Abstract
Cauliflower mosaic virus (CaMV) is a DNA-containing pararetrovirus replicating by means of reverse transcription of a terminally redundant pregenomic 35S RNA that is also used as a polycistronic mRNA. The leader of 35S RNA is long, highly structured, and contains multiple short ORFs (sORFs), which strongly interfere with the ribosome scanning process. Translation of this RNA is initiated by a ribosome shunt mechanism, in which ribosomes translate the most 5′-proximal short ORF (sORF A), then skip a large region of the leader containing a putative RNA encapsidation signal and reinitiate translation at the first long viral ORF. Here, we demonstrate that the efficiency of the sORF A-mediated ribosome shunt is an important determinant of viral infectivity. Point mutations in sORF A, which reduced the basal level of shunt-dependent expression and the degree of shunt enhancement by a CaMV-encoded translation transactivator (TAV), consequently reduced infectivity of the virus in turnip plants. First- or second-site reversions appeared in the viral progeny. The second-site reversions restored shuntdependent expression to an extent correlating with their relative abundance in the progeny. Mutations that abolished both the basal and TAV-activated components of shunting proved to be lethal. Finally, by using an artificial stem structure that blocks scanning, we obtained direct evidence that ribosome shunt operates during CaMV infection.
Cauliflower mosaic virus (CaMV) is a plant pararetrovirus with an 8-kbp double-stranded DNA genome (1). It replicates by means of reverse transcription of a terminally redundant pregenomic RNA (35S RNA) transcribed in the nucleus by host RNA polymerase II. The 35S RNA and its spliced derivatives serve as polycistronic mRNAs for viral proteins (2–4). Polycistronic translation depends on the presence of a CaMV-encoded regulator protein, TAV, which is believed to be a translation reinitiation factor (5, 6) (for review see ref. 7). TAV is produced from a monocistronic 19S RNA, the second major viral transcript.
Translation of 35S RNA is initiated by a ribosome shunt (8, 9), in which scanning ribosomes bypass most of the 612-nt-long leader sequence with an extended hairpin structure (10) and multiple short ORFs (sORFs) (11). The bypassed region also includes a putative RNA encapsidation signal that specifically interacts with the viral coat protein (12). It is thought that the ribosome shunt regulates the usage of 35S RNA for both translation and packaging followed by reverse transcription (13).
For shunting to occur, ribosomes must translate a short ORF (sORF A, the most 5′-proximal in the CaMV leader) in a defined distance upstream of the hairpin structure; after the translocation step, they resume scanning and reinitiate at the start sites (14–16). Shunt-dependent translation is enhanced by TAV (15).
Our previous analysis of the 35S RNA leader revealed that mutations in sORF A frequently revert on passage of the respective CaMV mutants in planta (11). This provided evidence that the sORF A-mediated shunt might be important for virus infectivity. In the present work, we show that viability and competitiveness of the virus indeed strongly correlate with the efficiency of TAV-activatable shunting, and we present direct evidence that ribosome shunt operates during CaMV infection.
Methods
Construction of Plasmids.
Plasmid “wild type” used in this study as a baseline control has been previously described as pLC20 (8); it contains a chloramphenicol acetyltransferase (CAT) ORF between the 35S RNA promoter/leader region and the CaMV terminator region. Mutant and revertant versions of the leader were subcloned into pLC20 from pV322 (11) by using the unique sites EcoRV and ClaI. All point mutations were introduced into the CaMV leader sequence of pV322 by the PCR ligation method; their sequences are given in respective figures. The flanking primers and PCR conditions used have been described previously (11). Construction of a simplified, shunt-competent, and TAV-responsive CaMV leader has been described elsewhere (15). In this leader, an extended hairpin structure above stem section 1 was replaced by a short but very stable stem (Kozak stem) that strongly inhibits scanning (17). The Kozak stem in the center of the full-length leader constructs wild type and MABC (11) was created by replacing a fragment between two BglII sites (positions 218 and 238) by a self-complementary oligonucleotide 5′-GATCgggcgcgtggtggcggctgcagccgccaccacgcgccc-3′, yielding KS-Bgl and MABCKS, respectively.
Protoplast Transfections and Reporter Gene Assays.
Protoplasts were prepared from suspension cultures of Orychophragmus violaceus (the Japanese violet, a CaMV host plant) and transfected with plasmid DNA by electroporation as described elsewhere (15). A 10-μg CAT plasmid was always cotransfected with 2.5 μg of a β-glucuronidase (GUS)-expressing plasmid to serve as an internal standard of transfection efficiency. For transactivation, 5 μg plasmid pHELP7 (5) expressing the TAV protein was also added. For each CAT construct, transfections were repeated at least three times with fresh DNA preparations of independent clones and in new protoplast batches. Additional transfections were performed if deviations in the normalized CAT expression were more than 10% of the average value. CAT and GUS assays were performed as in ref. 15.
Virus and Plants.
Construction of CaMV mutants, mechanical inoculation of turnip plants, DNA preparation and PCR, and cloning and sequencing of viral progeny from infected plants were performed as described in detail previously (11). Turnip plants were propagated in a phytobox with illumination for 16 h/day at 22–24°C. The recombinant, aphid nontransmissible CaMV strain Ca540 was used to avoid cross-contamination by aphids. Progeny of viable mutants was passaged at least two times to new turnip plants and sequenced. Usually, the infected tissues of two plants were pooled together for passaging and total DNA isolation. The CaMV leader region was amplified by PCR using Vent polymerase (Biolabs, Northbrook, IL). The resulting PCR product was trimmed by EcoRV and BstEII and cloned into pV322. Eight to ten individual clones for each progeny were sequenced (for further details, see ref. 11).
Results and Discussion
Ribosome Shunt During Virus Infection.
Removal of sORFs A, B, and C abolishes shunting but concomitantly increases the flow of scanning ribosomes migrating through the central region of the CaMV leader (15). This increased flow can overcome the barrier of the remaining sORFs and significantly contribute to downstream expression in plant protoplasts (Fig. 1A, line 2). A CaMV mutant lacking sORFs A, B, and C (11) exhibits a delay in the appearance of symptoms and eventually restores sORF A. We hypothesize that the significant level of scanning-dependent expression could explain the nonlethal phenotype of this CaMV shunt-negative mutant. To test this hypothesis, a short, artificial sequence [“Kozak stem” (KS)] known to block scanning (6, 17), was introduced into the center of the CaMV leader. The combination of KS insertion and sORF A, B, and C mutations completely abolished both expression downstream of the leader in plant protoplasts and infectivity of the corresponding CaMV mutant in turnip plants (Fig. 1A, cf. lines 2 and 3).
Figure 1.
(A) Effects of the Kozak stem (KS) element inserted into the center of the CaMV leader on shunt- (line 4 vs. 1) and scanning-dependent (line 3 vs. 2) expression in plant protoplasts as well as on infectivity of the corresponding CaMV mutants in turnip plants. Several sORFs in the leader are shown by boxes, point mutations of the sORFs A, B, and C indicated by crosses. Relative CAT expression levels in the presence or the absence of TAV are given. (B) Deletions and insertions generated in planta on passage of the CaMV mutant containing the 38-nt KS sequence (in lowercase) between positions 222 and 239 of the 35S RNA leader. Direct repeats at the borders of four deletions are shown by arrows. Insertions in the two deletion sites are indicated by triangles. (C) An example of restoration of stem section 3 in the CaMV leader structure because of the deletions/insertions. The extended hairpin structure of the wild-type leader comprising three stem sections and the bowl region with the putative RNA encapsidation signal is depicted schematically. The ribosome shunt pathway bypassing the hairpin is indicated by a curved arrow; the shunt-mediating sORF A is shown by a box. Close ups of the stem section 3 region of wild-type, mutant, and revertant leaders are shown. Nucleotides differing from the wild-type sequence are in lowercase. Numbering is from the 5′ end of 35S RNA (new numbering for the deletion/insertion variants is in italics).
In contrast, the KS element alone did not drastically reduce downstream expression (Fig. 1A, cf. lines 1 and 4), confirming that ribosome shunt is a main initiation mechanism on the CaMV leader. The corresponding CaMV mutant was infectious; all four plants inoculated developed normal symptoms, although with a significant delay (Fig. 1A, line 4).
Sequence analysis of the progeny of the mutant virus revealed that the KS sequence was unstable. After the first infection cycle, five types of deletions ranging from 41 to 55 nt were found that removed this sequence fully or partially (Fig. 1B, deletions 1–5). Four of these deletions could be assigned to forward jumps of the reverse transcriptase (RT) complex with the nascent DNA minus strand by using short direct repeats (shown by arrows in Fig. 1B). However, deletion 5 must have occurred by means of a more complicated scenario, because a 3-nt insertion was found at the site of deletion (Fig. 1B). A mechanism for such deletions/insertions has been proposed for RNA viruses (18). In CaMV, transient template misalignment and premature template switch by the RT complex are the most likely mechanisms explaining deletion/insertion and recombination events during viral evolution (19, 20). Finding these characteristics, which suggest the involvement of reverse transcription in the removal of the KS sequence, provides supportive evidence for ribosome shunt in CaMV, because translation of the KS-containing RNAs had to occur before reverse transcription.
After two passages, the revertants with deletions 3 and 5 evolved further by acquisition of a 7-nt insertion in the previous deletion site (insertion 3 in Fig. 1B) and a 21-nt deletion in a second site (from T404 to G424), respectively. This was indicative of possible restoration of the original RNA secondary structure affected by the Kozak stem. By using the MFOLD program (Wisconsin Package, Version 9.0; Genetics Computer Group, Madison, WI) we analyzed the secondary structures of the mutant and revertant CaMV leaders. Indeed, a tendency for most of the deletions and insertions to restore integrity of stem section 3 was observed (Fig. 1C, and other results not shown). The importance of stem section 3 was also inferred from a previous study, where compensatory point mutations generated in planta restored the formation of a 10-bp helix in this region (11). We have also observed that a deletion of 14 nt between the two BglII sites, which were replaced in this study by the KS sequence, itself led to the appearance of insertions of 16 or 29 nt in planta by duplication of the sequence located upstream of the deletion site, most likely by backward jumps of the RT complex, which restored stem section 3 (M.M.P., unpublished observations). We assume that integrity of stem section 3 is important for proper formation and/or exposure of a putative RNA encapsidation signal comprising the upper part of stem section 3 and the bowl region on its top (Fig. 1C). This part of the leader can specifically interact with the viral coat protein (12), an event thought to initiate 35S RNA encapsidation into the previrion where reverse transcription occurs. Thus, the Kozak stem most likely interferes with packaging/reverse transcription rather than gene expression.
In summary, we obtained direct evidence that shunting operates during CaMV infection, because insertion of the KS element that blocks scanning in the CaMV leader did not abolish infectivity. However, the KS sequence was unstable in viral progeny, possibly because of interference with the formation of secondary structure in the central part of the leader.
Effects of Mutations in the Termination Site and Coding Content of sORF A on CaMV Infectivity.
According to the ribosome shunt model, the proper position of the sORF A stop codon a few nucleotides upstream of the CaMV leader hairpin is a critical parameter (14, 15). Indeed, a point mutation in the stop codon (UAG to UAC) that elongates sORF A by an additional 23 codons almost abolished shunt-dependent expression both in the absence and the presence of TAV (Fig. 2, line 2) and severely reduced infectivity of the virus (11). In contrast, a mutation of the same nucleotide that only changed the nature of the stop codon (UAG to UAA) affected neither shunt-dependent expression nor infectivity of the virus (Fig. 2, line 3). This mutation was stable for two passages in turnip plants.
Figure 2.
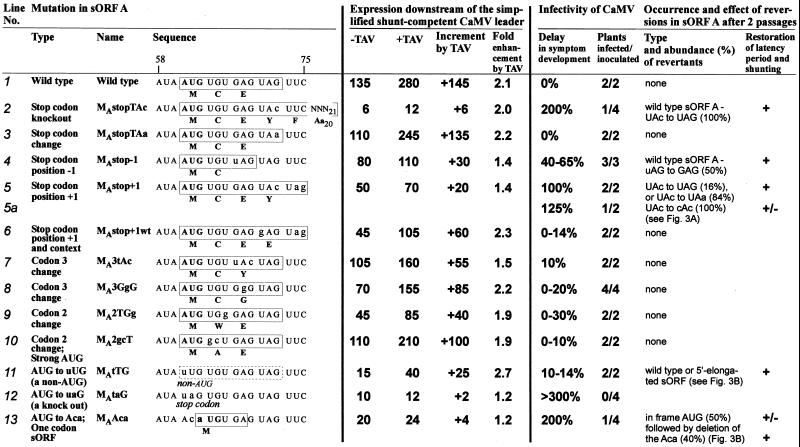
Correlation between the efficiency of the sORF A-mediated ribosome shunt and infectivity of CaMV. Types, names, and sequences of the sORF A point mutations are shown. Mutated nucleotides are in lowercase. sORF variants are boxed; amino acid sequences indicated below. Expression levels of a CAT reporter ORF placed downstream of the simplified, shunt-competent and TAV-responsive CaMV leader (described in ref. 15 and Methods) are given relative to the nontransactivated expression downstream of the wild-type CaMV leader set to 100% (Fig. 1A). With the simplified leader, a contribution of scanning-dependent expression because of mutations in sORF A could be almost completely excluded (15). Consequently, most of the mutations resulted in a slightly less pronounced negative effect in the context of the wild-type leader (Fig. 3, and other results not shown). Infectivity of the CaMV mutants was studied in turnip plants as described previously (11); delays in symptom development after the first inoculation with viral DNA are given in percent with respect to the time required for symptom appearance with the wild-type virus (i.e., 20 days = 100%). All of the viable mutants developed symptoms visually indistinguishable from the wild-type symptoms characteristic of strain Ca540 (see Methods) even after long delays and reversions.
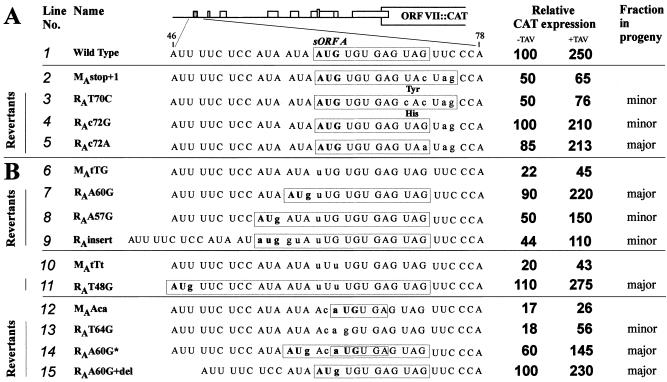
Displacement of the sORF A stop codon by one triplet upstream or downstream moderately reduced the basal level of shunt-dependent expression and, more strongly, the level of TAV-activated expression (Fig. 2, lines 4 and 5). Both of these mutations led to delayed symptom development and appearance of reversions in the progeny. For the first mutant, 50% of the second passage progeny contained a true reversion of the original sORF A by a T to G substitution, whereas the rest of the sequenced genomes retained the introduced mutation (Fig. 2, line 4). The second mutant with less efficient shunting had reversions in all cases. In one mixed progeny of two infected plants, two types of reversions were found; both restored a stop codon at the wild-type position (UAC to UAG or UAA; Fig. 2, line 5). Thus, both revertant sequences included two stop codons in a row and, when tested in the protoplast system, almost fully restored shunt-dependent expression and its responsiveness to TAV (Fig. 3A, lines 4 and 5 vs. 1). Another progeny that appeared only in one of the two plants inoculated by an independent clone of this mutant evolved a second site mutation changing the mutated UAC triplet to CAC (Fig. 2, line 5a). This reversion, however, did not fully restore the latency period after two passages (the delay was still 28%). Also, it did not improve the decreased basal level of expression and only slightly restored TAV-activated expression (Fig. 3A, cf. lines 2 and 3). Finding of such a reversion suggested that a suboptimal position of the stop codon might be partially compensated by the nature of the preceding codon. To evaluate this hypothesis, the UAC codon preceding the shifted stop codon was converted to GAG, which is the penultimate codon in the wild-type sORF A. Although the basal level of shunt-dependent expression was not restored with this mutation, confirming that the shifted position of the stop codon is indeed suboptimal, an effective response to TAV was restored and the corresponding CaMV mutant exhibited only a slight delay in symptom development; the introduced mutation was stable for two passages (Fig. 2, line 6).
Figure 3.
Reversions in the sORF A stop (A) and start (B) sites generated in planta restore shunt-dependent expression in plant protoplasts. Mutations in sORF A and the corresponding reversions are depicted, as well as their relative abundance in the progeny. The sequence of the sORF A region preceding the hairpin structure is shown; nucleotides differing from the wild-type sequence are in lowercase; AUG codons are in bold. sORFs are boxed.
Previous work on the shunt mechanism has established that the particular coding content of sORF A is not critical for efficient shunting, unless sORF A is replaced by a regulatory sORF that can conditionally repress downstream reinitiation [e.g., by the “MAGDIS” peptide-encoding sORF (16); or by the yeast GCN4 sORF 4 (15)]. In the present study, we observed that four different point mutations, which modify either the second or the third amino acid of sORF A without changing the position of the start and stop codons, only slightly increased the latency period of the virus and were stable after two passages in turnip plants (Fig. 2, lines 7–10). This confirms that the sORF Aencoded peptide is not required for infectivity. In the protoplast system, we did observe a moderate decrease in shunt-dependent expression because of some of these mutations (Fig. 2, lines 7–9). In two of these cases, the basal level of shunting was decreased (lines 8 and 9), and, in one case, the response to TAV was selectively compromised (line 7). Notably, in this latter mutant as well as in the mutant with shifted stop codon (Fig. 2, line 5), a UAC codon at the penultimate position correlated with a reduced responsiveness to TAV.
In summary, we obtained in planta evidence that a proper position of the sORF A stop codon is critical for viral infectivity, which strongly correlated with efficiency of shunt-dependent expression in plant protoplasts. We also demonstrated that the degree of shunt enhancement by TAV influenced by the penultimate codon of the sORF is another important parameter that could improve infectivity when the position of the stop codon was suboptimal.
Effects of Mutations in the Initiation Site of sORF A.
We have previously observed that replacement of the sORF A AUG start codon by UUG still allows some shunting both in the in vitro translation system (14) and in plant protoplasts in the presence of TAV (15). Also, CaMV mutants lacking the sORF A AUG start codon because of point mutations to UUG or ACG did not exhibit any drastic delay of symptom development; first- or second-site reversions creating an in-frame AUG arose slowly and dominated in the progeny only after the third passage (11). Because both UUG and ACG have been reported to function as non-AUG initiation codons in plant cells (21), and given that a stem structure downstream of a non-AUG start codon favors its use (22), a reasonably substantial rate of initiation might be expected allowing a level of sORF A-mediated shunt sufficient to rescue infectivity. To completely destroy ORF A, we replaced its start codon with the UAG stop codon. This codon should exclude any initiation at the given position and terminate translational events that may have originated at the two in-frame AUA triplets located directly upstream of the original start codon as well as at other non-AUG start codons located still further upstream (see the sequence in Fig. 3). As expected, the resulting viral mutant was not infectious at all (Fig. 2, line 12), thus verifying that sORF A translation is essential for CaMV viability.
Comparison of the effects of the UUG and UAG mutations on expression in protoplasts revealed an important difference: both mutations drastically reduced the basal level of shunting, but an effective response to TAV was observed only in the case of the UUG mutation (Fig. 2, cf. lines 11 and 12), indicating activation of shunting.
The UUG mutation and other mutations modifying the start site of sORF A resulted in a number of second site reversions in planta creating in-frame AUG codons that open N-terminally extended variants of sORF A (11) (Fig. 2). The effect of these reversions on shunt-dependent expression was tested. All of them restored expression fully or partially and allowed effective transactivation by TAV (Fig. 3B). The relative abundance of revertants in the progeny correlated well with the degree of restoration of expression (Fig. 3B).
The expression level supported by sORF A variants with different length or coding content varied. The longest sORF generated by a second site reversion in the mutant MAtTt (11) encoded eight amino acids and fully restored expression (Fig. 3B, line 11), whereas some shorter versions only partially restored expression (e.g., line 9). This again indicates that the coding potential of sORF might modulate efficiency of shunting/reinitiation.
Mutation of the sORF A AUG start codon to ACA created a one-codon (start/stop) sORF, which severely reduced downstream translation and viral infectivity (Fig. 2, line 13). Reversions appeared already in the first infection cycle (11). Again, the most frequently recovered revertants, or those dominating in later passages, restored expression significantly (Fig. 3B, lines 14 and 12) or completely (lines 15 and 1). A reversion observed in only one clone destroyed the one-codon sORF without introducing any start codon. This reversion increased expression only slightly and only in the presence of TAV (Fig. 3B, lines 13 and 12), thus resembling the sORF start codon knockout mutants described above. Apparently, the one-codon sORF without peptide elongation step cannot functionally substitute sORF A in mediating ribosome shunt (see ref. 15) and is, therefore, deleterious for CaMV infectivity.
In summary, the analysis of second site reversions of the sORF A AUG start codon revealed the close correlation between ribosome shunt efficiency and revertant competitiveness in progeny. This analysis also established that, in contrast to the stop codon position, the location of the sORF A start codon is not critical for efficient shunting. It is conceivable, however, that increasing the length of sORF A beyond a certain threshold would abolish shunt-dependent expression, because the ability of a ribosome to reinitiate translation is inversely correlated with the length of upstream ORF (23).
TAV-Induced Component of Shunt-Dependent Translation Is an Important Determinant of CaMV Infectivity.
An increased latency period of sORF A mutants and the appearance of reversions always correlated with a defect in shunting (Fig. 2). Conversely, not every reduction of shunt efficiency also led to a big delay in symptom development and to reversions (e.g., Fig. 2, lines 6, 9 and 11). Only those mutations that reduced the responsiveness of shunt-dependent expression to TAV exhibited a drastic reduction of infectivity (e.g., Fig. 2, lines 4, 5, 13).
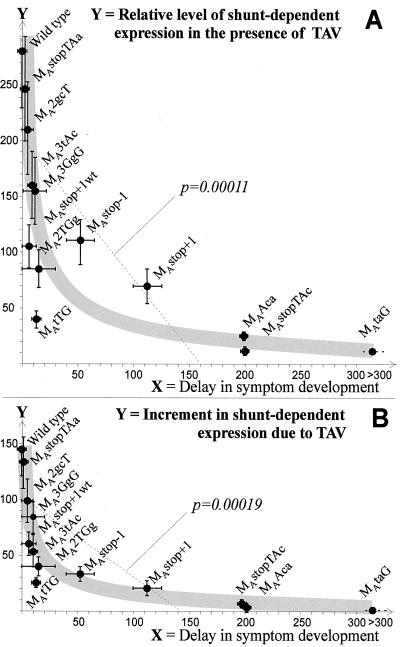
Mathematical calculations revealed a statistically significant inverse correlation (with a linear regression coefficient P < 0.05) between the delay in symptom development and both the TAV-activated expression level (P = 0.00011, Fig. 4A) and the increment of the expression because of TAV (P = 0.00019, Fig. 4B). In the latter case, a hyperbolic regression curve reflects the correlation best, with only one point falling out (corresponding to the UUG mutant; Fig. 4B). However, this particular mutant had a high reversion rate that reflects a defect in infectivity despite only a slight delay in the appearance of symptoms. Notably, all sORF A mutations with an increment value <40, i.e., 27.5% of the wild-type increment, were either unstable in planta leading to reversions restoring shunting, or lethal. Thus, we conclude that the TAV-activated component of shunt-dependent expression is a particularly important determinant of CaMV infectivity.
Figure 4.
The hyperbolic correlations between the delay in CaMV symptom development and both (A) transactivated shunt-dependent expression and (B) the increment in shunt-dependent expression because of TAV (shown with gray curves). A statistically significant (P < 0.05) linear regression that roughly reflects this inverse correlation in both cases is represented by a dashed line with the calculated regression coefficient P value. Delays are according to Fig. 2. Increments were calculated as the expression value in the presence of TAV minus the value in the absence of TAV (Fig. 2). Deviations from the average values are shown with error bars. The names of corresponding CaMV mutants are given.
It has to be considered that the analyses of shunting and infectivity use two different assay systems. Translational regulation events often respond to cellular conditions (e.g., ref. 24) and are therefore variable in different systems. For example, it is unknown how efficiently non-AUG codons like the UUG in the mutant MAtTG are recognized in vivo. Furthermore, we have shown before that the efficiency of shunt-dependent expression can be very variable in different types of plant cells (25) and the efficiency of the basal shunt in virus-infected cells is difficult to establish. Shunting requires translation reinitiation (15, 16), and work in other systems has shown that reinitiation efficiency can depend on metabolic conditions (e.g., ref. 26). It is therefore possible that, in infected cells, the nonactivated shunt-dependent expression is lower than in our assay system and that the reinitiation enhancement by TAV is more important to obtain expression at all. In this case, TAV would not only be required to allow translation of the further downstream ORFs on the CaMV 35S RNA (2, 3) but would be already involved in determining whether any considerable translation occurs beyond sORF A.
Possible Role for Ribosome Shunt in CaMV.
Several lines of evidence presented here lead us to conclude that the sORF A-mediated shunt mechanism is essential for CaMV infectivity. What could be the role of ribosome shunt? We hypothesize that this mechanism regulates the usage of 35S RNA for both translation and replication. Shunting leaves the upper part of the leader structure, which harbors the putative encapsidation signal (12), free of scanning ribosomes and therefore exposed for the interaction with the viral coat protein. In fact, the CaMV mutants with the AUG-free leader or the leader lacking AUGs of sORF A, B, and C, which allow efficient scanning-dependent translation (15) (Fig. 1A), exhibit significant delays in symptom development (11). This could be explained by the negative effect of scanning ribosomes on the coat protein binding, either because of direct steric hindrance, or because scanning prevents structure formation. The pattern of dominant reversions in these leaders generated in planta clearly shows that sORF A is most frequently restored, whereas other reversions mainly tend to restore the secondary structure, especially in the upper part of the leader hairpin (11). Taken together, our findings support the key role of sORF A in a shunt mechanism that diverts ribosomes from migration through the center of the leader and are also indicative for the importance of the structure formation for other processes, most likely including packaging.
A growing number of reports describe ribosome shunt or related processes operating on viral RNAs (27–31) and a few cellular mRNAs (31, 32), suggesting that shunting is a general translation mechanism reflecting an intrinsic property of the eukaryotic ribosome. The present study provides an example of ribosome shunt functioning in a biologically relevant context. We predict that the role of shunting in preserving RNA structural elements during translation initiation, as suggested here, will prove to be of general importance.
Acknowledgments
We thank Matthias Müller, Sandra Corsten, and Herbert Angliker for expert technical assistance. Special thanks go to Sebastian Bonhoeffer for mathematical calculations and discussions. We are grateful to Helen Rothnie for critical reading of the manuscript. This work was supported with an EMBO short-term and FEBS long-term fellowships (to M.M.P.) and also by the International Association for the promotion of cooperation with scientists from the New Independent States of the former Soviet Union Grant 96.857.
Abbreviations
- CaMV
cauliflower mosaic virus
- sORF(s)
short ORF(s)
- TAV
a CaMV-encoded translation transactivator protein
- CAT
chloramphenicol acetyltransferase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Hohn T, Fütterer J. Crit Rev Plant Sci. 1997;16:133–161. [Google Scholar]
- 2.Fütterer J, Bonneville J-M, Gordon K, De Tapia M, Karlsson S, Hohn T. In: Post-Transcriptional Control of Gene Expression. McCarthy J E G, Tuite M F, editors. Berlin: Springer; 1990. pp. 347–357. [Google Scholar]
- 3.Scholthof H B, Gowda S, Wu F C, Shepherd R J. J Virol. 1992;66:3131–3139. doi: 10.1128/jvi.66.5.3131-3139.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiss-László Z, Blanc S, Hohn T. EMBO J. 1995;14:3552–3562. doi: 10.1002/j.1460-2075.1995.tb07361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonneville J-M, Sanfaçon H, Fütterer J, Hohn T. Cell. 1989;59:1135–1143. doi: 10.1016/0092-8674(89)90769-1. [DOI] [PubMed] [Google Scholar]
- 6.Fütterer J, Hohn T. EMBO J. 1991;10:3887–3896. doi: 10.1002/j.1460-2075.1991.tb04958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pooggin M M, Ryabova L A, Hohn T. In: Plant Viruses as Molecular Pathogens. Khan J A, Dijkstra J, editors. New York: Harworth; 2000. , in press. [Google Scholar]
- 8.Fütterer J, Gordon K, Sanfaçon H, Bonneville J-M, Hohn T. EMBO J. 1990;9:1697–1707. doi: 10.1002/j.1460-2075.1990.tb08293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fütterer J, Kiss-László Z, Hohn T. Cell. 1993;73:789–802. doi: 10.1016/0092-8674(93)90257-q. [DOI] [PubMed] [Google Scholar]
- 10.Hemmings-Mieszczak M, Steger G, Hohn T. J Mol Biol. 1997;267:1075–1088. doi: 10.1006/jmbi.1997.0929. [DOI] [PubMed] [Google Scholar]
- 11.Pooggin M M, Hohn T, Fütterer J. J Virol. 1998;72:4157–4169. doi: 10.1128/jvi.72.5.4157-4169.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guerra-Peraza O, De Tapia M, Hohn T, Hemmings-Mieszczak M. J Virol. 2000;74:2067–2072. doi: 10.1128/jvi.74.5.2067-2072.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pooggin M M, Fütterer J, Skryabin K G, Hohn T. J Gen Virol. 1999;80:2217–2228. doi: 10.1099/0022-1317-80-8-2217. [DOI] [PubMed] [Google Scholar]
- 14.Dominguez D I, Ryabova L A, Pooggin M M, Schmidt-Puchta W, Fütterer J, Hohn T. J Biol Chem. 1998;273:3669–3678. doi: 10.1074/jbc.273.6.3669. [DOI] [PubMed] [Google Scholar]
- 15.Pooggin M M, Hohn T, Fütterer J. J Biol Chem. 2000;275:17288–17296. doi: 10.1074/jbc.M001143200. [DOI] [PubMed] [Google Scholar]
- 16.Ryabova L A, Hohn T. Genes Dev. 2000;14:817–829. [PMC free article] [PubMed] [Google Scholar]
- 17.Kozak M. Mol Cell Biol. 1989;9:5134–5142. doi: 10.1128/mcb.9.11.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilipenko E V, Gmyl A P, Agol V I. Nucleic Acids Res. 1995;23:1870–1875. doi: 10.1093/nar/23.11.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chenault K D, Melcher U. Biochimie. 1994;76:3–8. doi: 10.1016/0300-9084(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 20.Hohn T. In: Homologous Recombination and Gene Silencing in Plants. Paszkowski J, editor. Dordrecht, The Netherlands: Kluwer; 1994. pp. 25–38. [Google Scholar]
- 21.Gordon K, Fütterer J, Hohn T. Plant J. 1992;2:809–813. [PubMed] [Google Scholar]
- 22.Kozak M. Proc Natl Acad Sci USA. 1990;87:8301–8305. doi: 10.1073/pnas.87.21.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fütterer J, Hohn T. Nucleic Acids Res. 1992;20:3851–3857. doi: 10.1093/nar/20.15.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ling J, Wells D R, Tanguay R L, Dickey L F, Thompson W F, Gallie D R. Plant Cell. 2000;12:1213–1228. doi: 10.1105/tpc.12.7.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fütterer J, Gordon K, Pfeifer P, Sanfaçon H, Pisan B, Bonneville J-M, Hohn T. Virus Genes. 1989;3:45–55. doi: 10.1007/BF00301986. [DOI] [PubMed] [Google Scholar]
- 26.Hinnebusch A G. J Biol Chem. 1997;272:21661–21664. doi: 10.1074/jbc.272.35.21661. [DOI] [PubMed] [Google Scholar]
- 27.Fütterer J, Potrykus I, Bao Y, Li L, Burns T M, Hull R, Hohn T. J Virol. 1996;70:2999–3010. doi: 10.1128/jvi.70.5.2999-3010.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Latorre P, Kolakofsky D, Curran J. Mol Cell Biol. 1998;18:5021–5031. doi: 10.1128/mcb.18.9.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Remm M, Remm A, Ustav M. J Virol. 1999;73:3062–3070. doi: 10.1128/jvi.73.4.3062-3070.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yueh A, Schneider R J. Genes Dev. 1996;10:1557–1567. doi: 10.1101/gad.10.12.1557. [DOI] [PubMed] [Google Scholar]
- 31.Yueh A, Schneider R J. Genes Dev. 2000;14:414–421. [PMC free article] [PubMed] [Google Scholar]
- 32.Carter P S, Jarquin-Pardo M, DeBenedetti A. Oncogene. 1999;18:4326–4335. doi: 10.1038/sj.onc.1202890. [DOI] [PubMed] [Google Scholar]