively insensitive: they are only slightly affected by 2 mM Ca2+ and about 40% inhibited by 10 mM at pH 6.
Figure 3.
Only ASIC3 mimics kinetics and Ca2+ inhibition of cardiac afferents. Representative currents were evoked from a cardiac afferent (A) or from COS-7 cells expressing ASIC3 (B), ASIC1a (C), or ASIC1b (D) by steps of pH from 8.0 to 6.0 in the indicated extracellular Ca2+ concentrations (mM). Cardiac and ASIC3 channels parallel each other in activation kinetics, desensitization kinetics, and Ca2+ inhibition. Vertical scales: 1.1 nA (A), 14 nA (B), 1.5 nA (C), and 2 nA (D). Horizontal scales: 1 s for all traces.
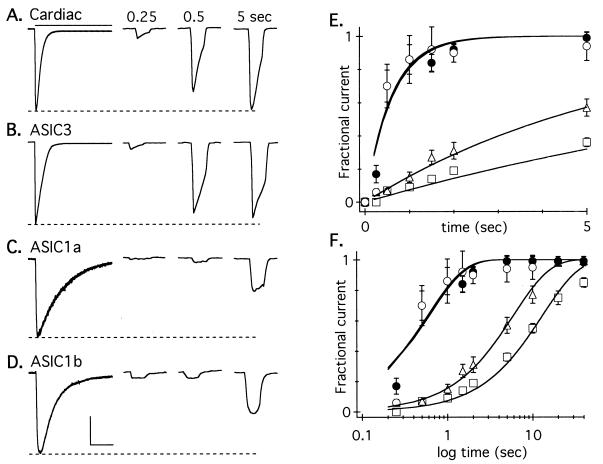
Recovery from desensitization also clearly distinguished ASIC3 from the other clones. Cells were exposed to prolonged (7–10 s) applications of pH 6, which caused complete current desensitization (leftmost traces in Fig. 4 A–D) and were then returned to pH 7.4. At various times thereafter, cells received a brief (600-ms) application of pH 6 to test the fraction of channels that had recovered from desensitization. The native channel and ASIC3 both recovered very rapidly compared with the other channels. The average recovered peak current relative to the initial peak value is plotted against the time of recovery for the first 5 s (linear scale, Fig. 4E) and 40 s (log scale, Fig. 4F). Single exponentials fit to the data have time constants of 0.61 and 0.58 s for the native channel and ASIC3, and 13 and 6 s for ASIC1a and ASIC1b, respectively.
Figure 4.
Only ASIC3 mimics recovery kinetics of cardiac afferents. Current was completely desensitized by a prolonged pulse to pH 6.0 (bar), and then the cell was returned to pH 7.4. Recovery from desensitization was tested by a 600-ms pH pulse triggered at the indicated times. (Only one pulse was given after each desensitizing prepulse, thereby avoiding accumulating desensitization; peak current occurs early in the 600-ms pulse, and this time to peak was not added to the recovery time.) Currents in a cardiac afferent (A) and ASIC3 (B) recovered rapidly; currents in ASIC1a (C) and 1b (D) recovered slowly. Horizontal scales: 2 s for desensitizing currents (leftmost), 1 s for test currents. Vertical scales: 1 nA (A), 1.25 nA (B), 0.45 nA (C), and 0.5 nA (D). The recovery of average (± SEM) currents, normalized to initial amplitude, for the first 5 s (E) (linear) and 40 s (F) (log scale) is given. Solid lines are fits of single exponentials. ●, cardiac afferents (τ = 0.61 s, n ≥ 4 for each data point); ○, ASIC3 (τ = 0.58 s, n ≥ 5); □, ASIC1a (τ = 12.99 s, n ≥ 4); ▵, ASIC1b (τ = 5.88 sec, n ≥ 3).
The above data rule out ASIC1a and 1b and leave ASIC3 as a candidate for the native channel. To further test ASIC3, we made a number of other measurements, which are summarized in Table 1. All channel types share two traits: selection of Na+ over K+ and block by fairly high concentrations of amiloride. However, the following six properties of the native channel are shared only by ASIC3: high proton sensitivity, rapid rates of activation, desensitization, and recovery, weak Ca2+ inhibition, and low but detectable Ca2+ permeability (see next section). Because all these functional features match in near-perfect detail, we conclude that ASIC3 is the major component of the native acid-gated channel in cardiac afferents.
Ca2+ Permeability of ASICs.
Our measurements reveal some complexities of Ca2+ permeation and inhibition that deserve attention. Both ASIC3 and the native channel passed inward currents when 10 mM Ca2+ was the only external permeant ion. The amplitude of these currents increased by about 3-fold in 30 mM extracellular Ca2+, confirming that both channels can pass Ca2+ (Table 1). Current through ASIC1a did not increase with this solution change, but this need not imply low Ca2+ permeability. Indeed, Waldmann et al. (17) reported substantial Ca2+ permeability in ASIC1a with 1.8 mM Ca2+ as the current carrier. To investigate this result, we measured the voltage at which current changed from inward to outward when the current carriers were 10 mM external Ca2+ and 15 mM internal Na+. These reversal potentials (−23 ± 7 mV for ASIC1a, −80 ± 2 mV for ASIC3) indicate that ASIC1a is substantially more permeable to Ca2+ than is ASIC3 (PNa/Pca = 16 for ASIC1a; PNa/Pca > 100 for ASIC3). The native channel also has low Ca2+ permeability under these ionic conditions (PNa/Pca > 100). The inhibition, permeation, and reversal potential measurements are consistent with the presence of an intrapore binding site in ASIC1a that is fully saturated at 10 mM Ca2+ and that allows Ca2+ to compete effectively with Na+ for occupancy of the pore. A relatively high Ca2+ permeability appears unique to ASIC1a, as there is no apparent Ca2+ permeation in ASIC1b (19). We found greater Ca2+ inhibition than previously reported (19) for ASIC1b. Perhaps this greater Ca2+ inhibition is due to the fact that our measurements were made at pH 6, whereas the published measurements were made at pH 5.1. An alternative explanation is the single amino acid difference between our clone and the sequence deposited in GenBank (see Methods). Because pH sensitivity and gating kinetics sufficiently distinguish both forms of ASIC1b from the native channel (Table 1), we did not investigate these issues further.
A Sustained Current That Is Unidentified.
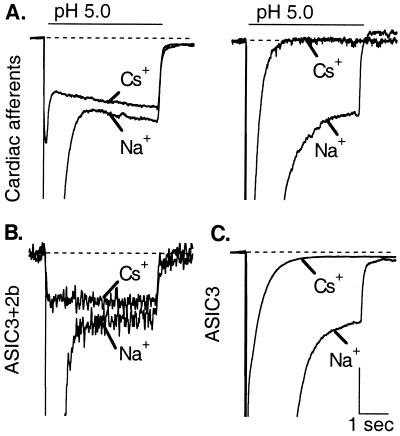
Acid-sensing ion channels select Na+ over other ions (13, 28), but prolonged application of very low pH (≤ 5.0) evokes a distinctly different current in some sensory neurons. This current is sustained, is carried equally by various monovalent cations, and is not blocked by amiloride (29). We previously described such a current in cardiac afferents with an apparent half-activation at pH 3.7 (7). However, Fig. 5A shows that this sustained current has different properties in different cardiac afferents. In all cases, its amplitude is small compared with the transient current (Isust/Itrans = 0.14 ± 0.04 at pH 5, n = 13), but its selectivity varies in different cells. In response to prolonged (4 s) application of pH 5, the cardiac afferent on the left of Fig. 5A had a sustained current that passed Cs+ about as well as Na+, whereas the one on the right had a sustained current that was Na+ selective. Nine of 13 cardiac afferents surveyed with this protocol exhibited some degree of Cs+ permeability to the sustained current, but the Cs+/Na+ current ratio varied unpredictably. In all cases, the transient current (far off scale with Na+ as the current carrier in Fig. 5) was greatly diminished when Cs+ was the current carrier, indicating its high Na+ selectivity.
Figure 5.
Variable expression of a sustained, nonselective current in cardiac afferents is consistent with variable expression of ASIC2b. Peak currents are off-scale to emphasize the smaller sustained component. (A) Currents, evoked by steps to pH 5 for 4 s, from two cardiac afferents that display sustained components that differ in selectivity. (Left) The sustained component is nonselective (passes Cs+ and Na+ equally). (Right) The sustained component is Na+ selective. (B) Coexpression of ASIC3 and 2b yields a nonselective sustained component. (C) The ASIC3 homomer yields a Na+-selective sustained component. Vertical scales: 600 pA (A Left), 150 pA (A Right), 35 pA (B), 500 pA (C). Horizontal scale: 1 s.
ASIC3 homomers have a Na+-selective sustained current (23), but if ASIC3 is coexpressed with ASIC2b, a nonselective current appears, presumably because of ASIC3/2b heteromer formation (22). Fig. 5 (B and C) reproduces these observations. So, a possible explanation of the variable sustained current in Fig. 5A is that ASIC3 is highly expressed in all cardiac afferents, whereas ASIC2b is expressed in some, but not all. As discussed below, this difference in expression between ASIC3 and ASIC2b is not the only possible mechanism.
Discussion
Extracellular acid appears to be a significant part of the signal that triggers pain during cardiac ischemia (3, 4), and rat cardiac sensory neurons display extremely large Na+ currents through an acid-sensing ion channel (7). Because the ion channels that mediate this current are probably molecular sensors for cardiac ischemic pain, we attempted to determine which, if any, of the five cloned ASICs mediates the acid sensitivity of rat cardiac sensory neurons. Our results point to ASIC3 for the following reasons: (i) the native channel and ASIC3 both open at pH 7, whereas other ASICs are less sensitive to protons (some dramatically so); (ii) the native channel and ASIC3 both gate between closed, open, and desensitized states clearly faster than other ASICs; (iii) the native channel and ASIC3 share aspects of permeation to and inhibition by Ca2+ that are unlike the other ASICs.
The high proton sensitivity is perhaps the most critical of these features. During coronary artery blockade, extracellular pH drops to neutral in a few minutes but is unlikely to drop below about pH 6.7 (3, 5, 6). Such moderate pH changes readily open ASIC3 channels, which have a very steep activation curve consistent with a requirement that four protons bind to open the channel. This requirement makes the channel 4-fold more sensitive than a pH electrode to protons over the range of pH that changes during cardiac ischemia. Perhaps coincidentally, most evidence indicates that channels in this family are tetramers (30–32), although other evidence argues for different numbers of subunits (33).
ASIC3 mRNA is abundant in rat sensory ganglia and scarce elsewhere (19, 23). Moreover, we show in Fig. 1 that low threshold mechanosensors (cells uninvolved with detecting ischemia) have no functional ASIC3 channels, whereas cells specialized to sense cardiac ischemia are enriched in them. Such specificity in expression suggests that ASIC3 would be a useful pharmaceutical target for selectively suppressing angina and maybe other forms of ischemic pain. The human homolog (84% sequence identity) of ASIC3 has been cloned and displays high proton sensitivity (34), but there is no information yet about its expression pattern in humans.
One property of the response of cardiac afferents to pH (a small, nonselective, amiloride-insensitive conductance seen at pH 5 in some, but not all, cardiac afferents) is not mimicked when ASIC3 is expressed alone. It is mimicked on coexpression of ASIC3 and 2b, as previously reported by Lingueglia et al. (22), and the two subunits coimmunoprecipitate when cotransfected (35). However, we cannot conclude that cardiac afferents express ASIC heteromers because other channel types could generate such currents. For example, vanilloid receptors generate sustained, nonselective currents at such extreme pH (36) and are present at low levels in cardiac afferents (7). Furthermore, the extreme acidity required to evoke the sustained current might modify other ion channels to generate such small nonselective currents. We are pessimistic that a molecular identity can be provided to this current that varies in expression and has no kinetics, no selectivity, and no pharmacology. Moreover, because the sustained current occurs only at extremely low pH, its relevance to cardiac ischemia is questionable.
Roles for Other Channels.
There are several reasons to hesitate to conclude that ASIC3 is the sole sensor for cardiac ischemic pain. First, acidity is only one of a number of signals that lead to cardiac pain, and acidity can activate molecules besides ion channels (see below). Second, we previously reported that a few cardiac afferents exhibit a less sensitive acid-evoked current with smaller amplitude and slower kinetics than the large, transient current that we studied here (7). Thus, several channels may sense acid in rat cardiac afferents, ASIC3 being the most abundant. It is also possible that ASIC3 forms heteromers with other ASIC subtypes if these heteromers have the same properties as the ASIC3 homomer. A final issue concerns an inherent limit in our study: we can only compare the native channel to cloned channels. Could the native channel be an uncloned ASIC rather than ASIC3? We consider this unlikely because each of the known channels has a distinct fingerprint of functional properties that is unmatched by the others. The variability of these properties among the known channels argues against an unknown channel with the precise properties of ASIC3 and, thus, the precise properties of the native channel.
Search for the Mediator of Ischemic Pain.
Although our efforts have focused on acid as a mediator of ischemic pain, there are other important candidates that should not be ignored. Adenosine is released from cells whenever there is a decline in intracellular ATP. There is conflicting literature about adenosine action on cardiac afferents, with some groups supporting a role in cardiac pain (37–39) and others not (40, 41). Bradykinin increases firing of cardiac afferents in vivo (40, 42) by acting at B2 receptors (43). It is cleaved from kininogens by the enzyme kallikrein, which can be triggered by a drop in pH. Thus, kallikrein is an acid sensor that generates a pain signal, bradykinin, that appears more slowly than ASIC3 current but might persist longer [tissue half-life of 15 s (44)]. Among other suggested mediators of cardiac pain are serotonin (45), ATP (46), histamine (47), and oxygen radicals (48). It may be possible to test in intact animals the relative importance of ASICs in cardiac pain by using amiloride; however, the concentrations necessary to block ASICs are very high, roughly 100-fold more than those used to block ENaCs.
Defining the mediator of ischemic pain, identifying the sensor, and finding a blocker are important for several reasons. First, angina is common: 12 million people in the United States have coronary artery disease, and 6 million suffer from chronic angina. Second, a drug that inhibits cardiac sympathetic afferents may be cardioprotective by suppressing the injurious sympathetic hyperactivity that accompanies chronic cardiac ischemia (10, 12). Finally, if, as suggested by Thomas Lewis (1), cardiac and skeletal ischemic pains are triggered by the same mechanism, a blocker might be useful against other vasocclusive pain such as sickle cell anemia. Its properties and expression pattern suggest that ASIC3 is a good target for developing drugs that are selective against ischemic pain.
Acknowledgments
We thank Drs. R. Waldmann and M. Lazdunski for kindly providing ASIC clones and Drs. D. Immke and S. Cook for reviewing the manuscript and figures.
Abbreviations
- ASIC
acid-sensing ion channel
- ENaC
epithelial sodium channel
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 395.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.011404498.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.011404498
References
- 1.Lewis T. Arch Intern Med (Moscow) 1932;49:713–727. [Google Scholar]
- 2.Armour J A. Cardiovasc Res. 1999;41:41–54. doi: 10.1016/s0008-6363(98)00252-1. [DOI] [PubMed] [Google Scholar]
- 3.Pan H L, Longhurst J C, Eisenach J C, Chen S R. J Physiol (London) 1999;518:857–866. doi: 10.1111/j.1469-7793.1999.0857p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uchida Y, Murao S. Am J Physiol. 1975;228:27–33. doi: 10.1152/ajplegacy.1975.228.1.27. [DOI] [PubMed] [Google Scholar]
- 5.Cobbe S M, Poole-Wilson P A. J Mol Cell Cardiol. 1980;12:745–760. doi: 10.1016/0022-2828(80)90077-2. [DOI] [PubMed] [Google Scholar]
- 6.Cobbe S M, Poole-Wilson P A. Br Heart J. 1982;47:369–374. doi: 10.1136/hrt.47.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benson C J, Eckert S P, McCleskey E W. Circ Res. 1999;84:921–928. doi: 10.1161/01.res.84.8.921. [DOI] [PubMed] [Google Scholar]
- 8.Foreman R D. Annu Rev Physiol. 1999;61:143–167. doi: 10.1146/annurev.physiol.61.1.143. [DOI] [PubMed] [Google Scholar]
- 9.Meller S T, Gebhart G F. Neuroscience. 1992;48:501–524. doi: 10.1016/0306-4522(92)90398-l. [DOI] [PubMed] [Google Scholar]
- 10.Malliani A. Basic Res Cardiol. 1990;85:243–252. doi: 10.1007/978-3-662-11038-6_20. [DOI] [PubMed] [Google Scholar]
- 11.Rendig S V, Chahal P S, Longhurst J C. Am J Physiol. 1997;272:H791–H796. doi: 10.1152/ajpheart.1997.272.2.H791. [DOI] [PubMed] [Google Scholar]
- 12.Leenen F H. Can J Cardiol. 1999;15, Suppl. A:2A–7A. [PubMed] [Google Scholar]
- 13.Krishtal O A, Pidoplichko V I. Neuroscience. 1980;5:2325–2327. doi: 10.1016/0306-4522(80)90149-9. [DOI] [PubMed] [Google Scholar]
- 14.Krishtal O A, Pidoplichko V I. Brain Res. 1981;214:150–154. doi: 10.1016/0006-8993(81)90446-7. [DOI] [PubMed] [Google Scholar]
- 15.Korkushko A, Krishtal O. Neirofiziologiia. 1984;16:557–561. [PubMed] [Google Scholar]
- 16.Kovalchuk Yu N, Krishtal O A, Nowycky M C. Neurosci Lett. 1990;115:237–242. doi: 10.1016/0304-3940(90)90461-h. [DOI] [PubMed] [Google Scholar]
- 17.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. Nature (London) 1997;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Anoveros J, Derfler B, Neville-Golden J, Hyman B T, Corey D P. Proc Natl Acad Sci USA. 1997;94:1459–1464. doi: 10.1073/pnas.94.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen C C, England S, Akopian A N, Wood J N. Proc Natl Acad Sci USA. 1998;95:10240–10245. doi: 10.1073/pnas.95.17.10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price M P, Snyder P M, Welsh M J. J Biol Chem. 1996;271:7879–7882. doi: 10.1074/jbc.271.14.7879. [DOI] [PubMed] [Google Scholar]
- 21.Waldmann R, Champigny G, Voilley N, Lauritzen I, Lazdunski M. J Biol Chem. 1996;271:10433–10436. doi: 10.1074/jbc.271.18.10433. [DOI] [PubMed] [Google Scholar]
- 22.Lingueglia E, de Weille J R, Bassilana F, Heurteaux C, Sakai H, Waldmann R, Lazdunski M. J Biol Chem. 1997;272:29778–29783. doi: 10.1074/jbc.272.47.29778. [DOI] [PubMed] [Google Scholar]
- 23.Waldmann R, Bassilana F, de Weille J, Champigny G, Heurteaux C, Lazdunski M. J Biol Chem. 1997;272:20975–20978. doi: 10.1074/jbc.272.34.20975. [DOI] [PubMed] [Google Scholar]
- 24.Waldmann R, Lazdunski M. Curr Opin Neurobiol. 1998;8:418–424. doi: 10.1016/s0959-4388(98)80070-6. [DOI] [PubMed] [Google Scholar]
- 25.Mano I, Driscoll M. Bioessays. 1999;21:568–578. doi: 10.1002/(SICI)1521-1878(199907)21:7<568::AID-BIES5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 26.Waldmann R, Champigny G, Lingueglia E, De Weille J R, Heurteaux C, Lazdunski M. Ann NY Acad Sci. 1999;868:67–76. doi: 10.1111/j.1749-6632.1999.tb11274.x. [DOI] [PubMed] [Google Scholar]
- 27.Eckert S P, Taddese A, McCleskey E W. J Neurosci Methods. 1997;77:183–190. doi: 10.1016/s0165-0270(97)00125-8. [DOI] [PubMed] [Google Scholar]
- 28.Davies N W, Lux H D, Morad M. J Physiol (London) 1988;400:159–187. doi: 10.1113/jphysiol.1988.sp017116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bevan S, Yeats J. J Physiol (London) 1991;433:145–161. doi: 10.1113/jphysiol.1991.sp018419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Firsov D, Gautschi I, Merillat A M, Rossier B C, Schild L. EMBO J. 1998;17:344–352. doi: 10.1093/emboj/17.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coscoy S, Lingueglia E, Lazdunski M, Barbry P. J Biol Chem. 1998;273:8317–8322. doi: 10.1074/jbc.273.14.8317. [DOI] [PubMed] [Google Scholar]
- 32.Kosari F, Sheng S, Li J, Mak D O, Foskett J K, Kleyman T R. J Biol Chem. 1998;273:13469–13474. doi: 10.1074/jbc.273.22.13469. [DOI] [PubMed] [Google Scholar]
- 33.Snyder P M, Cheng C, Prince L S, Rogers J C, Welsh M J. J Biol Chem. 1998;273:681–684. doi: 10.1074/jbc.273.2.681. [DOI] [PubMed] [Google Scholar]
- 34.de Weille J R, Bassilana F, Lazdunski M, Waldmann R. FEBS Lett. 1998;433:257–260. doi: 10.1016/s0014-5793(98)00916-8. [DOI] [PubMed] [Google Scholar]
- 35.Dyer N, Srinivasan M, Amagasu S, Tzoumaka E, Rabert D, Smith J, Sangameswaran L. Society for Neuroscience. Vol. 25. Miami, FL: Soc. Neurosci.; 1999. p. 2255. [Google Scholar]
- 36.Tominaga M, Caterina M J, Malmberg A B, Rosen T A, Gilbert H, Skinner K, Raumann B E, Basbaum A I, Julius D. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 37.Crea F, Pupita G, Galassi A R, el-Tamimi H, Kaski J C, Davies G, Maseri A. Circulation. 1990;81:164–172. doi: 10.1161/01.cir.81.1.164. [DOI] [PubMed] [Google Scholar]
- 38.Gnecchi-Ruscone T, Montano N, Contini M, Guazzi M, Lombardi F, Malliani A. J Auton Nerv Syst. 1995;53:175–184. doi: 10.1016/0165-1838(94)00169-k. [DOI] [PubMed] [Google Scholar]
- 39.Thames M D, Kinugawa T, Dibner-Dunlap M E. Circulation. 1993;87:1698–1704. doi: 10.1161/01.cir.87.5.1698. [DOI] [PubMed] [Google Scholar]
- 40.Pan H L, Longhurst J C. Am J Physiol. 1995;269:H106–H113. doi: 10.1152/ajpheart.1995.269.1.H106. [DOI] [PubMed] [Google Scholar]
- 41.Abe T, Morgan D, Sengupta J N, Gebhart G F, Gutterman D D. J Auton Nerv Syst. 1998;71:28–36. doi: 10.1016/s0165-1838(98)00060-5. [DOI] [PubMed] [Google Scholar]
- 42.Baker D G, Coleridge H M, Coleridge J C, Nerdrum T. J Physiol (London) 1980;306:519–536. doi: 10.1113/jphysiol.1980.sp013412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tjen A L S C, Pan H L, Longhurst J C. J Physiol (London) 1998;510:633–641. doi: 10.1111/j.1469-7793.1998.633bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilman A, Goodman L, Rall T, Murid F. The Pharmacological Basis of Therapeutics. New York: Macmillan; 1985. [Google Scholar]
- 45.James T N, Rossi L, Hageman G R. Trans Am Clin Climatol Assoc. 1988;100:81–99. [PMC free article] [PubMed] [Google Scholar]
- 46.Huang M H, Horackova M, Negoescu R M, Wolf S, Armour J A. Cardiovasc Res. 1996;32:503–515. [PubMed] [Google Scholar]
- 47.Ginsburg R, Bristow M R, Kantrowitz N, Baim D S, Harrison D C. Am Heart J. 1981;102:819–822. doi: 10.1016/0002-8703(81)90030-2. [DOI] [PubMed] [Google Scholar]
- 48.Huang H S, Pan H L, Stahl G L, Longhurst J C. Am J Physiol. 1995;269:H888–H901. doi: 10.1152/ajpheart.1995.269.3.H888. [DOI] [PubMed] [Google Scholar]