Abstract
Society invests billions of dollars in the development of new drugs and technologies but comparatively little in the fidelity of health care, that is, improving systems to ensure the delivery of care to all patients in need. Using mathematical arguments and a nomogram, we demonstrate that technological advances must yield dramatic, often unrealistic increases in efficacy to do more good than could be accomplished by improving fidelity. In 2 examples (the development of anti-platelet agents and statins), we show that enhanced efficacy failed to achieve the health gains that would have occurred by delivering older agents to all eligible patients. Society’s huge investment in technological innovations that only modestly improve efficacy, by consuming resources needed for improved delivery of care, may cost more lives than it saves. The misalignment of priorities is driven partly by the commercial interests of industry and by the public’s appetite for technological breakthroughs, but health outcomes ultimately suffer. Health, economic, and moral arguments make the case for spending less on technological advances and more on improving systems for delivering care.
Keywords: Allocation of resources, delivery of health care, priority setting, disparities, quality of health care
INTRODUCTION
Although modern medicine can be proud of its successes in the prevention and treatment of disease, much more can be done to alleviate morbidity and premature mortality. Two transcendent problems pre-dominate. First, available care is not delivered well: Americans do not always obtain the interventions that would improve their health or prevent illness. By one account, Americans receive only 55% of recommended health care services.1 Gaps in delivery are even greater for the poor and for racial and ethnic minorities.2 Second, the interventions that Americans do receive have limited efficacy in improving outcomes. More lives could be saved by developing better drugs, technologies, and procedures. In effect, society faces a choice between these 2 strategies for bettering health and must strike a prudent balance in how many resources it allocates to each endeavor.
Fidelity
The first endeavor addresses what might be described as the fidelity of health care. Independent of the efficacy or effectiveness of interventions, fidelity is the extent to which the system provides patients the precise interventions they need, delivered properly, precisely when they need them. Fidelity is lacking when patients cannot make known their need for care (eg, there are barriers in access or communication), when clinicians cannot recognize that an intervention is indicated (eg, there is a lack of time, knowledge, attention, or memory), and when the intervention cannot be delivered properly (eg, there is inadequate infrastructure, procedures, safety, coordination, or information). Fidelity has less to do with the properties of interventions than with the functionality of the system that delivers them. It requires systems of care (eg, practice groups, hospitals) to have intelligent designs, skilled professionals, coordinated teams, adequate resources, competent information systems, reminders and other decision support tools, cooperation across organizations to achieve seamlessness, and a leadership culture committed to patient-centered care.3–5 Assembling these conditions is one major way for society to improve the health of the population.
Efficacy and Effectiveness
The second strategy to alleviate disease is to enhance the efficacy (and effectiveness) of interventions. No treatment is perfect. Health can be improved if screening and diagnostic procedures are made more accurate and if treatments can perform better in reducing morbidity and mortality. This enterprise involves basic biomedical research; the translation from basic science to human application; and clinical trials to evaluate effectiveness, safety, adverse effects, and costs. The effort to improve efficacy and effectiveness involves the development of new agents and products in university-based and private industrial laboratories, and the dissemination of these products through licensing, advertising, and other channels. This prodigious technological investment to perfect new drugs and procedures is the second major way for society to improve the health of the population.
SOCIETY’S PRIORITIES
An objective observer would concede that the United States devotes most of its resources to the second aim, the enhancement of efficacy. The pharmaceutical industry spends $32 billion annually to develop new drugs and biologics.6 This amount exceeds the entire $29 billion budget of the National Institutes of Health, which, in turn, spends most of its research dollars on basic science and translational research to bring new drugs and technologies to market.7
In contrast, our society spends relatively little on fidelity. Health systems spend greatly on delivering care—both on its administration and on competition for patients—but they spend relatively little on the important system redesigns that are essential to deliver care well. Although progressive institutions—exemplified by the Veterans Administration,8 “breakthrough collaboratives,”9,10 and leading health systems11—have enacted bold system redesigns and achieved notable success in delivering the right care at the right time, most health systems and private practices have moved less boldly.12,13 Unlike the leaders of other industries who have committed themselves to quality, managers in health care have not embraced the need for system restructuring and have not committed resources to optimize service to their clients.14 In addition, policy makers have not resolved the barriers to access and health insurance that deny care to at least 45 million Americans.15
Society’s investment in research also includes little for fidelity. The annual budget of the federal agency with chief responsibility for this type of research, the Agency for Healthcare Research and Quality, is approximately $300 million, $1 for every $100 appropriated to the National Institutes of Health.16 Private industry spends as much to develop just 1 new drug.17,18 The spirit of inquiry and innovation that scientists apply to the creation of new technologies could yield ingenious solutions to the problems of health care delivery if similar levels of intellectual energy and financial resources were brought to bear.19 Society’s choice to channel billions of dollars into the race for new drugs and devices suggests that it values efficacy over fidelity as a priority for improvement.
HEALTH GAINS OF EFFICACY AND FIDELITY IN PERSPECTIVE
Is this strategy good for the population? The question is posed not to argue against the need for improving efficacy, which is vital, but rather to examine whether the balance is correct. The ethical principle of utilitarianism20 compels a thoughtful examination of how efficacy and fidelity should be prioritized to accomplish the greatest good for the population’s health (and the corollary, the extent to which imbalanced priorities contribute to disease).
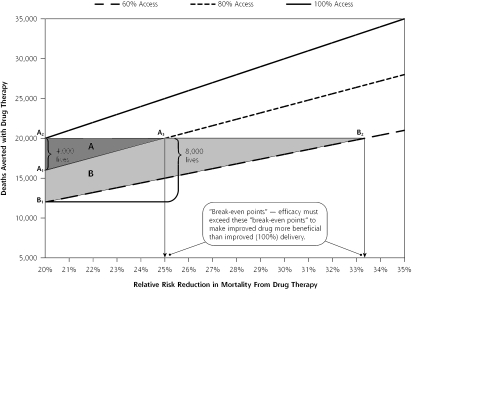
Suppose a disease claims 100,000 lives each year and a drug is available that reduces the mortality rate from that disease by 20% (relative risk reduction [RRR] = 0.20). The drug therefore has the potential to save 20,000 lives each year, but if only 80% of eligible patients receive the drug, only 16,000 deaths will be averted (Figure 1 ▶, A1). If society made no effort to improve the efficacy of the drug but managed to deliver it to 100% of eligible persons, 20,000 (4,000 additional) lives would be saved (Figure 1 ▶, A2). But if society retains the 20% gap in delivery and works to enhance the efficacy of the drug, the RRR would have to rise above 25% (100,000 × 0.25 × 0.8 = 20,000), what we call the break-even point, to do as much good (ie, to save 4,000 additional lives) (Figure 1 ▶, A3).
Figure 1.
“The Break-even Point” (for a drug that reduces mortality by 20%).
Triangle A. If 100,000 patients are destined to die from a disease, a drug that reduces death rates by 20% (relative risk reduction [RRR] = 0.20) will save 16,000 lives (A1) if delivered to 80% of eligible patients. Increasing delivery to 100% would save 4,000 more lives (A2). To save as many lives with without improving upon the delivery rate of 80% (A3), the RRR of the drug must be increased to at least 25% (‘break-even point”).
Triangle B. Delivering the drug to only 60% of patients would save only 12,000 lives (B1), and improving delivery to 100% would save 8,000 additional lives. To save 8,000 additional lives without improving upon the delivery rate of 60% (B2), the RRR of the drug must be increased to at least 33.3% (“break-even point”). Developing a more efficacious drug is more beneficial than improving access only if the new relative risk reduction (RRR) exceeds the existing RRR divided by the proportion of the population exposed to treatment. The complete nomogram from which the figure derives is provided by the Annals in an online appendix available at http://www.annfammed.org/cgi/content/full/3/6/545/DC1.
The greater the gaps in delivery, the more efficacy must be increased to make that enterprise more beneficial than improving delivery, as shown in Figure 1 ▶. For example, if the assumptions in the above scenario are held constant, but 60%, rather than 80%, of the eligible population receives the medication, the RRR would have to rise from 20% to 33.3% to make the enhancement of drug efficacy more beneficial than improved delivery. When access to efficacious interventions is poor, large and unrealistic increases in efficacy must be achieved to surpass the potential gains from improving fidelity.
IS THE COMPARISON FAIR?
It might be argued that investments to enhance efficacy have a better track record of success than perfecting the delivery of care, making these hypothetical calculations unrealistic. Medicine can point to dramatic gains in improving efficacy, whereas the intractable barriers to achieving fidelity make the goal of 100% access seem untenable. But the assumptions underlying this argument deserve scrutiny.
First, although biotechnological research does yield stunning advances, the denominator is populated by a much larger number of failures. Only 1 of 10,000 compounds investigated by the pharmaceutical industry becomes a new drug. Only 20% of new drug applications to the Food and Drug Administration progress through clinical trials and are approved as new drugs.17,18 Much biotechnological research yields negative results or produces new agents or technologies of minimal value over standard care (eg, “me-too” drugs). Only 22% of the drugs approved by the Food and Drug Administration represent “significant improvement compared with marketed products.”18 When stunning advances in efficacy are set against the denominator of all private and public research efforts, the net public health benefit from the endeavor is modest.
Second, the notion that fidelity cannot be markedly improved is mistaken. A large body of trial evidence suggests otherwise.21 According to some reviews, the probability of providing the right care can be increased by as much as 68% through educational outreach and social marketing, 250% by offering feedback to clinicians about their performance, and 420% by instituting reminder systems.22,23 Quality is improved and errors are reduced by building systems with integrated, multifaceted features that “make it hard for people to do the wrong thing.”24 Integrated system redesign, popularized by the work of Berwick3 and Shortell et al,25 is embodied in the Chronic Care Model and has improved outcomes for patients with chronic diseases.26
Large-scale system redesign has been recognized for some time as an urgent public health priority. Warning in 2001 that the health care system was fundamentally flawed, a landmark Institute of Medicine report, Crossing the Quality Chasm, urged the nation and systems of care to undertake bold design changes to close the gap between what is and what should be done in health care.24 The report called for major changes to create a system of care that was safe, effective, patient centered, timely, efficient, and equitable.
In the 5 years since that stark warning was issued, billions of dollars have flowed into the development of drugs and technologies, but a national resolve to rebuild systems to improve delivery is lacking. This imbalance in the priority given to efficacy vs fidelity has potentially serious consequences to population health, as the following 2 examples illustrate.
Antiplatelet Therapy to Prevent Recurrent Stroke
A systematic review by the Antithrombotic Trialists Collaboration27 reported that the use of aspirin by patients who had previously experienced a stroke or transient ischemic attack reduces the incidence of recurrent nonfatal strokes by 23%. That is, in a population in which 100,000 people were destined to have strokes, 23,000 events could be prevented if all eligible patients took aspirin. McGlynn et al1 reported, however, that antiplatelet therapy is given to only 58% of eligible patients. At that rate, only 13,340 strokes would be prevented in the hypothetical population, whereas achieving 100% fidelity in offering aspirin would prevent 23,000 strokes (ie, 9,660 additional strokes).
Not addressing the fidelity of aspirin delivery and opting instead to develop better drugs makes sense (given the reasoning outlined above) only if the newer agents can lower stroke incidence by at least 40% (100,000 × 0.40 × 0.58 = 23,000), but this increased efficacy would require a proportional improvement over aspirin of 74%. The pharmaceutical industry has invested heavily in alternative antiplatelet therapies; clopidogrel and ticlopidine underwent extensive testing in trials involving 22,976 subjects. Rather than demonstrating a 74% improvement over aspirin, however, these drugs were only 10% to 12% more effective in preventing vascular events.27 It is worth asking whether the resources expended for the antiplatelet trials might have prevented more vascular events if they were invested in better systems for the delivery of aspirin.
Statin Use by Patients With Coronary Artery Disease
The use of simvastatin or pravastatin by patients with coronary artery disease reduces 5-year coronary artery disease mortality by as much as 24%.28–30 McGlynn et al1 report, however, that statins are prescribed to only 33% of eligible patients. Using the logic outlined above, we can posit that developing statins that surpass simvastatin or pravastatin is better for population health than achieving 100% uptake only if the new agents are 3 times as potent, reducing 5-year coronary artery disease mortality by at least 72% (100,000 × 0.72 × 0.33 = 24,000).
The degree to which the new generation of statins (eg, atorvastatin, rosuvastatin) lower mortality is unknown, pending the results of ongoing trials, but the evidence regarding their effects on lipid levels suggests that they are not 3 times as potent. Rosuvastatin is only 26% more effective than pravastatin and 12% to 18% more effective than simvastatin in lowering levels of low-density lipoprotein cholesterol.31 Although this superiority is clinically significant, we conclude that developing these agents has done less to save lives than would robust delivery systems that bring the older statins to all eligible patients.
That care processes can be modified to improve the delivery of cardiac drugs is hardly unrealistic.32 Even simple interventions can have dramatic effects. One trial—conducted in a setting wherein 33% of patients with coronary artery disease were receiving lipid-lowering therapy—demonstrated the effectiveness of a simple reminder system: affixing a red notice to the front of the chart, citing current guidelines and the changes necessary to restore adherence.33 Drug therapy was instituted or increased in 94% of patients in the intervention group but in only 10% of control patients, for whom no reminders were used.
Consider the choice of investing society’s resources in making such reminder systems routine vs developing more potent drugs. If rosuvastatin is 26% more effective than pravastatin in lowering lipid levels,31 we postulate (in the absence of direct evidence about mortality benefits) that the drug would reduce 5-year coronary artery disease mortality by an additional 6% (0.24 × 0.26), saving an additional 2,000 lives in our hypothetical population (100,000 × 0.06 × 0.33). In contrast, if older statins were retained but reminder systems were made routine, 14,640 additional lives might be saved (100,000 × 0.24 × [0.94 – 0.33]). In essence, forfeiting rosuvastatin to improve delivery of existing statins would have saved 7 times as many lives (14,640/2,000).
Establishing reminder systems in the office of every physician in the nation would be costly, but developing new drugs is also exorbitant, with a cost of approximately $800 million per agent.17 Spending on statins might be higher, given the size of the trials.34 Even an investment of $800 million to develop a new statin would provide $28 for each of the estimated 28.4 million Americans35 who ought to receive statins, a sum that could subsidize the cost of reminder systems for eligible patients.
LIMITATIONS
This thesis encounters problems at both the methodological and policy levels. The first methodological limitation is that relative risk rates and the estimates of proportion of the eligible population who receive recommended treatments are subject to error. The point estimates we used obscure the heterogeneity with which interventions perform across agents and settings. Second, the calculations assume that patients not receiving interventions face the same risk, and benefit equivalently, as those with access. Third, 100% uptake is rarely attainable, and the calculations should be adjusted for more realistic expectations. Formidable challenges impede the delivery of care to disadvantaged and minority populations, who are disproportionately affected by inadequate access and health insurance and by disparities in care.2 Fourth, the hard endpoint of receipt of a service does not capture whether the service was delivered well, with quality, safety, and compassion.
Beyond its methodological limitations, our thesis encounters its greatest difficulties at the policy level. First, we pose a false dichotomy by suggesting that the pursuit of efficacy and fidelity are mutually exclusive, when one endeavor may enhance the other. For example, uptake of therapy can be improved if industry develops more acceptable products or if advertising campaigns alert patients and their clinicians to the need for treatment. A major reason why eligible patients do not take lipid-lowering medication—lack of awareness or agreement that the drugs are necessary36—may be mitigated by pharmaceutical companies’ billion-dollar advertising campaigns.37,38
Second, this article focuses on investment in new products when often the trade-off involves the intensity of care. For example, while many women receive mammograms and Papanicolaou tests more frequently than needed,39,40 47% of eligible American Indian and Alaskan Native women have not had a recent mammogram.41 Resources consumed by the overuse of care might do more good if deployed to serve those receiving inadequate care. Allocation of resources based on the relative effectiveness of health care services and the size of the population at risk (eg, allocating for childhood immunizations vs heart transplantations) could further enhance population health.42,43 A British study calculated that reducing cardiac risk factors had saved 731,720 life-years in England and Wales, compared with 194,145 life-years gained by cardiac treatments.44
Third, our analysis focuses only on selected health benefits of improving efficacy and fidelity. A fuller comparison would consider other health outcomes, harms, and costs, and would elucidate trade-offs in priority populations (eg, racial and ethnic minorities, children, the elderly).45
Fourth and most important, the thesis envisions a nonexistent decision maker in American society, one who is responsible and controls resources for both technology development and systems to improve care delivery. In our health care system, private-sector manufacturers are largely responsible for allocating resources to drug and technology development, whereas managers of health systems and government agencies decide how much to spend on quality improvement. The wealth engine in private industry is not a resource shared with health plans to redesign systems of care.
COMPETING AGENDAS
It may be idealistic to expect decision makers in health systems or private industry to retain a global perspective—considering which strategy serves the greater good for the population—when other priorities influence their resource allocation decisions. Pharmaceutical companies care about population health but are also accountable to shareholders. Promoting statins is not just a public health exercise but also a $22 billion industry.46 AstraZeneca’s investment in rosuvastatin was an effort more to compete with Pfizer’s atorvastatin than to meet a health need in the population.47
Capitalism and altruism can work at cross-purposes. A company faced with potential blockbuster earnings—such as the $2 to $3 billion per year that both clopidogrel and rosuvastatin generate48—is not expected to forgo its profits and donate its development budget to help health systems improve fidelity, potentially improving uptake of competitors’ products, even if these campaigns will ultimately do more for population health.
Industry’s technological advancement finds support with the American public, which marvels over scientific discovery and technological breakthroughs.49 Robotic devices and genome mapping are more thrilling than bland quality improvement efforts, such as reminder systems and organizational redesign, irrespective of whether the latter saves more lives.50
The state is responsible for population health, but it can control only public budgets and cannot ensure that the vast and widely distributed resources of the health care system are deployed in rational proportions. Rarely are the complexities of health optimization first on the minds of government decision makers. Legislators and political appointees focus on pleasing constituents. Regulatory requirements, not health optimization, guide the choices of many agency officials. Neither the Food and Drug Administration nor the Centers for Medicare and Medicaid Services explicitly considers how the resources consumed by the interventions it approves compromise more effective strategies to improve public health.51,52
Although it may be appropriate to their work cultures for decision makers to base their choices on parochial concerns, the public ultimately suffers if, in the end, interventions that will do the most good are displaced by less beneficial measures. The ethic of utilitarianism20 and the duty to provide care to all in need53 create moral imperatives to design systems to ensure that care is delivered well to everyone. This imperative compels society to marshal the resources, intellectual energy, and national resolve for the reconfiguration of structure and process that excellence in care requires.24
Policy makers committed to fidelity would orchestrate the system solutions that expert panels24,54 have recommended but that only selected health centers and communities have implemented.55 They would establish universal health insurance,15,56 remove financial barriers to care for the poor,57 and address other causes of disparities.2 They would transform today’s visionary ideas for a new model of care58 into tomorrow’s reality. They would restructure delivery systems and realign reimbursement to promote the most effective treatments and to replace current fragmentation with seamless delivery.9,25 They would provide open access,59 e-mail consultations,60 and other innovations to ensure timely assistance and fewer errors. They would invest in information systems to connect patients with the finest educational resources, decision aids, and electronic health records.61,62 Communities would build integrated linkages between health care professionals and civic partners—for example, work sites, schools, and churches—to help patients implement medical advice after they leave the clinic.25,63
The future may bring greater public interest in fidelity as frustration with health care deepens.64 A national survey found that 55% of Americans were dissatisfied with the quality of health care in 2004, up from 44% in 2000.65 In time, the public may come to view new technological wizardry as less of a “medical advance” than provision of prompt clinical attention; responsiveness; preventive services; skilled care that is coordinated, evidence based, and error free; timely reminders; clear communication; immediate access to information; cultural sensitivity; equity; and respect.2,9,25,59,62,66–74
That private and public leaders have chosen instead to invest comparatively little in achieving these aims and to commit the bulk of resources to making better treatments for those who receive care is not only problematic in terms of equity, but, as we have shown, a potential contributor to excess deaths and morbidity. Both lives and ethics are thus at stake.
Individual physicians can press their leaders, both civic and professional, to make fidelity a higher priority,75 and they can promote fidelity in daily practice. This article shows that prescribing the latest drug may help patients less than adopting office systems to ensure that all eligible individuals receive recommended care.76 Clinicians should look past catchy drug advertisements and the promotions of pharmaceutical representatives to consider whether the incremental magnitude of benefit offered by newer agents crosses the break-even point (Figure 1 ▶).
As a society, we should confront the price we pay—in human lives—by maintaining a health care system that is not designed to deliver care well. Society can realign its priorities. It can spend less profligately on the enhancement of drugs and technology, and redesign systems of care to ensure a standard of excellence that fulfills the attributes of quality outlined in the Chasm report24 and the Future of Family Medicine project.58 Failure to act to correct deficiencies in fidelity is, in effect, an affirmative choice to subject patients to greater illness and suffering. To do so while investing vast wealth in technology should weigh heavily on society’s collective conscience.
Acknowledgments
The authors thank Leif I. Solberg, MD, Russell Glasgow, PhD, and the anonymous reviewers for their helpful comments on this manuscript.
Conflicts of interest: none reported
REFERENCES
- 1.McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348:2635–2645. [DOI] [PubMed] [Google Scholar]
- 2.Smedley BD, Stith AY, Nelson AR, eds. Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care BoHSP, Institute of Medicine. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: National Academies Press; 2003. [PubMed]
- 3.Berwick DM. A primer on leading the improvement of systems. BMJ. 1996;312:619–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Von Korff M, Gruman J, Schaefer J, Curry SJ, Wagner EH. Collaborative management of chronic illness. Ann Intern Med. 1997;127:1097–1102. [DOI] [PubMed] [Google Scholar]
- 5.Berwick DM. Improvement, trust, and the healthcare workforce. Qual Saf Health Care. 2003;12:448–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pharmaceutical Research and Manufacturers of America. Washington, DC. Pharmaceutical R&D spending: PhRMA Annual Membership Survey, 2002. 2002. Available at: http://www.phrma.org/issues/research-dev/. Accessed October 8, 2003.
- 7.Sung NS, Crowley WF Jr, Genel M, et al. Central challenges facing the national clinical research enterprise. JAMA. 2003;289:1278–1287. [DOI] [PubMed] [Google Scholar]
- 8.Jha AK, Perlin JB, Kizer KW, Dudley RA. Effect of the transformation of the Veterans Affairs Health Care System on the quality of care. N Engl J Med. 2003;348:2218–2227. [DOI] [PubMed] [Google Scholar]
- 9.Wagner EH, Glasgow RE, Davis C, et al. Quality improvement in chronic illness care: a collaborative approach. Jt Comm J Qual Improv. 2001;27:63–80. [DOI] [PubMed] [Google Scholar]
- 10.Berwick D, Rothman M. Pursuing perfection: an interview with Don Berwick and Michael Rothman. Interview by Andrea Kabcenell and Jane Roessner. Jt Comm J Qual Improv. 2002;28:268–278, 209. [PubMed] [Google Scholar]
- 11.Solberg LI, Hroscikoski MC, Sperl-Hillen JM, O’Connor PJ, Crabtree BF. Key issues in transforming health care organizations for quality: the case of advanced access. Jt Comm J Qual Saf. 2004;30:15–24. [DOI] [PubMed] [Google Scholar]
- 12.Becher EC, Chassin MR. Improving the quality of health care: who will lead? Health Aff (Millwood). 2001;20:164–179. [DOI] [PubMed] [Google Scholar]
- 13.Berwick DM. Disseminating innovations in health care. JAMA. 2003;289:1969–1975. [DOI] [PubMed] [Google Scholar]
- 14.Chassin MR. Is health care ready for Six Sigma quality? Milbank Q. 1998;76:565–591, 510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Committee on the Consequences of Uninsurance, Board on Health Care Services, Institute of Medicine. Care Without Coverage: Too Little, Too Late. Washington, DC: National Academy Press; 2002.
- 16.Clancy CM. AHRQ’s FY 2005 budget request: new mission, new vision. Health Serv Res. 2004;39:xi–xviii.14965073 [Google Scholar]
- 17.DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22:151–185. [DOI] [PubMed] [Google Scholar]
- 18.Public Citizen. Rx R&D Myths: The Case Against The Drug Industry’s R&D “Scare Card.” 2001. Available at: http://www.citizen.org/publications/release.cfm?ID=7065.
- 19.Berwick DM. Crossing the boundary: changing mental models in the service of improvement. Int J Qual Health Care. 1998;10:435–441. [DOI] [PubMed] [Google Scholar]
- 20.Gillon R. Utilitarianism. Br Med J (Clin Res Ed). 1985;290:1411–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agency for Healthcare and Research Quality. Translating Research Into Practice (TRIP)-II. Fact sheet. Rockville, Md: AHRQ; 2001. Available at: http://www.ahrq.gov/research/trip2fac.htm.
- 22.Thomson O’Brien MA, Oxman AD, Davis DA, et al. Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2000:CD000409. [DOI] [PubMed]
- 23.Bennett JW, Glasziou PP. Computerised reminders and feedback in medication management: a systematic review of randomised controlled trials. Med J Aust. 2003;178:217–222. [DOI] [PubMed] [Google Scholar]
- 24.Committee on Quality of Health Care in America, Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
- 25.Shortell SM, Gillies RR, Anderson DA. Remaking Health Care in America. 2nd ed. San Francisco, Calif: Jossey-Bass; 2000.
- 26.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002;288:1775–1779. [DOI] [PubMed] [Google Scholar]
- 27.Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. [DOI] [PubMed] [Google Scholar]
- 29.Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001–1009. [DOI] [PubMed] [Google Scholar]
- 30.Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med. 1998;339:1349–1357. [DOI] [PubMed] [Google Scholar]
- 31.Jones PH, Davidson MH, Stein EA, et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial). Am J Cardiol. 2003;92:152–160. [DOI] [PubMed] [Google Scholar]
- 32.Miller NH, Hill M, Kottke T, Ockene IS. The multilevel compliance challenge: recommendations for a call to action. A statement for healthcare professionals. Circulation. 1997;95:1085–1090. [DOI] [PubMed] [Google Scholar]
- 33.Stamos TD, Shaltoni H, Girard SA, Parrillo JE, Calvin JE. Effectiveness of chart prompts to improve physician compliance with the National Cholesterol Education Program guidelines. Am J Cardiol. 2001;88:1420–1423, A1428. [DOI] [PubMed] [Google Scholar]
- 34.McKillop T. The statin wars [letter]. Lancet. 2003;362:1498. [DOI] [PubMed] [Google Scholar]
- 35.Jacobson TA, Griffiths GG, Varas C, et al. Impact of evidence-based “clinical judgment” on the number of American adults requiring lipid-lowering therapy based on updated NHANES III data. National Health and Nutrition Examination Survey. Arch Intern Med. 2000;160:1361–1369. [DOI] [PubMed] [Google Scholar]
- 36.Pearson TA, Feinberg W. Behavioral issues in the efficacy versus effectiveness of pharmacologic agents in the prevention of cardiovascular disease. Ann Behav Med. 1997;19:230–238. [DOI] [PubMed] [Google Scholar]
- 37.Noonan D. You want statins with that? Newsweek. July 14, 2003:34–48. [PubMed]
- 38.Barnett M. Pill profits. US News and World Report. December 22, 2003;135:40. [PubMed] [Google Scholar]
- 39.Kerlikowske K, Smith-Bindman R, Sickles EA. Short-interval follow-up mammography: are we doing the right thing? J Natl Cancer Inst. 2003;95:418–419. [DOI] [PubMed] [Google Scholar]
- 40.Ostbye T, Greenberg GN, Taylor DH Jr, Lee AM. Screening mammography and Pap tests among older American women 1996–2000: results from the Health and Retirement Study (HRS) and Asset and Health Dynamics Among the Oldest Old (AHEAD). Ann Fam Med. 2003;1:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Health, United States, 2003 With Chartbook on Trends in the Health of Americans. Hyattsville, Md: National Center for Health Statistics; 2003. Available at: http://www.cdc.gov/nchs/data/hus/hus03.pdf. Accessed September 6, 2005. [PubMed]
- 42.Murray CJL, Evans DB, eds. Health Systems Performance Assessment: Debates, Methods and Empiricism. Geneva, Switzerland: World Health Organization; 2003.
- 43.Stevens A, Raftery J, eds. Health Care Needs Assessment. Oxford, England: Radcliffe Medical Press; 1997.
- 44.Unal B, Critchley JA, Fidan D, Capewell S. Life-years gained from modern cardiological treatments and population risk factor changes in England and Wales, 1981–2000. Am J Public Health. 2005;95:103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leatherman S, Berwick D, Iles D, et al. The business case for quality: case studies and an analysis. Health Aff (Millwood). 2003;22:17–30. [DOI] [PubMed] [Google Scholar]
- 46.Herper M. Statin makers bet big on imaging technique. Forbes. November 10, 2003. Available at: http://www.forbes.com/home_europe/2003/11/10/cx_mh_1110merck.html.
- 47.Barrett A, Carey J. A bare knuckle battle over cholesterol drugs. Business Week. July 21, 2003:28.
- 48.Foster G. Bears sink teeth into AstraZeneca. This Is London. November 11, 2003. Available at: http://www.thisislondon.co.uk/news/business/articles/timid70360?source=.
- 49.Gergen and McCurry advise on politics of health care policy: poll confirms public’s “insatiable appetite” for information on medical technology. Presented at: 26th Annual Meeting of the Health Industry Manufacturers Association; March 25, 2000. Available at: http://www.advamed.org/2000annualmeeting/newstories.html. Accessed April 4, 2005.
- 50.Milstein A, Adler NE. Out of sight, out of mind: why doesn’t widespread clinical quality failure command our attention? Health Aff (Millwood). 2003;22:119–127. [DOI] [PubMed] [Google Scholar]
- 51.Medicare program: Negotiated rulemaking: Coverage and administrative policies for clinical diagnostic laboratory services; final rule. CFR 42 66: 410: 58788–58836. Available at: http://cms.hhs.gov/ncd/lab1.pdf. [PubMed]
- 52.Meadows M. The FDA’s drug review process: ensuring drugs are safe and effective. FDA Consumer Magazine. July–August 2002. FDA publication No. 02–3242. [PubMed]
- 53.Smith R, Hiatt H, Berwick D. Shared ethical principles for everybody in health care: a working draft from the Tavistock group. BMJ. 1999;318:248–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adams K, Corrigan JM, eds. Committee on Identifying Priority Areas for Quality Improvement. Board on Health Care Services, Institute of Medicine. Priority Areas for National Action: Transforming Health Care Quality. Washington, DC: National Academies Press; 2003. [PubMed]
- 55.Corrigan JM, Greiner A, Erickson SM, eds. Committee on Rapid Advance Demonstration Projects: Health Care Finance and Delivery Systems, Institute of Medicine. Fostering Rapid Advances in Health Care: Learning from System Demonstrations. Washington, DC: National Academies Press; 2002. [PubMed]
- 56.Committee on the Consequences of Uninsurance, Board on Health Care Services, Institute of Medicine. Insuring America’s Health: Principles and Recommendations. Washington, DC: National Academies Press; 2004. [PubMed]
- 57.Himmelstein DU, Warren E, Thorne D, Woolhandler S. MarketWatch: Illness and injury as contributors to bankruptcy. Health Aff (Millwood). February 2, 2005 [Web exclusive]. Available at: http://content.healthaffairs.org/cgi/reprint/hlthaff.w5.63v1. Accessed September 6, 2005. [DOI] [PubMed]
- 58.Martin JC, Avant RF, Bowman MA, et al. The future of family medicine: a collaborative project of the family medicine community. Ann Fam Med. 2004;2(Suppl 1):S3–S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murray M, Bodenheimer T, Rittenhouse D, Grumbach K. Improving timely access to primary care: case studies of the advanced access model. JAMA. 2003;289:1042–1046. [DOI] [PubMed] [Google Scholar]
- 60.Car J, Sheikh A. Email consultations in health care: 1—scope and effectiveness. BMJ. 2004;329:435–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bodenheimer T, Grumbach K. Electronic technology: a spark to revitalize primary care? JAMA. 2003;290:259–264. [DOI] [PubMed] [Google Scholar]
- 62.Glasgow RE, Bull SS, Piette JD, Steiner JF. Interactive behavior change technology: a partial solution to the competing demands of primary care. Am J Prev Med. 2004;27:80–87. [DOI] [PubMed] [Google Scholar]
- 63.Safran DG. Defining the future of primary care: what can we learn from patients? Ann Intern Med. 2003;138:248–255. [DOI] [PubMed] [Google Scholar]
- 64.Murphy J, Chang H, Montgomery JE, Rogers WH, Safran DG. The quality of physician-patient relationships: patients’ experiences 1996–1999. J Fam Pract. 2001;50:123–129. [PubMed] [Google Scholar]
- 65.The Kaiser Family Foundation/Agency for Healthcare Research and Quality/Harvard School of Public Health. National Survey on Consumers’ Experiences With Patient Safety and Quality Information. Washington, DC: Henry J. Kaiser Family Foundation; 2004. Publication No. 7209.
- 66.Kuzel AJ, Woolf SH, Gilchrist VJ, et al. Patient reports of preventable problems and harms in primary health care. Ann Fam Med. 2004;2:333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coffield AB, Maciosek MV, McGinnis JM, et al. Priorities among recommended clinical preventive services. Am J Prev Med. 2001;21:1–9. [DOI] [PubMed] [Google Scholar]
- 68.Woolf SH, George JN. Evidence-based medicine: interpreting studies and setting policy. Hematol Oncol Clin North Am. 2000;14:761–784. [DOI] [PubMed] [Google Scholar]
- 69.Kohn LT, Corrigan JM, Donaldson MS, eds. Committee on Quality of Health Care in America, Institute of Medicine. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000. [PubMed]
- 70.Szilagyi PG, Bordley C, Vann JC, et al. Effect of patient reminder/recall interventions on immunization rates: a review. JAMA. 2000;284:1820–1827. [DOI] [PubMed] [Google Scholar]
- 71.Schmittdiel J, McMenamin SB, Halpin HA, et al. The use of patient and physician reminders for preventive services: results from a National Study of Physician Organizations. Prev Med. 2004;39:1000–1006. [DOI] [PubMed] [Google Scholar]
- 72.Nielsen-Bohlman L, Panzer AM, Kindig DA, eds. Committee on Health Literacy, Board on Neuroscience and Behavioral Health, Institute of Medicine. Health Literacy: A Prescription to End Confusion. Washington, DC: National Academies Press; 2004. [PubMed]
- 73.Committee on Communication for Behavior Change in the 21st Century: Improving the Health of Diverse Populations, Board on Neuroscience and Behavioral Health, Institute of Medicine. Speaking of Health: Assessing Health Communication Strategies for Diverse Populations. Washington, DC: National Academies Press; 2002.
- 74.Gallagher A. Dignity and respect for dignity—two key health professional values: implications for nursing practice. Nurs Ethics. 2004;11:587–599. [DOI] [PubMed] [Google Scholar]
- 75.Gruen RL, Pearson SD, Brennan TA. Physician-citizens—public roles and professional obligations. JAMA. 2004;291:94–98. [DOI] [PubMed] [Google Scholar]
- 76.Dickey LL, Gemson DH, Carney P. Office system interventions supporting primary care-based health behavior change counseling. Am J Prev Med. 1999;17:299–308. [DOI] [PubMed] [Google Scholar]