Abstract
Interstellar gas and dust constitute the primary material from which the solar system formed. Near the end of the hot early phase of star and planet formation, volatile, less refractory materials were transported into the inner solar system as comets and interplanetary dust particles. Once the inner planets had sufficiently cooled, late accretionary infall seeded them with complex organic compounds [Oró, J. (1961) Nature (London) 190, 389–390; Delsemme, A. H. (1984) Origins Life 14, 51–60; Anders, E. (1989) Nature (London) 342, 255–257; Chyba, C. F. & Sagan, C. (1992) Nature (London) 355, 125–131]. Delivery of such extraterrestrial compounds may have contributed to the organic inventory necessary for the origin of life. Interstellar ices, the building blocks of comets, tie up a large fraction of the biogenic elements available in molecular clouds. In our efforts to understand their synthesis, chemical composition, and physical properties, we report here that a complex mixture of molecules is produced by UV photolysis of realistic, interstellar ice analogs, and that some of the components have properties relevant to the origin of life, including the ability to self-assemble into vesicular structures.
Keywords: vesicle, amphiphile, origin of life, interstellar ice, cometary delivery
Infrared observations coupled with laboratory studies have shown that H2O, CH3OH, CO, CO2, and NH3 are major components of ices in molecular clouds (1–3). Energetic in situ processing of these interstellar ices into more complex species can be driven by cosmic ray-induced UV in dense clouds (4), the significantly enhanced UV field in star-forming regions, and high energy particle bombardment and UV radiation from the T-Tauri phase in stellar birth (5). Laboratory studies have shown that such energetic processing produces many new organic compounds in these ices, including species far more complex than the starting materials (6–8). Given that this processing occurs wherever new stars are being created and that there is isotopic evidence from meteorites and cosmic dust that these materials can survive incorporation into forming stellar systems and subsequent delivery to planetary surfaces (9), this photochemical processing could potentially play a significant role in prebiotic chemistry.
In this paper we investigate the chemical and physical properties of complex organic compounds formed in the laboratory under simulated interstellar conditions. We particularly focus on those properties supporting self-assembly processes, in the presence of water, that are relevant to meteoritic studies and perhaps to the origin of life. We found that complex molecules capable of self-assembly are produced by UV photolysis of simple, realistic, interstellar ice analogs.
Materials and Methods
Sample Preparation.
Because the experimental sample preparation technique is described in detail elsewhere (10), only the salient features are summarized here. The gas mixture was deposited under vacuum onto a rotatable, cold (≈15 K) nickel, aluminum, or brass substrate, resulting in the formation of an amorphous mixed-molecular ice. (Substrates were cleaned by either of the following three methods with no change in results: buffing with steel wool, followed by a wash with HPLC grade methanol; buffing with cotton, followed by a wash with HPLC grade methanol; or heating to 550°C in air for 18 h.) The sample was then irradiated in situ with UV from a microwave-powered, flowing hydrogen discharge lamp that illuminates an area several centimeters on a side with 1015 photons/sec. The UV output flux is nearly evenly divided between the hydrogen Lyman α-line at 121.6 nm and a 20-nm-wide molecular transition centered near 160 nm. Under such conditions, the time that elapses between photons arriving in the same molecular neighborhood is ≈13 orders of magnitude longer than molecular relaxation times, so only single-photon processes are relevant.
The selected gas mixture was deposited at a rate of ≈0.02 mmol/hr onto the cold substrate with simultaneous irradiation over the course of up to 2 days. After this time, the ice was allowed to slowly warm to room temperature under static vacuum. This repetitive warming process, which typically took several hours, served three purposes. First, by keeping the ice thin, a more homogeneous temperature can be maintained. Second, by minimizing the amount of volatile compounds, the risk of losing large quantities of residue because of explosive sublimation of ice is diminished. Third, by warming under static vacuum, the loss of compounds of moderate volatility is reduced. This process of warm-up after 2 days of simultaneous deposit and photolysis leaves a thin organic film on the substrate. The chamber was then re-evacuated, and the substrate with residue was recooled to 15 K, followed by the next 2-day cycle of deposition with photolysis, followed by warm-up under static vacuum. This process was repeated up to 10 times for a typical total sample preparation period of 5 weeks. Experiments in which there are no intermediate warmups, i.e., continuous photolysis and deposition for 4–7 days, produce similar samples but with lower yields.
When sufficient sample was generated, the substrate with the organic film was removed from the vacuum chamber, and the organic residue was washed from the substrate with a few drops of a 1:1 methanol:chloroform mixture. (Infrared spectra and the self-assembly behavior of the material have not been observed to change with and without the exclusion of air.) The total mass of a typical room-temperature residue after ≈20 cycles of simultaneous deposition/photolysis plus warm up was nearly 1 mg. The material was then dried under flowing dry nitrogen and redissolved in 50 μl of 1:1 methanol:chloroform mixture to obtain a consistent volume.
HPLC.
In an attempt to isolate the amphiphilic component of the residue, we chromatographically separated the residue into fractions that were individually analyzed by microscopy. Chromatography was performed on a Hewlett Packard 1100 series HPLC with parallel diode array UV/vis and fluorescence detectors and a manual injector with a 20 μl loop. Separation was achieved via a Vydac C-18 4.6 × 250 mm 5 μm resin analytical column and a mobile phase of 50% methanol and 50% water to 100% methanol in 15 min, then 100% methanol for 20 min. Fractions were of 1 min or an individual fluorescent or UV absorbing peak, whichever was smaller. Typically 70 to 80 fractions were collected. In addition, samples of the HPLC effluent before injection and during blank injections were also analyzed. These fractions were dried and analyzed via some of the techniques described below.
Surface Activity.
For initial analysis of self-assembly processes driven by hydrophobic effects in an aqueous phase, aliquots (20 μl) of the 50 μl of 1:1 methanol:chloroform solution containing the residue were removed and spotted on a freshly cleaned microscope slide and allowed to dry at room temperature. Drying was followed by addition of 10 μl of a dilute alkaline buffer (10 mM sodium phosphate, pH 8.5) and a coverslip. (These are conditions in which organic compounds extracted from carbonaceous meteorites produce a variety of self-assembled structures; ref. 11) The resulting droplets were viewed at ×400 via phase microscopy and, in the same field of view, with fluorescence (excitation = 400–430; emission = 450–520). Photographs were taken with Kodak ISO 400 color slide film with automatic exposure. The fluorescence photos were typically exposed for several minutes.
All amphiphiles have the capacity to form monolayers at air-water interfaces. To determine the amphiphilic character of the residues, aliquots of the residue-containing solution were spread on dilute sulfuric acid (0.05 N) by using a 4-cm-diameter plastic Petri dish as a trough and a Wilhelmy plate to measure surface tension changes (12).
Dye Encapsulation.
A 20-μl aliquot of the extract was dried in the presence of 1.0 μM pyranine (8-hydroxy-1,3,6-pyrenesulfonic acid), a strongly anionic dye that does not permeate membranes. Under these conditions, if impermeable membranes are present, dye molecules are in close proximity to amphiphilic molecules and are captured on rehydration (13).
Results and Discussion
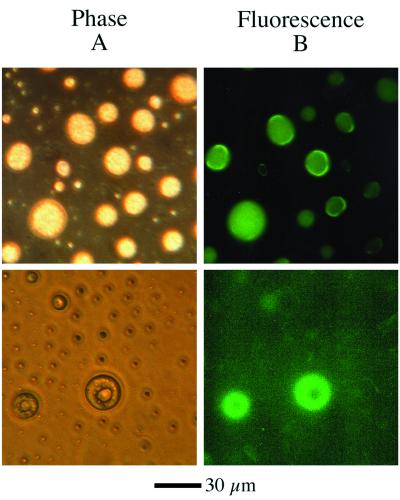
The starting gas-mixture (H2O:CH3OH:NH3:CO = 100:50:1:1) was chosen as a simple mixture that reflects the composition and concentrations of the major interstellar ice components (1, 6, 14). Different concentrations of the same mixture (all H2O-rich) have also been studied and produce analogous results. This mixture was deposited onto a 15 K substrate and irradiated with vacuum UV to simulate the processing of icy grains in dense molecular clouds. When the ice was warmed to room temperature, there is an oily organic residue which remains (6). This material was extracted, dried, and analyzed in aqueous media via microscopy. We found that some components of the photochemical product produced water-insoluble fluorescent droplets under these conditions. Fig. 1A shows the droplets by phase microscopy, and Fig. 1B shows the emission at 450–520 nm when excited at 400–430 nm. The droplets are roughly 10–50 μm in diameter and have apparent internal structures on a 1-μm scale, which are presumably related to phase separations that occur within the organic components of the droplets.
Figure 1.
(A) Residue droplets viewed at ×400 via phase microscopy. (B) The same field of view with fluorescence (excitation = 400–430; emission = 450–520). The bottom panels have been exposed to the UV light from the microscope for 15 min and have developed internal structures.
The formation of insoluble droplets implies that the residue contains nonpolar compounds and their derivatives, which are organic species considerably more complex than the water-soluble compounds present at the start of the experiment. Furthermore, the chain lengths must contain eight carbon atoms or more, because shorter chain alkanes such as pentane or hexane would be expected to either evaporate or to be water soluble if they contain polar groups such as alcohols or carboxylates. Significantly, the fact that internal structures were observed suggested that phase separation had occurred within the droplets, and that some of the components may be amphiphilic. An amphiphile is defined here as an organic compound, such as a soap, that has both nonpolar and polar groups on the same molecule. Virtually all lipids are amphiphilic compounds.
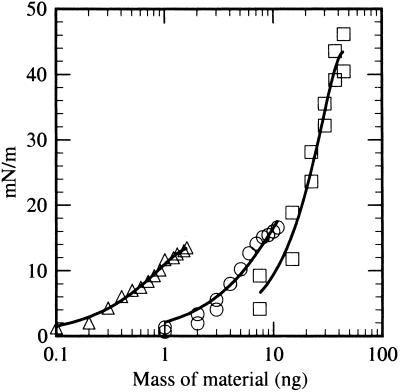
To determine the amphiphilic character of the residue, we used a standard Langmuir isotherm test for surface activity. For comparison, force-area isotherms of decanoic acid and an organic extract of the Murchison meteorite were measured in addition to the residue. These are shown in Fig. 2. Because of its shorter chain length and relative solubility in the aqueous subphase, pure decanoic acid forms unstable monolayers that collapse at surface pressures exceeding 15 mN/m. The mixture of amphiphilic compounds present in the photochemical product formed relatively stable monolayers that collapse at surface pressures exceeding 20 mN/m, whereas the mixture of amphiphiles in the Murchison extract produce stable monolayers that collapse at 45 mN/m. These results confirmed that the photochemical product includes amphiphilic compounds with chain lengths sufficient to form monolayers at air–water interfaces.
Figure 2.
Surface pressure of monolayers of the residue produced by the UV photolysis of an H2O:CH3OH:NH3:CO = 100:50:1:1 ice (circle), Murchison extract (square), and decanoic acid (triangle).
To eliminate the possibility of sample contamination and to ensure that the residue produced by ice photolysis is responsible for all of the behavior described above, a number of control experiments were performed. For such long-duration cryogenic experiments, one must eliminate the possibility of contamination from materials that may be present in the vacuum system even at trace levels, such as pump oil vapor. To test this, the gas mixture was deposited onto a 15 K foil without photolysis for 3 weeks. No residual material could be seen on the substrate after warm-up of this sample under vacuum, and, after washing the substrate surface with the same solvent mixture and employing the same microscopy techniques described above, no droplet formation or fluorescence was observed. Similar negative results were found when only H2O:NH3 = 100:1 and H2O:NH3:CO = 100:1:1 ices were deposited and photolyzed as described above. These controls demonstrate that the amphiphilic material is unlikely to be a contaminant from the vacuum pumps or any other part of the sample preparation apparatus, nor is it an artifact of our sample analysis protocols. This series of experiments also indicates that the presence of CH3OH in the ice is critical to the production of both the complex amphiphilic compounds and the fluorescent material.
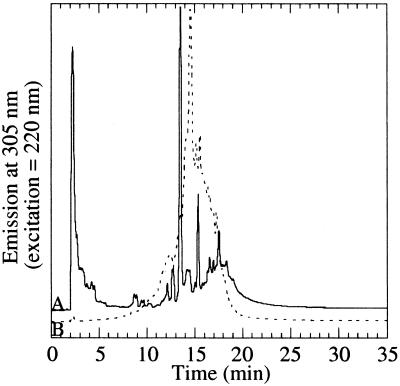
Photolysis of H2O:CH3OH:CO = 100:50:1 ices produces a visible residue. This residue shows a diffuse overall fluorescence but fails to generate any droplets on the addition of water. HPLC was performed on this sample as well as the residue made in the presence of ammonia. Although there were similarities, significant differences were apparent (Fig. 3). The most profound difference was the great reduction in size of the largest fluorescent peaks at 13.5 min and at the solvent front and the appearance of a peak at 14.6 min. The differences in behavior between the three-component mixture and the same mixture with NH3 indicates that the presence of ammonia is also critical to the formation of the amphiphilic material.
Figure 3.
Fluorescence HPLC (excitation = 220, emission = 305) of (A) the photolysis residue from an H2O:CH3OH:NH3:CO = 100:50:1:1 ice (solid line) and (B) the photolysis residue from an H2O:CH3OH:CO = 100:50:1 ice (dotted line).
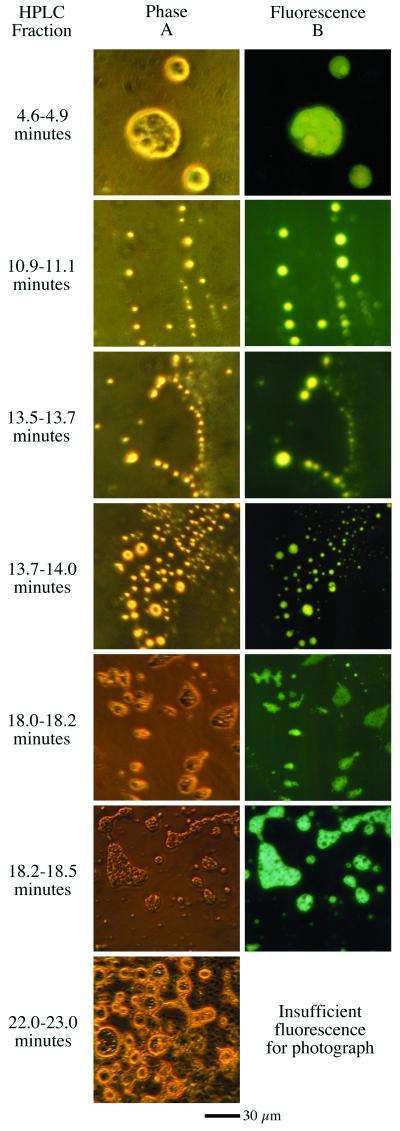
Fractions of the H2O:CH3OH:NH3:CO = 100:50:1:1 residue were collected from the HPLC, and each was individually analyzed by phase and fluorescence microscopy. The samples include a number of fractions that form water-insoluble droplets with different morphologies, some of which are shown in Fig. 4. These are interspersed by fractions that do not form insoluble droplets. Thus, there are many distinct amphiphilic molecules in the residue. The fractions that form droplets do not necessarily correspond to fluorescent- or UV-absorbing peaks on the HPLC. However, the fractions that form droplets and occur with fluorescent peaks exhibit brighter fluorescence than droplet-forming fractions that lie on the tails of fluorescent peaks. This pattern suggests that the amphiphilic molecules are not the same compounds as the fluorescent molecules, but that instead the relatively nonpolar fluorescent components can become incorporated into the membranous (amphiphilic) phase. Unfortunately, there was insufficient material for further analysis of most of these fractions. It is clear, however that each fraction contains numerous compounds, with possibly hundreds of different molecules contained in the residue.
Figure 4.
Photos of fractions collected at various times. (A) Viewed at ×400 via phase microscopy. (B) The same field viewed in fluorescence (excitation = 400–430; emission = 450–520).
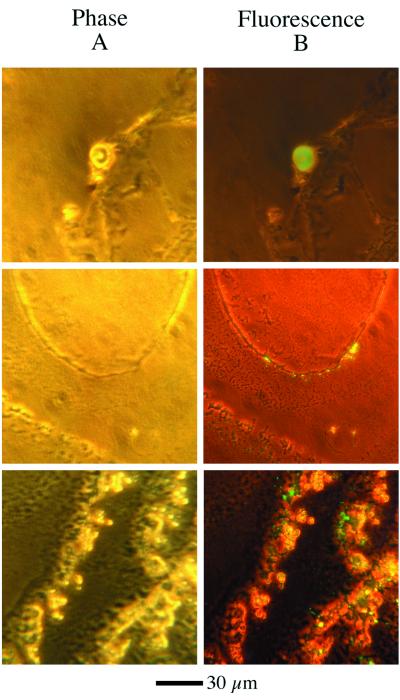
To determine whether the amphiphilic components of the droplets can assemble into membranous vesicles having interior spaces, we encapsulated a polar dye via a cycle of wetting and drying. This wetting–drying cycle has been proposed as a probable mechanism by which relatively impermeant molecules could be captured by membranes in the prebiotic environment (15). We found that substantial numbers of highly fluorescent vesicles were present, most in the range of 1–10 μm (Fig. 5 Middle and Bottom), but some are considerably larger (Fig. 5 Top). True encapsulation of the dye was demonstrated by addition of 5 mM Triton X-100, a nonionic detergent that is known to permeabilize lipid bilayers (16). The detergent was introduced at one edge of the microscope slide, and, as it slowly spread under the coverslip, the vesicles became nonfluorescent, indicating that pyranine was released and diluted in the surrounding medium through defects induced in the membranes by the Triton X-100. This release confirms that the dye was trapped inside the vesicles, rather than merely dissolved within their membranes.
Figure 5.
Pyranene dye encapsulated in various sizes of vesicles made from a residue. (A) Viewed at ×400 via phase microscopy. (B) The same field viewed in fluorescence (excitation = 400–430; emission = 450–520). Note that, in the Bottom Right panel, both the bright fluorescence of encapsulated pyranine and the dimmer autofluorescence of the residue can be seen.
We also compared the behavior of the laboratory-produced residue with the extraterrestrial organic material extracted from a carbonaceous meteorite. Although the Murchison meteorite has a complex history and was derived from a more complex mixture than the residue in our simulations, Fig. 6 shows that strikingly similar vesicular structures self-assemble from chloroform-methanol extracts of the Murchison meteorite sample when a phosphate buffer is added to the organic extract. It is also interesting to note that, like the droplets found in extracts of the Murchison meteorite, the residue droplets show an increased granular or foamy texture during UV (but not visible light) exposure in the microscope, as evident in the larger droplets in Figs. 1 and 3. The physical changes in the droplets on illumination indicate that UV-driven photochemical reactions can occur in the organic compounds that compose the droplets. The nature of this photochemistry may have prebiotic significance, in that pigments present in the mixture are apparently able to capture UV light energy and use it to produce increasingly complex structures in the droplets.
Figure 6.
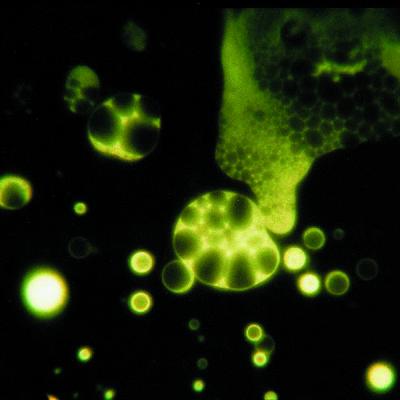
Pyranene dye encapsulated in various sizes of vesicles made from an acid extract of the Murchison meteorite.
The ready formation of these organic species from simple starting mixtures, the ice chemistry that ensues when these ices are mildly warmed, and the observation that the more complex refractory photoproducts show lipid-like behavior and self-organize into droplets on exposure to liquid water suggest that extraterrestrial materials could exhibit a far greater range in chemical properties and behavior than previously thought. Given that these materials are readily created under simulated interstellar conditions and that they seem very similar to materials in primitive meteorites, it seems reasonable to seriously consider whether organic material generated in the interstellar medium could indeed have been delivered to the early Earth and could have contributed to the origin and early evolution of life. Delivery of this material via meteorites, comets, or interplanetary dust particles may have augmented the endogenously generated molecules for the origin or the early evolution of life. For example, because there are only a few known prebiotic synthesis routes for lipids, exogenous material may have been an important source of amphiphilic molecules or, in hydrated environments, vesicle-like structures.
The experiments described here used organic solvents to efficiently dissolve the organic compounds, followed by drying and dispersion in aqueous phases. The amphiphilic compounds in Murchison can be extracted by using only dilute buffered or unbuffered water (11). Organic compounds can certainly be released from carbonaceous meteorites by hot water, for example, under hydrothermal conditions (17).
Conclusions
This paper presents the laboratory simulation of an interstellar ice mixture proven to produce amphiphilic vesicle-forming compounds similar to those found in primitive meteorites, such as the Murchison carbonaceous chondrite. Starting from a very simple, yet astrophysically relevant, ice mixture (water, methanol, ammonia, and carbon monoxide), a very complex mixture of compounds, including amphiphiles and fluorescent molecules, is generated on low temperature photolysis. The ready formation of these insoluble compounds from photolyzed ices comprised of simple molecules suggests not only that this process might be the source of their origin in meteorites, but that the delivery of such compounds by comets, meteorites, and interplanetary dust particles during the late heavy bombardment period may have played an influential role in the origin of life on Earth. Because our experimental conditions for ice photolysis were designed to simulate the environments of dense interstellar molecular clouds (the birth sites of new stars and planetary systems), the delivery of these materials to the surfaces of newly formed planets may be a universal process. The level of extraterrestrial molecular complexity is just now becoming apparent, and the full implications of this chemical input to the early Earth, and by implication to other habitable planets, are likely to be far reaching.
Acknowledgments
We thank Max Bernstein for many helpful scientific discussions and for help with the laboratory procedures. We also acknowledge Bob Walker for his excellent technical support, without which these long duration experiments would not have been possible. This work was supported by the National Aeronautics and Space Administration Exobiology Program (Grant 344-38-12-04), Astrobiology Program (Grant 344-50-92-02), and Origins of Solar Systems Program (Grant 344-37-44-01).
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Allamandola L J, Bernstein M P, Sandford S A, Walker R M. Space Sci Rev. 1999;90:219–232. doi: 10.1007/978-94-011-4211-3_20. [DOI] [PubMed] [Google Scholar]
- 2.d'Hendecourt L B, Jourdain de Muizon M, Dartois E, Demyk K, Ehrenfreundt P, Heras A. In: The Universe as Seen by ISO. Cox P, Kessler M F, editors. Noordwijk, The Netherlands: ESA Publications Division; 1999. pp. 589–597. [Google Scholar]
- 3.Dartois E, Schutte W, Geballe T R, Demyk K, Ehrenfreund P, D'Hendecourt L. Astron Astrophys. 1999;342:L32–L35. [Google Scholar]
- 4.Prasad S S, Tarafdar S P. Astrophys J. 1983;267:603–609. [Google Scholar]
- 5.Giampapa M S, Imhoff C L. In: Protostars and Planets II. Black D C, Matthews M S, editors. Tucson: Univ. Arizona Press; 1985. pp. 386–404. [Google Scholar]
- 6.Bernstein M P, Sandford S A, Allamandola L J, Chang S, Scharberg M A. Astrophys J. 1995;454:327–344. [Google Scholar]
- 7.Moore M H, Ferrante R F, Nuth J A. Planet Space Sci. 1996;44:927–935. [Google Scholar]
- 8.Tegler S C, Weintraub D A, Allamandola L J, Sandford S A, Rettig T W, Campins H. Astrophys J. 1993;411:260–265. doi: 10.1086/172825. [DOI] [PubMed] [Google Scholar]
- 9.Bernstein M P. In: Laboratory Astrophysics and Space Research. Ehrenfreund P, Kraft C, Kochan H, Pirronello V, editors. Dordrecht, The Netherlands: Kluwer; 1999. pp. 105–120. [Google Scholar]
- 10.Allamandola L J, Sandford S A, Valero G. Icarus. 1988;76:225–252. [Google Scholar]
- 11.Deamer D W, Pashley R M. Orig Life Evol Biosph. 1989;19:21–38. doi: 10.1007/BF01808285. [DOI] [PubMed] [Google Scholar]
- 12.Volkov A G, Deamer D W, Tanelian D L, Markin V S. Liquid Interfaces in Chemistry and Biology. New York: Wiley; 1998. pp. 76–77. [Google Scholar]
- 13.Shew R, Deamer D W. Biochim Biophys Acta. 1985;816:1–8. doi: 10.1016/0005-2736(85)90386-4. [DOI] [PubMed] [Google Scholar]
- 14.Sandford S A. Meteoritics Planet Sci. 1996;31:449–476. doi: 10.1111/j.1945-5100.1996.tb02088.x. [DOI] [PubMed] [Google Scholar]
- 15.Deamer D W, Barchfeld G L. J Mol Evol. 1982;18:203–206. doi: 10.1007/BF01733047. [DOI] [PubMed] [Google Scholar]
- 16.Seufert W D. Nature (London) 1965;207:174–176. doi: 10.1038/207174a0. [DOI] [PubMed] [Google Scholar]
- 17.Mautner M, Leonard R, Deamer D W. Planet Space Sci. 1995;43:139–147. doi: 10.1016/0032-0633(94)00205-6. [DOI] [PubMed] [Google Scholar]