Abstract
The theory of exponential dispersion models was applied to construct a stochastic model for heterogeneities in regional organ blood flow as inferred from the deposition of labeled microspheres. The requirements that the dispersion model be additive (or reproductive), scale invariant, and represent a compound Poisson distribution, implied that the relative dispersion (RD = standard deviation/mean) of blood flow should exhibit self-similar scaling in macroscopic tissue samples of masses m and mref such that RD(m) = RD(mref). (m/mref)1−D, where D was a constant. Under these circumstances this empirical relationship was a consequence of a compound Poisson-gamma distribution that represented macroscopic blood flow. The model also predicted that blood flow, at the microcirculatory level, should also be heterogeneous but obey a gamma distribution—a prediction supported by observation.
Keywords: exponential dispersion model, fractals
Regional organ blood flow exhibits a significant degree of spatial heterogeneity when measured by using labeled microspheres or by other means (1, 2). This heterogeneity is apparently not random (there exist correlations between neighboring regions of an organ) and over a certain range the relative dispersion of blood flow reveals a self-similar scaling with respect to the size of the region (3–5). Bassingthwaighte and colleagues (1, 6), based on their observations from the deposition of labeled microspheres, have inferred that the relative dispersion RD(m) of blood flow (ml/min⋅g) within tissue pieces of mass m will scale relative to that from pieces of reference mass mref according to the equation,
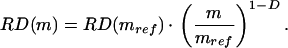 |
1 |
In their work, the constant D has been identified as a spatial fractal dimension.
This empirical scaling has been related to the self-similar branching of vascular trees (3), and to the demands placed by local tissue metabolism (4). As the regions under observation are scaled down to the level of the microcirculation, this relationship presumably would require some modification. We know that the capillary network can contribute to the heterogeneity of capillary perfusion through a complex and variable pattern of redistributed blood flow (7). Blood flow, as indexed by erythrocyte velocity within individual capillaries, also appears quite heterogeneous; the related velocity distribution has been characterized by a gamma distribution (8, 9). How this microcirculatory heterogeneity might relate to the observed macroscopic heterogeneity will be considered here.
In this article, the theory of exponential dispersion models (10) will be employed to provide a stochastic description for the macroscopic and microscopic heterogeneities in regional organ blood flow. The scaling relationship (Eq. 1) will be shown to be a direct consequence of an exponential dispersion model based on a scale invariant compound Poisson-gamma distribution. A hypothesis, relating this Poisson-gamma distribution to possible microcirculatory dynamics, will be presented.
Exponential Dispersion Models
A brief introduction to the relevant aspects of exponential dispersion models that closely follows the work of Jørgensen (10, 11) will be presented here. These models serve as error distributions for generalized linear models (12), and they provide a means to study a large variety of non-normal data. The term exponential dispersion model reflects, in part, the exponential form of the generalized linear model. One class of such models may be expressed by the canonical equation using the interrelated measures νλ,
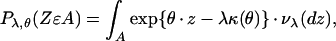 |
which describes the distribution Pλ,θ corresponding to the random variable Z defined upon the measurable sets A; θ is the canonical parameter, κ(θ) = 1/λ log ∫ eθ⋅z⋅vλ(dz) is the cumulant function, λ the index parameter, and z the canonical statistic. Pλ,θ, for a range of values of θ, represents a family of distributions ED*(θ,λ) completely determined by the parameters (θ,λ) and the cumulant function. This family has the property that the distribution of the sum of independent random variables, Z+ = Z1 + … +Zn with Zi ∼ ED*(θ,λi) corresponding to fixed θ and various values of λ, belongs to the family of distributions with the same θ, Z+ ∼ ED*(θ,λ1 + … + λn). These distributions, ED*(θ,λ), are called additive. An additive exponential dispersion model will be employed below to describe the quantity of radioactivity deposited by blood flow within individual tissue fragments of equal mass.
Returning now to the cumulant function, κ(θ), this may be used to construct what is known as a cumulant generating function K*(s) for the additive exponential dispersion model corresponding to the random variable Z:
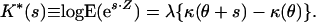 |
2 |
Here, s is a variable used to construct the generating function, and E represents the expectation operator. The cumulant generating function determines the distribution function of Z; it is particularly useful when the distribution function is not easily expressed in closed form (as is the case with the blood flow model). In addition, the first two derivatives of K*(s) at s = 0 provide the mean and variance of Z. And, K*(s) can be used to construct new random variables from simpler variables, a property exploited in the blood flow model.
The function τ(θ) = κ′(θ) gives the relationship between the canonical parameter θ and the mean, μ = κ′(θ), is called the mean value mapping. Using this mapping we can define the variance function, V(μ) ≡ τ′ {τ−1(μ)}, where τ−1(μ) denotes the inverse function rather than the reciprocal. This function is designed to isolate how the variance behaves with respect to the mean. The mean and variance of an additive random variable can be expressed by using these quantities as E(Z) = λμ and var(Z) = λV(μ). In the model for deposited radioactivity given below, both Z and E(Z) will be expressed in units of counts, whereas var(Z) will be in units of counts2.
At this point, a second class of exponential dispersion models should be introduced, with random variable Y = Z/λ ∼ ED(μ,σ2), where σ2 = 1/λ. These models, ED(μ,σ2), are termed reproductive exponential dispersion models, and they are characterized by a convolution property: For n independent random variables Yi ∼ ED(μ,σ2/wi), where the weighting factors wi are summed thus, w = Σi=1n wi, we have under the weighted averaging of the variables 1/w Σi=1n wiYi ∼ ED(μ,σ2/w). This weighted average of independent random variables, corresponding to fixed μ and σ2 and various values of wi, belongs to the family of distributions with the same μ and σ2. A reproductive exponential dispersion model will be employed in the discussion to describe blood flow when expressed in the physiologic units, ml/min⋅g.
Another property that certain exponential dispersion models may possess is called scale invariance. For a reproductive exponential dispersion model ED(μ,σ2) we can require that for any positive constant c, c ⋅ ED(μ,σ2) = ED(cμ,c2−p σ2), where p is a real-valued and unitless constant. Under this scale transformation, the new random variable Ŷ = cY belongs to the same family of distributions with fixed μ and σ2, but different values of c. Under such transformation the variance function obeys the equation, V(cμ) = g(c) ⋅ V(μ), for an appropriate function g(c). Scale invariance implies that g(c) = V(c) and V(μ) = μp. Scale invariant exponential dispersion models have been termed Tweedie models, in recognition of M. C. K. Tweedie's contribution (13) in this area, and p has been called the Tweedie exponent (10).
Two differential equations follow from the properties of exponential dispersion models. The first equation,
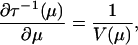 |
gives the relationship between the mean value mapping τ(θ) and the variance function; the second equation,
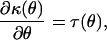 |
expresses how the mean value mapping relates to the cumulant function κ(θ). These two equations may be solved under specific conditions to obtain the cumulant function κ(θ), and thus specify different exponential dispersion models.
In the scale invariant case where V(μ) = μp and p ≠ 1 or 2 the cumulant function takes the form (10, 14),
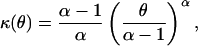 |
3 |
where the parameter α = (p − 2)/(p − 1). In this case, the cumulant generating function for the additive form of the distribution, K*(s) is (10, 14):
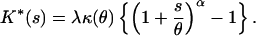 |
4 |
By referring to the properties of cumulant generating functions, Eq. 4 can be shown to represent a compound Poisson distribution generated by a gamma distribution (10, 14, 15). This is essentially the distribution of the sum of N independent identically distributed random variables with a gamma distribution where N is a random variable with a Poisson distribution. Eq. 4, with the use of Eq. 3, yields for this Poisson-gamma distribution a variance to mean power function: var(Z) = a ⋅ E(Z)p, where a = λ1/(α−1) is a proportionality constant.
In general, the Tweedie exponent p characterizes the related distribution (10). Table 1 gives the different distributions that correspond to the values of p along with the domains of support for the associated random variable, and the distributional parameters a, θ, and μ. In the case of regional blood flow, a compound Poisson-gamma distribution is employed with 1 < p < 2 and the random variables being supported as positive real values with 0. The value of p > 1, indeed, implies a degree of clustering of blood flow within certain regions of a given organ.
Table 1.
Summary of Tweedie exponential dispersion models (10)
| Distributions | p Domain on R | Support | α Domain on R | θ Domain | μ Domain |
|---|---|---|---|---|---|
| Extreme stable | p < 0 | R | 1 < α < 2 | R0 | R+ |
| Normal | p = 0 | R | α = 2 | R | R |
| (do not exist) | 0 < p < 1 | nil | α > 2 | R0 | R+ |
| Poisson | p = 1 | N0 | α = −∞ | R | R+ |
| Compound Poisson | 1 < p < 2 | R0 | α < 0 | R− | R+ |
| Gamma | p = 2 | R+ | α = 0 | R− | R+ |
| Positive stable | 2 < p < 3 | R+ | 0 < α < ½ | (−∞,0] | R+ |
| Inverse Gaussian | p = 3 | R+ | α = ½ | (−∞,0] | R+ |
| Positive stable | p > 3 | R+ | ½ < α < 1 | (−∞,0] | R+ |
R ≡ real numbers, R0 ≡ positive real numbers with zero, R+ ≡ positive real numbers, R− ≡ negative real numbers, N0 ≡ positive integers with zero.
From a practical standpoint, the basic mathematical tools needed to construct a blood flow model would include the properties of additivity and scale invariance, and the resultant variance to mean power function. With these tools it is possible to construct a model characterized by Bassingthwaighte's equation (Eq. 1). Because of its nonlinearity, the Poisson-gamma distribution is more difficult to work with. The reader is referred to Jorgensen's book (10) for details regarding exponential dispersion models.
Regional Blood Flow Described by an Exponential Dispersion Model
Here, a stochastic model for regional organ blood flow will be presented that yields Bassingthwaighte's relationship (Eq. 1). One of the most common methods for measuring regional blood flow employs radiolabeled microspheres (16). Here, microspheres 10–15 μm in diameter are injected intravascularly and then become entrapped in the capillaries [diameters 2.5–9 μm (17)] of the target organ (16). The injected animals are killed, the target organ is removed and sectioned into equally sized pieces of tissue, and the amount of radioactivity deposited in each piece is measured. The amount of deposited radioactivity within each piece is assumed proportional to the relative amount of blood flow (ml/min). Blood flow through larger conduit vessels would not be associated with any significant deposition, and thus would be excluded by this approach. In this initial formulation, blood flow will be considered in the context of engineering units (ml/min) so that it can be treated as an additive quantity. A generalization to include physiologic units (ml/min⋅g) will be provided in the discussion to follow. In strict terms, the stochastic model to be derived here describes the heterogeneity in the deposited radioactivity within organs. Since the deposited activity has been accepted in the context of experimental blood flow measurements (16), it should similarly be considered a reasonable surrogate for blood flow in the theoretical model.
To proceed with the model, consider an organ that can be subdivided into n pieces of equal mass. The deposited radioactivity within these pieces will be modeled using an additive exponential dispersion model, and the deposited radioactivity within the individual pieces (measured in terms of the number of radioactive counts per piece) will be represented by the independent random variables Z1, … , Zn. Therefore, it should be possible to sum the activity contained within adjacent organ pieces to reconstitute larger pieces. Thus, in theory, one may consider the regional distribution of deposited radioactivity within a series of reconstituted organ pieces, where the reconstructed pieces can be scaled upwards in size. For simplicity, the development of this model was restricted to the case where the volumes were scaled radially, through one degree of freedom. Thus, the organ tissue was assumed isotropic with respect to all of its properties, including the heterogeneity of deposited radioactivity.
If the model were required to be scale invariant, the variance of the deposited radioactivity within the reconstituted pieces would relate to the mean radioactivity within the pieces according to a power function,
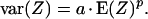 |
5 |
The variances for deposited radioactivity as measured for two different sizes of reconstituted tissue pieces would then be related by the scaling relationship,
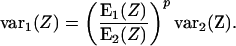 |
Some further manipulation gives a relationship resembling Bassingthwaighte's:
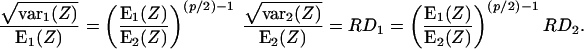 |
Here the exponent p corresponds to Bassingthwaighte's dimension D such that p = 4 − 2D, and the ratio of means corresponds to that of the masses.
The distribution function for the model can now be specified. If the deposited radioactivity in the ith tissue piece Zi is represented by an additive and scale invariant exponential dispersion model, and if the exponent p is restricted such that 1 < p < 2, then the compound Poisson-gamma distribution in the form given by Eq. 4 would be used to describe the distribution of radioactivity among the tissue pieces.
The restricted range of the exponent p implies that Bassingthwaighte's spatial dimension D will also be restricted: 1 < D < 1.5. This restriction was consistent with the usual range observed for D (18). Other values for p (that would be consistent with Eq. 1) were possible, but would have required the formulation of different dispersion models (10, 14).
A relationship resembling Bassingthwaighte's equation (Eq. 1), therefore, can be derived to describe the regional heterogeneity in the macroscopic deposition of labeled microspheres within an organ. This relationship can be viewed as a consequence of an additive and scale invariant exponential dispersion model based on a compound Poisson-gamma distribution. How appropriate this dispersion model is to the description of blood flow (in both engineering terms and physiologic terms) and how it might relate to the microcirculation will be discussed in the sections below, but first we will examine the implications of this model at the level of the capillary circulation.
Implications for the Microcirculation
An exponential dispersion model thus seems reasonable to describe the macroscopic heterogeneities in the regional deposition of radioactivity as a result of organ blood flow. To understand how this model might relate to the microcirculation we need to first review how the deposition of microspheres relates to blood flow, and to how blood flow is defined. The sizes of the radiolabeled microspheres are chosen so that virtually all injected microspheres become entrapped at restrictive sites within the microcirculation. The probability that a restrictive site might entrap a microsphere presumably depends upon the blood flow through the site relative to the flow through all other restrictive sites within the catchment volume (1). In the context of an individual restrictive site, blood flow would be best expressed in normal engineering units such as ml/min. It is this flow through the restrictive site that presumably relates to the chance that a microsphere might be carried to and entrapped within the site.
To interpret the exponential dispersion model in the context of the capillary entrapment of microspheres requires a number of assumptions: (i) Within each equally sized sample of tissue, there exists a random number (i.e., Poisson-distributed) of restrictive sites; (ii) the blood flow (ml/min), at the level of the restrictive sites, obeys a gamma distribution; (iii) the probability of entrapment is directly proportional to the blood flow through the potential traps; and (iv) the blood flow between the individual traps is uncorrelated. (Uncorrelated blood flow between traps does not imply that the blood flow between adjacent pieces of tissue is also uncorrelated—this will be discussed below.) Granted these assumptions, a compound Poisson-gamma distribution may be constructed, in accordance with the cumulant generating function of Eq. 4. Scale invariance and additivity represented additional properties that were imposed for reason of macroscopic requirements to yield Eq. 1.
A relationship resembling Bassingthwaighte's equation (Eq. 1) can therefore be derived, granted certain assumptions regarding both blood flow at the microscopic level and the macroscopic deposition of radioactivity consequent to this blood flow. The biophysical justification for these assumptions, and the validity of the derived equations, will be discussed below.
Discussion
A number of assumptions were made that warrant closer examination. First, there was the use of an exponential dispersion model to describe the deposition of labeled microspheres. Exponential dispersion models have been particularly useful in describing overdispersed distributions, and they allow the dependence of the variance upon the mean to be closely examined (10). Because the observed range for D (1–5) specified an overdispersed distribution and because Bassingthwaighte's equation (Eq. 1) implied a particular relationship between the variance and the mean, an exponential dispersion model seemed appropriate. Moreover, the use of an exponential dispersion model allowed for the imposition of additivity and scale invariance, two properties that were useful in characterizing the macroscopic distribution of deposited radioactivity.
The assumption of additivity was reasonable, because the total deposition of microspheres within a number of tissue samples should represent the sum from individual samples. However, this assumption did limit the description of blood flow to that of engineering terms (ml/min), and it was not immediately obvious that a model so derived would generalize to blood flow measured in physiologic units (ml/min⋅g). Scale invariance was justified on the basis of the scale invariance inherent to Bassingthwaighte's empirical relationship (Eq. 1), and that arising from work with artificial networks (19).
In this model, the deposition of microspheres was considered an indicator of macroscopic blood flow. Blood flow though large conduit vessels is not associated with significant deposition of microspheres, nor physiologically relevant materials, and thus was excluded from consideration.
The physiologic definition of blood flow (ml/min⋅g) can be problematic, particularly if one wishes to consider regional organ blood flow within tissue pieces of nonuniform mass. Because of the normalization with respect to mass, a flow measurement is specific to the whole piece; one cannot apportion out regional contributions of the (mass-normalized) flow from various segments of the piece. Similarly, to reconstitute the blood flow for a collection of tissue pieces requires that the flow (ml/min⋅g) of each piece be weighted by its individual mass, the weighted flows summed, and then the sum divided by the total mass to obtain the reconstituted flow (ml/min⋅g). The additive exponential dispersion model, presented above, did not account for blood flow measured in this manner.
Nevertheless, a closely related exponential dispersion model can be applied to physiologic blood flow measurements. Let the independent random variables Y1, … , Yn, describe the blood flow (ml/min⋅g) in n pieces of tissue that make up an organ, each with corresponding masses w1, … , wn. We can estimate the blood flow in the reconstituted organ Y+ = 1/w Σi=1n wiYi. With a nonadditive variable like Y, a reproductive exponential dispersion models is appropriate. The reproductive model can also be made scale invariant, and thus can possess a variance to mean power function. Scale invariant reproductive models are also Tweedie models, and they have a direct correspondence with their additive counterparts. The reproductive form of the cumulant generating function K(s), that corresponds to Eq. 2 is (10):
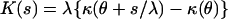 |
The imposition of scale invariance and the requirement that p ≠ 1 or 2 gives an equation analogous to Eq. 4 (10):
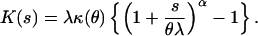 |
6 |
In short, regional blood flow in physiologic terms (ml/min⋅g) can be represented by a scale invariant reproductive exponential dispersion model that is characterized by a power function relationship between the variance and the mean, var(Y) = E(Y)p/λ, resembling that seen with the additive model presented above (Eq. 5). Bassingthwaighte's relationship can be derived from this exponential dispersion model for blood flow expressed in physiologic units (remembering that E(Y) = E(Z)/λ). The probability distribution that arises from this reproductive model (Eq. 6) is also a compound Poisson-gamma distribution, albeit parameterized differently.
The different parameterizations for the reproductive model should be emphasized. The mean and variance of the reproductive random variable Y are, respectively, E(Y) = μ and var(Y) = σ2V(μ). Despite the different parameterizations used in the additive and reproductive models for blood flow, it is the underlying scale invariance that yields the power function relationship seen for the relative dispersion of blood flow. This relationship is apparent for blood flow defined either in engineering terms (ml/min) or physiologic terms (ml/min⋅g). Both models are closely related and they rest upon the same biophysical assumptions.
Another assumption that requires justification was that the blood flow at the different restrictive sites should be uncorrelated. If significant correlations were to exist between the blood flow of neighboring capillaries this could prevent the construction of a compound distribution. Nevertheless, light microscopic studies of the microcirculation done by Ellis et al. (7) revealed a highly complex and uncorrelated movement of blood cells within adjacent vessels. In addition, Tyml (20) measured the coefficient of variation of red cell velocity in skeletal muscle capillaries and found this to range from 49% to 60%, reflecting a considerable heterogeneity in the spatial distribution of the microcirculatory blood flow. And finally, Groom et al. (21) observed a significant temporal heterogeneity in capillary blood flow within skeletal muscle, and they proposed that “even if arteriolar flow were homogeneous, there would still be heterogeneity of capillary flow, because of the structure of the capillary network.” On the basis of these observations, the presumption that any such correlations could be considered negligible therefore seemed reasonable.
The assumption of uncorrelated flow between neighboring capillary traps did not mean that the flows between adjacent macroscopic tissue pieces would also be uncorrelated. Macroscopic correlations in regional blood flow have been well documented (22). Moreover, such correlations are in fact implied by the Poisson-gamma distribution through the power variance function.
The distinction here is that uncorrelated flow at the level of the microcirculation does not imply a lack of correlation within macroscopic flows. In the exponential dispersion model, the gamma-distributed deposition of microspheres at the level of multiple capillaries level was necessarily uncorrelated so that the distributions could be summed to yield the Poisson-gamma distribution. The total deposited activity within macroscopic tissue fragments would involve the summed contributions from multiple capillaries, and it would be these macroscopic deposits that would exhibit near-neighbor correlations by virtue of the imposed scale invariance. Correlations such as these have been well studied for the one-dimensional case in the context of fractal stochastic processes (23).
The assumption that the chance of entrapment should be directly proportional to the blood flow through the restrictive sites in any tissue sample seemed reasonable. This assumption has been implicitly used and verified in macroscopic studies (2). As for the assumption that the number of entrapment sites should be distributed randomly within each tissue piece, according to a Poisson distribution, this too seemed reasonable as a first approximation. Indeed, the anatomic placement of microvessels seems to obey a Poisson distribution (24), and presumably the placement of restrictive sites would follow similar statistics.
We are left with the assumption that the blood flow through the sites of entrapment should be gamma-distributed. Capillary blood flow has been noted to obey a gamma distribution (8, 9). Since the entrapment sites are likely associated with capillaries, and the gamma distribution is additive, it seemed reasonable that the flow through the restrictive sites should also be gamma-distributed. Again, at this point it is important to emphasize the distinction between the flow observed between tissue fragments at the macroscopic level and that observed within capillaries. Bassingthwaighte has observed macroscopic flow distributions that are fairly symmetrical (1), and not as skewed as seen with those measured within individual capillaries by microscopic techniques (8, 9). As noted above, and according to the proposed model, these phenomena are described by two different distributions.
Some of these assumptions, made at the microcirculatory level, may not be strictly accurate. However, in the context of our incomplete understanding of microcirculatory hemodynamics, these assumptions seemed reasonable. The scale invariance inherent to Bassingthwaighte's relationship (Eq. 1) may reflect vascular structure; it may also reflect long-range and continuing demands of local tissue metabolism. These two processes, moreover, may be related. Other interpretations for the exponential dispersion model at the level of the microcirculation, nevertheless, are also conceivable.
As previously mentioned, Bassingthwaighte's relationship (Eq. 1) has been attributed to the branching structure of the vasculature (3), and to the demands of local tissue metabolism (4). The stochastic model presented here provided a kinematic description for the heterogeneities in blood flow, but it did not specify the biophysical origins of scale invariance or additivity. Presumably, these properties could be reflective of vascular structure. Similarly, the reasons why microcirculatory flow might be gamma-distributed remain unclear. A better understanding of microcirculatory dynamics and the dynamics of blood flow in a dichotomous vasculature would be required to pursue these concerns.
This article presents an application of exponential dispersion models, in particular Tweedie models (10, 13), to describe the self-similar pattern of heterogeneous organ blood flow. The dispersion model provided a framework from which Bassingthwaighte's empirical relationship for blood flow (Eq. 1) was a direct consequence of the scale invariance. Although the model was itself stochastic, it nevertheless possessed a nonrandom symmetry—scale invariance. This symmetry underlies Bassingthwaighte's empirical relationship for blood flow (Eq. 1), and it relates to the correlations, which appear between neighboring organ regions (22).
In conclusion, two stochastic models, based upon scale invariant Poisson-gamma distributions, have been derived to describe regional heterogeneities in organ blood flow. The additive model was applied to blood flow described in engineering units (ml/min); the reproductive model to blood flow in physiologic units (ml/min⋅g). Both models implied power-law scaling of the relative dispersion of their respective blood flows attributable to scale invariance. These models were explained on the basis of gamma-distributed blood flow through random restrictive sites within the microcirculation; the scale invariance was attributed to the structure of the vascular tree. The theory of exponential dispersion models, used to derive these models, provides a practical means to examine the effect of scale invariance upon stochastic models, and thus potentially may provide descriptions for other random processes with fractal symmetries.
Acknowledgments
I would like to acknowledge the support provided by the Beattie Library and the Department of Radiation Oncology, both of the Ottawa Regional Cancer Centre.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.021347898.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.021347898
References
- 1.Bassingthwaighte J B, King R B, Roger S A. Circ Res. 1989;65:578–590. doi: 10.1161/01.res.65.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King R B, Raymond G M, Bassingthwaighte J B. Ann Biomed Eng. 1996;24:357–372. doi: 10.1007/BF02660885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Beek J H G M, Roger S A, Bassingthwaighte J B. Am J Physiol. 1989;257:H1670–H1680. doi: 10.1152/ajpheart.1989.257.5.H1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bassingthwaighte J B, Li Z. Am J Cardiol. 1999;83:7H–12H. doi: 10.1016/s0002-9149(99)00250-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bassingthwaighte J B, Beard D A. Circ Res. 1995;77:1212–1221. doi: 10.1161/01.res.77.6.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King R B, Raymond G M, Bassingthwaighte J B. Ann Biomed Eng. 1996;24:352–372. doi: 10.1007/BF02660885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellis C G, Wrigley S M, Groom A C. Circ Res. 1994;75:357–368. doi: 10.1161/01.res.75.2.357. [DOI] [PubMed] [Google Scholar]
- 8.Honig C R, Feldstein M L, Frierson J L. Am J Physiol. 1977;233:H122–H129. doi: 10.1152/ajpheart.1977.233.1.H122. [DOI] [PubMed] [Google Scholar]
- 9.Burton K S, Johnson P C. Am J Physiol. 1972;223:517–524. doi: 10.1152/ajplegacy.1972.223.3.517. [DOI] [PubMed] [Google Scholar]
- 10.Jørgensen B. The Theory of Dispersion Models. London: Chapman & Hall; 1997. pp. 1–7. , 71–174. [Google Scholar]
- 11.Jørgensen B. J R Statist Soc B. 1987;49:127–162. [Google Scholar]
- 12.Nedler J A, Wedderburn R W M. J R Statist Soc A. 1972;135:370–384. [Google Scholar]
- 13.Tweedie M C K. In: Statistics: applications and new directions. Proceedings of the Indian Statistical Institute Golden Jubilee International Conference. Gosh J K, Roy J, editors. 1981. pp. 579–604. [Google Scholar]
- 14.Bar-Lev S, Enis P. Ann Stat. 1986;14:1507–1522. [Google Scholar]
- 15.Feller W. An Introduction to Probability Theory and its Applications. II. NY: Wiley; 1971. pp. 348–349. [Google Scholar]
- 16.Heymann M A, Payne B D, Hoffman J I E, Rudolf A M. Prog Cardiovasc Dis. 1977;20:55–79. doi: 10.1016/s0033-0620(77)80005-4. [DOI] [PubMed] [Google Scholar]
- 17.Kassab G S, Le K N, Fung Y-C B. Am J Physiol. 1999;277:H2158–H2166. doi: 10.1152/ajpheart.1999.277.6.H2158. [DOI] [PubMed] [Google Scholar]
- 18.Glenny R W, Robertson H T, Yamashiro S, Bassingthwaighte J B. J Appl Physiol. 1991;70:2351–2367. doi: 10.1152/jappl.1991.70.6.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beard D A, Bassingthwaighte J B. J Vasc Res. 2000;37:282–296. doi: 10.1159/000025742. [DOI] [PubMed] [Google Scholar]
- 20.Tyml K. Int J Microcirc Clin Exp. 1991;10:75–86. [PubMed] [Google Scholar]
- 21.Groom A C, Ellis C G, Wrigley S J, Potter R F. Int J Microcirc Clin Exp. 1996;15:223–230. doi: 10.1159/000179022. [DOI] [PubMed] [Google Scholar]
- 22.Bassingthwaighte J B, Beyer R P. Physica D (Amsterdam) 1991;53:71–84. [Google Scholar]
- 23.Teich M C, Heneghan C, Lowen S B, Ozaki T, Kaplan E. J Opt Soc Am A. 1997;14:529–546. doi: 10.1364/josaa.14.000529. [DOI] [PubMed] [Google Scholar]
- 24.Honig C R, Odoroff C L, Frierson J L. Am J Physiol. 1980;238:H31–H42. doi: 10.1152/ajpheart.1980.238.1.H31. [DOI] [PubMed] [Google Scholar]


