Abstract
The F1Fo-type ATP synthase is the smallest motor enzyme known. Previous studies had established that the central γ and ɛ subunits of the F1 part rotate relative to a stator of α3β3 and δ subunits during catalysis. We now show that the ring of c subunits in the Fo part moves along with the γ and ɛ subunits. This was demonstrated by linking the three rotor subunits with disulfide bridges between cysteine residues introduced genetically at the interfaces between the γ, ɛ, and c subunits. Essentially complete cross-linking of the γ, ɛ, and c subunits was achieved by using CuCl2 to induce oxidation. This fixing of the three subunits together had no significant effect on ATP hydrolysis, proton translocation, or ATP synthesis, and each of these functions retained inhibitor sensitivity. These results unequivocally place the c subunit oligomer in the rotor part of this molecular machine.
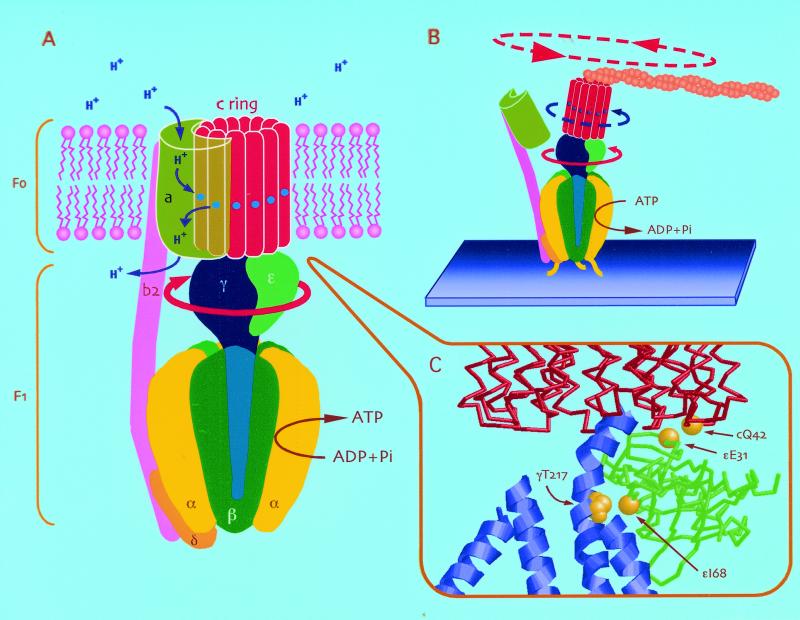
Most of the ATP required by a cell is produced by an F1Fo ATP synthase using the energy of a transmembrane proton electrochemical gradient that is generated by the respiratory chain or in photosynthesis. This enzyme can work in reverse as a proton pump by hydrolyzing ATP to ADP and Pi. F1Fo-type ATP synthases are composed of two parts, as the name implies: a cytoplasmic F1 part (α3β3γδɛ) and a membrane-embedded Fo part (ab2c10–12 in bacterial F1Fo) (Fig. 1A). F1 has been well characterized by high resolution structural studies of the α3β3γδɛ subunit complexes of beef heart and Escherichia coli (1–3). The α and β subunits alternate around a central cavity, within which is located a part of the γ subunit. The γ subunit extends from below the α3β3 domain into a central stalk some 40–45 Å long, where it interacts with the ɛ subunit. This central stalk is a major interaction point of F1 with Fo. In its simplest form, as in E. coli, Fo consists of a ring of 12 c subunits, to the outside of which are attached the a and b subunits (4, 5). The two copies of the b subunit extend to the top of F1, where they interact with the δ subunit (6, 7). They form the outer stalk now known to link the two domains (8–10).
Figure 1.
(A) Structural and functional model of the Escherichia coli F1Fo ATP synthase. The cytoplasmic F1 portion (α3β3γδɛ) and membrane-embedded Fo portion (ab2c12) are connected through two stalks, a central stalk of γ and ɛ, which links to the c subunit ring, and an outer stalk (δb2), linking α3β3 to the a subunit. It has been proposed that the a subunit and the c ring provide the pathway for proton translocation, with a proton binding to Asp-61 (shown with a blue circle) as each c subunit enters the interface with the a subunit (the proton inlet channel), and the protonated binding site then leaves the interface of the a subunit and moves into the lipid phase. After 12 steps of this event, the proton is released to the outlet channel in the a subunit as it reenters this interface. Inhibitors such as N,N′-dicyclohexylcarbodiimide (DCCD) and venturicidin are known to bind to the Asp-61 of subunit c and thereby interfere with the protonation of this residue. (B) Previous observations of ATP-driven c ring rotation by Sambongi et al. (25) and Pänke et al. (27) are flawed. Disruption by detergents of the interaction between the a subunit and the c ring as shown (and/or between the δ and b subunits) results in an ATP hydrolysis-driven rotation of γ and ɛ, which briefly and artificially moves the c ring. However, there is no proton pumping, and the enzyme is not affected by F1Fo-specific inhibitors (see ref. 26). (C) The structural model of the interface of the γ, ɛ, and c ring. The positions of Cys residues introduced to allow the γ-ɛ-cc′ cross-linking are shown with space-filling spheres. The model was created based on the unrefined Cα model (1qo1) and E. coli γ subunit coordinates (1d8s) in the Protein Data Bank.
There are three catalytic sites in F1, one at the interface of each of the three αβ subunit pairs (1). These catalytic sites must be linked functionally to proton translocation within Fo. Genetic studies indicate that proton translocation occurs at the interface between a and c subunits (11). The mechanism of this linkage, called coupling, is now becoming clear. It involves a rotation of the γ subunit driven by sequential ATP synthesis (or hydrolysis) such that the central stalk undergoes one full rotation in three 120° steps for every three ATP molecules synthesized or hydrolyzed (one per catalytic site; ref. 12). This rotary mechanism, predicted by Boyer and Kohlbrenner (13), has been dramatically demonstrated in single-molecule studies using F1 (α3β3γ part) (14). Rotational motion was visualized by attaching a fluorescently labeled actin filament to the γ subunit and observing this move relative to the α3β3 part, which had been immobilized on a glass surface. The ATP-driven rotation of the γ subunit was found to be unidirectional (i.e., counterclockwise when F1 is observed from the periplasmic side, that is from the side closest to Fo). Rotation of the ɛ subunit was subsequently observed by using the same method with F1 (α3β3γɛ) (15).
As the γ and ɛ subunits are intimately attached to the c subunit ring (16–18), the rotation of the central stalk can be predicted to accompany a rotation of the c subunit ring, which would bring each c subunit into interaction with the a subunit (19–21). Such a rotation of the c subunits with respect to the a subunit provides a testable model of coupling within F1Fo (see Fig. 1A). Thus, in ATP synthesis, a proton gradient would drive the rotation of the c subunit ring (clockwise when viewed from a periplasmic side), which would allow the γ subunit to interact sequentially with the three αβ pairs in a way that favors synthesis of ATP in the catalytic sites, each from already bound ADP and Pi. In this mechanism, three molecules of ATP would be synthesized in one full turn of the rotor (requiring translocation of 12 protons), which gives an ATP/proton ratio of 1:4, close to that observed experimentally (22–24). In the reverse direction (ATP hydrolysis), it can be imagined that rotation of the γ-ɛ-c rotor (counterclockwise) brings c subunits that had been protonated from a cytoplasmic side (at Asp-61 in E. coli numbering system) into contact with the a subunit for deprotonation and subsequent release of the proton on the opposite side of the membrane. Clearly, unequivocal evidence that the rotation of the c subunit ring does accompany rotation of the γɛ part would be strong support for the above mechanism.
Three recent studies have visualized rotation of the c subunit ring by using the same single-molecule technique used for showing the rotation of the γ and ɛ subunits (as shown in Fig. 1B; refs. 25–27). However, none of these studies were conducted with fully functional enzyme because in each case, the F1Fo being used no longer showed a significant sensitivity to the inhibitors generally used to show that the F1Fo ATP synthase is coupled (26, 28). Additionally, ATP synthesis was not measured directly in any of these studies. A complication of single-molecule studies is that isolation of monodisperse membrane protein requires using levels of detergent that remove most or all of the phospholipid, without which the protein complex is unstable and disrupted. We have suggested that lipid depletion results in disruption of the interaction between the a and c subunits. This proposal is supported by our own experiments with E. coli F1Fo (B. Schulenberg and R.A.C., unpublished results), and by crystallization studies of yeast F1Fo, which show that a complex of α3β3γδɛ and the c subunit ring is readily separated from other subunits, including the a subunit, by detergent treatments (16).
For the above reasons, we have sought an alternative approach to show that the c subunit ring moves with the γ and ɛ subunits, which can be conducted with membrane-embedded enzyme. The approach we have adopted is to fix the γ, ɛ, and c subunits with respect to one another so that one subunit cannot rotate without the others.
Materials and Methods
Strains and Plasmids and Isolation of E. coli F1Fo.
E. coli strains used were XL1-Blue (Stratagene) for site-directed mutagenesis and for cloning and the unc− RA1 (29) to express the ATP synthase. Plasmids used were pRA13 (30) containing the wild-type β, γ, and ɛ genes and pRA197 (31) containing the wild-type unc operon except that two c subunits were fused through an additional sequence, and a cysteine was introduced in position 42 in the latter of the two c subunits (cc′). The E. coli F1Fo mutant pST202, containing c dimer and cysteines in position 217 of γ, 31 and 68 of ɛ, and 42 of the second subunit of c dimer (cc′Q42C/γT217C/ɛE31C,I68C), was generated by employing Quick Change site-directed mutagenesis (Stratagene). Inner membrane was obtained from the strain RA1, and E. coli F1Fo was isolated as described by Foster and Fillingame (32) and modified as described by Aggeler et al. (33). The isolated F1Fo was reconstituted into egg-lecithin vesicles as described (30).
Formation of the γ−ɛ−cc′ Cross-Link Product.
The inner membrane or isolated F1Fo in vesiclesof 0.8 mg/ml in buffer containing 50 mM Mops-NaOH, 5 mM MgCl2, 10% glycerol (pH 7.0) was treated with 100 μM CuCl2 for 15 min at 23°C. For comparison with non-cross-linked enzyme, 1 mM DTT was added instead of CuCl2. Then, 7.5 mM EDTA was added to terminate oxidation. Cross-linked products were analyzed by gel electrophoresis (12–20% polyacrylamide) containing 0.1% SDS in the absence of reducing agent, followed by staining with Coomassie brilliant blue (CBB) R or immunoblotting for identification with monoclonal antibodies against γ, ɛ, and c subunits. The cross-link yield was determined from the decrease of the γ subunit band on CBB-stained gel and blotting membrane.
Other Methods.
ATP hydrolysis was measured at 37°C in the presence of an ATP-regenerating system. The assay mixture contained 25 mM Hepes-KOH, 25 mM KCl, 5 mM MgCl2, 5 mM KCN, 0.5 mM NADH, 2 mM phosphoenolpyruvate, 2 mM ATP, 30 units/ml pyruvate kinase, and 30 units/ml lactate dehydrogenase (pH 7.5). ATP-dependent proton translocation was determined by measuring the quenching of 9-amino-6-chloro-2-methoxyacridine (ACMA) on inner membrane. An 80-μg sample of the inner membranes was diluted 10-fold in buffer (50 mM Hepes-NaOH/5 mM MgCl2/100 mM KCl/0.5 mM NADH/10 mM KCN/3.6 μM valinomycin/1 μM ACMA, pH 7.5). Change of fluorescence at 480 nm (excitation at 410 nm) was monitored in response to 2 mM ATP followed by the addition of 3.6 μM nigericin. ATP synthesis was determined as follows: 16 μg of inner membranes in 25 mM Tris⋅HCl/5 mM MgCl2/10% glycerol/5 mM ADP/5 mM K2HPO4/2 mM NADH (pH 7.5) was incubated at 37°C for 0, 60, 120, and 180 sec, followed by addition of 0.1 M trichloroacetic acid on ice to stop the reaction. The amount of ATP was determined with a luciferin/luciferase system by measuring the emitted light with a chemiluminometer. The value of units/mg corresponds to hydrolyzed or synthesized μmol of ATP per min per mg of protein. Protein concentrations were determined by using the BCA (bicinchoninic acid) protein assay (Pierce).
Results
To covalently link the γ, ɛ, and c subunits, these subunits were each mutated to incorporate cysteines to allow disulfide bond formation. Thus, Cys residues were introduced at positions γ217 and ɛ68 to cross-link γ to ɛ. Also, a Cys was placed at position 31 of ɛ and at 42 of the c subunit to allow cross-linking between the ɛ and c subunits. The sites are indicated in Fig. 1C, which is based on x-ray diffraction and NMR data of yeast and E. coli F1 and Fo subunits (16, 34–36). The final mutant, γT217C≠ɛE31C≠ɛI68C≠cc′Q42C also produced the c subunit as a covalent dimer joined by an 11 amino acid linker (4, 31) and with the Q42C mutation present only in every second c subunit. This last alteration is necessary to prevent cross-linking via the Cys at 42 between neighbor c subunits, which inhibits ATPase activity and lowers the yield of the ɛ-c subunit products.
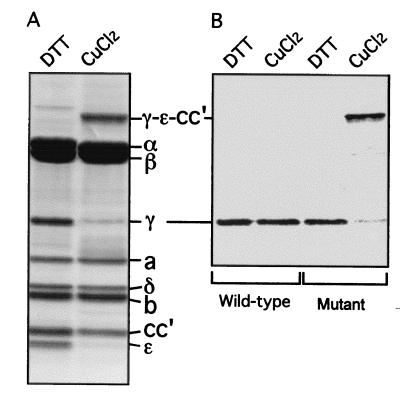
Both inner membranes and purified E. coli F1Fo were prepared from the mutant and studied. Oxidation of either preparation with CuCl2 treatment led to generation of one high molecular weight band with concomitant loss of the γ, ɛ, and cc′ subunits (Fig. 2A). This additional band was confirmed to be cross-linked γ−ɛ–cc′ subunits by Western blotting with anti-γ subunit (Fig. 2B), anti-ɛ subunit, and anti-c subunit antibodies. At 100 μM CuCl2, the yield of the γ−ɛ–cc′ cross-linked product was 85–90% based on quantitation of the disappearance of the γ subunit from gels of the purified enzyme and on Western blotting of the inner membrane preparations.
Figure 2.
Formation of the γ−ɛ−cc′ cross-link through disulfide bonds in the E. coli F1Fo mutant. (A) Purified E. coli F1Fo mutant was incubated with 100 μM CuCl2 to induce the cross-linking. As a control, 1 mM DTT was added instead of CuCl2. The samples were loaded on SDS/polyacrylamide (12–20%) gel, subjected to electrophoresis, and stained with Coomassie brilliant blue. The dissociation buffer for SDS/PAGE contained 40 mM N-ethylmaleimide but no reducing agent. (B) Anti-γ subunit immunoblotting. Inner membranes from the wild-type and mutant F1Fo were exposed to 100 μM CuCl2. The γ−ɛ–cc′ cross-link was confirmed with anti-γ, ɛ, and c subunit immunoblotting. (The data from the anti-ɛ and c subunit antibodies are not shown.)
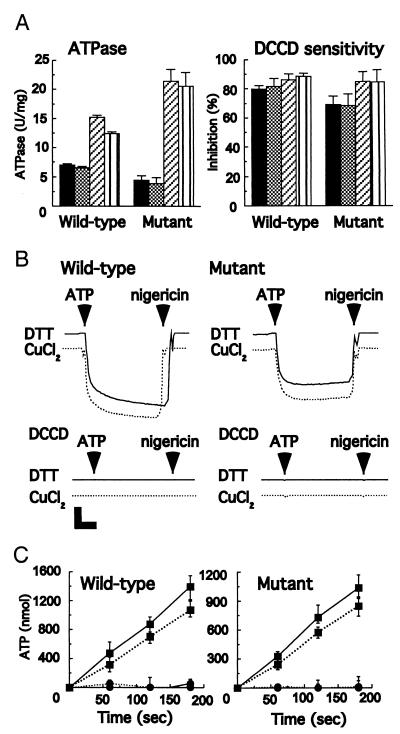
The F1Fo ATP synthase in mutant membranes had an ATPase activity of approximately 65% that of the wild-type enzyme (4.5 units/mg compared with 7.0 units/mg). This difference is because of the presence of c subunit dimers and not the introduction of cysteines (31). After high-yield cross-linking of γ, ɛ, and cc′ (90%), the mutant retained 85–95% of the original ATPase activity in both the inner membranes and the isolated enzyme (Fig. 3A). A small decrease also was observed with wild-type enzyme treated with 100 μM CuCl2 (Fig. 3A).
Figure 3.
Effect of cross-linking on activity. (A) Effect of the γ−ɛ–cc′ cross-linking on ATPase activity and inhibitor sensitivity. The inner membranes and purified F1Fo from the wild-type and the mutant were treated with CuCl2 or DTT as described in the legend for Fig. 2. The ATPase activity was measured in the presence of an ATP-regenerating system. Samples were also incubated for 60 min at 23°C with 40 μM DCCD, a specific inhibitor. Then, the ATPase activity was measured. The inhibition was estimated based on the activity without DCCD treatment. The inner membrane reacted with DTT (black) and CuCl2 (cross-hatched). Purified F1Fo reacted with DTT (diagonal stripes) and CuCl2 (vertical stripes). (B) Effect of γ−ɛ–cc′ cross-linking on ATP-driven proton translocation. The proton pumping ability of the inner membranes from both the wild-type and the mutant was determined by monitoring the decrease of the fluorescence intensity of ACMA. Before the assay, the inner membranes were treated with DTT or CuCl2 as described in the legend for Fig. 2, followed by incubation with or without 40 μM DCCD for 60 min at 23°C. At the times indicated by arrowheads, 2 mM ATP and 3.6 μM nigericin were added, respectively. The inner membrane was treated with DTT (solid line) and CuCl2 (dotted line). Vertical bar, 20% of relative fluorescence; horizontal bar, 20 sec. (C) Effect of γ−ɛ–cc′ cross-linking on ATP synthesis activity. The inner membranes from the wild-type and the mutant were exposed to 2 mM NADH to generate the proton gradient at 37°C. The data show the amount of ATP produced by 1 mg of inner membrane protein. Solid line, DTT; dotted line, CuCl2-treated membranes as described in the legend for Fig. 2. Before the assay, the samples were reacted with (●) or without (■) 40 μM DCCD for 60 min at 23°C.
DCCD is a well-characterized inhibitor of Fo. It reacts covalently with Asp-61 of the c subunit, an essential residue for proton translocation, to irreversibly block both ATP hydrolysis and ATP synthesis (37). The DCCD sensitivity of ATP hydrolysis by the mutant was unaltered by γ−ɛ–cc′ cross-linking (Fig. 3A). Moreover, ATP-dependent proton pumping was unaltered by cross-linking when measured by the ACMA quenching assay system (Fig. 3B). As shown in the figure, this activity was fully DCCD-sensitive.
Finally, the effect of covalently linking the γ, ɛ, and c subunits together on ATP synthesis was measured. Membranes from the mutant showed an ATP-synthesis activity of around 75% of that of the wild type in the absence of cross-linking (0.34 units/mg compared with 0.46 units/mg; again because of the presence of c dimers instead of monomers). After cross-linking in yields of 90%, inhibition of ATP-synthesis activity was less than 25% compared with untreated membranes (Fig. 3C), and this loss is not related to the cross-linking per se: the same extent of inhibition of ATP synthesis was observed with the equivalent treatment of wild-type membranes (Fig. 3C). DCCD abolished the ATP synthesis of both cross-linked and non-cross-linked F1Fo.
Discussion
Cross-linking studies in which disulfide bonds are generated between Cys residues genetically introduced into the F1Fo complex have provided considerable structural and functional information about this important enzyme (5, 38, 39). It had previously been shown by this approach that the ɛ subunit can be cross-linked to the c subunit without major effect on the coupling, while the tight relationship of the γ and ɛ subunits was well characterized (31, 40, 41). However, these experiments do not separately provide conclusive evidence that the γɛ and c subunits move together as the rotor because the function(s) of the ɛ subunit remain enigmatic. The primary role of the ε subunit appears to be to inhibit ATPase activity and ATP-driven proton translocation activity (42–44). Functional and structural studies have revealed that the ɛ subunit can take two different states (2, 3, 45, 46). Therefore, it is possible that, in one state, ɛ can remain bound to c subunits, freeing the γ subunit to rotate alone in the complex, while the γ and ɛ subunits rotate relative to the c ring in another. Cross-linking of the γ and c subunits has been shown to uncouple the enzyme for reasons discussed later (31). Therefore, the direct experiment to link the γ and c subunits is not possible.
Here we have introduced Cys residues to generate optimal cross-linking of both γ-to-ɛ and ɛ-to-c subunits at the same time based on insights provided by high-resolution structural data on these three subunits (Figs. 1C and 2). An important advantage of cross-linking over the previous studies that claim rotation of the c ring is that they can be conducted with enzymes in membranes, where F1Fo retains coupled functions that can be measured. We show that fixing γ, ɛ, and c subunits together does not block the functioning of the F1Fo ATP synthase as a molecular motor. The cross-linked enzyme retains high ATP-driven proton pumping. It functions as an ATP synthase, and both ATP hydrolysis and ATP synthesis retain full inhibitor sensitivity (Fig. 3). Therefore, as the γ and ɛ subunits rotate in 120° steps driven by sequential ATP synthesis or hydrolysis in the three catalytic sites (47, 48), the c subunit ring must move in unison.
It is important to emphasize that the essentially full inhibitor sensitivity of ATP hydrolysis as well as the high ATP synthesis activity and DCCD sensitivity of this function establishes unequivocally that the enzyme under study here is coupled. Previous work that showed rotation of the c subunit ring by using fluorescent actin filament technology failed to provide evidence of this. Pänke et al. (27) did not measure the effect of F1Fo-specific inhibitors on their observed rotation of the c subunit ring. Sambongi et al. (25) examined the effect of venturicidin, but their results have shown very little inhibition by this potent reagent. More recently, these authors have confirmed that their preparation is insensitive to DCCD (49). The rotation of the c subunit seen in the above two studies is probably artifactual and due to release of the c subunit ring from its critical interactions with the a subunit as shown schematically in Fig. 1B. In this connection it is interesting to note that the rotation of the c ring was lost rapidly according to Sambongi et al. (25). This can be explained if the c subunit ring, once displaced from the a subunit, is only weakly bound to the γɛ subunits and is quickly released by the viscous drag due to the torque of the rotation (26).
The γ, ɛ, and c ring could function as a rigid rotor. However, there are likely to be conformational changes transmitted within the rotor with each ATP synthesized or hydrolyzed, to facilitate the dissociation/rebinding of the γ and ɛ subunits with β subunits in the F1 and c subunits with the a subunit in the Fo. Such conformational changes are probably transmitted directly from γ to the c ring, as mutants at the interface between γ and c but not ɛ and c cause disruption of the coupling between catalytic sites in F1 and the proton channel in Fo (31, 50).
Acknowledgments
We thank Beth Monika for help in making enzyme preparations, Dr. Birte Schulenberg for helpful discussions, and Dr. Andrew C. Hausrath for providing the structural model in Fig. 1C. The c subunit antibody was a kind gift from Dr. Robert H. Fillingame. This work was supported by the Human Frontier Science Program Organization (RG 15/1998-M) and National Institutes of Health Grant HL24526 (to R.A.C.). S.P.T. was supported by a Research Fellowship of the Japan Society for the Promotion of Science for Young Scientists.
Abbreviation
- DCCD
N,N′-dicyclohexylcarbodiimide
- ACMA
9-amino-6-chloro-2-methoxyacridine
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.031564198.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.031564198
References
- 1.Abrahams J P, Leslie A G W, Lutter R, Walker J E. Nature (London) 1994;370:621–628. doi: 10.1038/370621a0. [DOI] [PubMed] [Google Scholar]
- 2.Gibbons C, Montgomery M G, Leslie A G W, Walker J E. Nat Struct Biol. 2000;7:1055–1061. doi: 10.1038/80981. [DOI] [PubMed] [Google Scholar]
- 3.Rodgers A J W, Wilce M C J. Nat Struct Biol. 2000;7:1051–1054. doi: 10.1038/80975. [DOI] [PubMed] [Google Scholar]
- 4.Jones P C, Fillingame R H. J Biol Chem. 1998;273:29701–29705. doi: 10.1074/jbc.273.45.29701. [DOI] [PubMed] [Google Scholar]
- 5.Fillingame R H, Jiang W, Dmitriev O Y. J Exp Biol. 2000;203:9–17. doi: 10.1242/jeb.203.1.9. [DOI] [PubMed] [Google Scholar]
- 6.Rodgers A J W, Wilkens S, Aggeler R, Morris M B, Howitt S M, Capaldi R A. J Biol Chem. 1997;272:31058–31064. doi: 10.1074/jbc.272.49.31058. [DOI] [PubMed] [Google Scholar]
- 7.Rodgers A J W, Capaldi R A. J Biol Chem. 1998;273:29406–29410. doi: 10.1074/jbc.273.45.29406. [DOI] [PubMed] [Google Scholar]
- 8.Wilkens S, Capaldi R A. Nature (London) 1998;393:29. doi: 10.1038/29908. (lett.). [DOI] [PubMed] [Google Scholar]
- 9.Böttcher B, Schwarz L, Gräber P. J Mol Biol. 1998;281:757–762. doi: 10.1006/jmbi.1998.1957. [DOI] [PubMed] [Google Scholar]
- 10.Karrasch K, Walker J E. J Mol Biol. 1999;290:379–384. doi: 10.1006/jmbi.1999.2897. [DOI] [PubMed] [Google Scholar]
- 11.Fillingame R H. In: Bacterial Energetics, The Bacteria. Krulwich T, editor. Vol. 12. New York: Academic; 1990. pp. 345–391. [Google Scholar]
- 12.Boyer P D. Annu Rev Biochem. 1997;66:717–749. doi: 10.1146/annurev.biochem.66.1.717. [DOI] [PubMed] [Google Scholar]
- 13.Boyer P D, Kohlbrenner W E. Energy Coupling in Photosynthesis. New York: Elsevier/North–Holland; 1981. pp. 231–240. [Google Scholar]
- 14.Noji H, Yasuda R, Yoshida M, Kinosita K., Jr Nature (London) 1997;386:299–302. doi: 10.1038/386299a0. [DOI] [PubMed] [Google Scholar]
- 15.Kato-Yamada Y, Noji H, Yasuda R, Kinosita K, Jr, Yoshida M. J Biol Chem. 1998;273:19375–19377. doi: 10.1074/jbc.273.31.19375. [DOI] [PubMed] [Google Scholar]
- 16.Stock D, Leslie A G, Walker J E. Science. 1999;286:1700–1705. doi: 10.1126/science.286.5445.1700. [DOI] [PubMed] [Google Scholar]
- 17.Watts S D, Zhang Y, Fillingame R H, Capaldi R A. FEBS Lett. 1995;368:235–238. doi: 10.1016/0014-5793(95)00658-v. [DOI] [PubMed] [Google Scholar]
- 18.Hermolin J, Dmitriev O Y, Zhang Y, Fillingame R H. J Biol Chem. 1999;274:17011–17016. doi: 10.1074/jbc.274.24.17011. [DOI] [PubMed] [Google Scholar]
- 19.Vik S B, Antonio B J. J Biol Chem. 1994;269:30364–30369. [PubMed] [Google Scholar]
- 20.Rastogi V K, Girvin M E. Nature (London) 1999;402:263–268. doi: 10.1038/46224. [DOI] [PubMed] [Google Scholar]
- 21.Dimroth P, Wang H, Grabe M, Oster G. Proc Natl Acad Sci USA. 1999;96:4924–4929. doi: 10.1073/pnas.96.9.4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicholls D G, Ferguson S J. Bioenegetics 2. London: Academic; 1992. [Google Scholar]
- 23.van Walraven H S, Strotmann H, Schwarz O, Rumberg B. FEBS Lett. 1996;379:309–313. doi: 10.1016/0014-5793(95)01536-1. [DOI] [PubMed] [Google Scholar]
- 24.Berry S, Rumberg B. Biochim Biophys Acta. 1999;1410:248–261. doi: 10.1016/s0005-2728(99)00003-1. [DOI] [PubMed] [Google Scholar]
- 25.Sambongi Y, Iko Y, Tanabe M, Omote H, Iwamoto-Kihara A, Ueda I, Yanagida T, Wada Y, Futai M. Science. 1999;286:1722–1724. doi: 10.1126/science.286.5445.1722. [DOI] [PubMed] [Google Scholar]
- 26.Tsunoda S P, Aggeler R, Noji H, Kinosita K, Jr, Yoshida M, Capaldi R A. FEBS Lett. 2000;470:244–248. doi: 10.1016/s0014-5793(00)01336-3. [DOI] [PubMed] [Google Scholar]
- 27.Pänke O, Gumbiowski K, Junge W, Engelbrecht S. FEBS Lett. 2000;472:34–38. doi: 10.1016/s0014-5793(00)01436-8. [DOI] [PubMed] [Google Scholar]
- 28.Böttcher B. EMBO Rep. 2000;1:223–224. doi: 10.1093/embo-reports/kvd062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aggeler R, Ogilvie I, Capaldi R A. J Biol Chem. 1997;272:19621–19624. doi: 10.1074/jbc.272.31.19621. [DOI] [PubMed] [Google Scholar]
- 30.Aggeler R, Haughton M A, Capaldi R A. J Biol Chem. 1995;270:9185–9191. doi: 10.1074/jbc.270.16.9185. [DOI] [PubMed] [Google Scholar]
- 31.Schulenberg B, Aggeler R, Murray J, Capaldi R A. J Biol Chem. 1999;274:34233–34237. doi: 10.1074/jbc.274.48.34233. [DOI] [PubMed] [Google Scholar]
- 32.Foster D L, Fillingame R H. J Biol Chem. 1979;254:8230–8236. [PubMed] [Google Scholar]
- 33.Aggeler R, Zhang Y Z, Capaldi R A. Biochemistry. 1987;26:7107–7113. doi: 10.1021/bi00396a036. [DOI] [PubMed] [Google Scholar]
- 34.Hausrath A C, Grüber G, Matthews B W, Capaldi R A. Proc Natl Acad Sci USA. 1999;96:13697–13702. doi: 10.1073/pnas.96.24.13697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uhlin U, Cox G B, Guss J M. Structure (London) 1997;5:1219–1230. doi: 10.1016/s0969-2126(97)00272-4. [DOI] [PubMed] [Google Scholar]
- 36.Girvin M E, Rastogi V K, Abildgaard E, Markley J L, Fillingame R H. Biochemistry. 1998;37:8817–8824. doi: 10.1021/bi980511m. [DOI] [PubMed] [Google Scholar]
- 37.Linnett P E, Beechey R B. Methods Enzymol. 1979;55:473–518. doi: 10.1016/0076-6879(79)55061-7. [DOI] [PubMed] [Google Scholar]
- 38.Capaldi R A, Schulenberg B, Murray J, Aggeler R. J Exp Biol. 2000;203:29–33. doi: 10.1242/jeb.203.1.29. [DOI] [PubMed] [Google Scholar]
- 39.Duncan T M, Bulygin V V, Zhou Y, Hutcheon M L, Cross R L. Proc Natl Acad Sci USA. 1995;92:10964–10968. doi: 10.1073/pnas.92.24.10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang C, Capaldi R A. J Biol Chem. 1996;271:3018–3024. doi: 10.1074/jbc.271.6.3018. [DOI] [PubMed] [Google Scholar]
- 41.Watts S D, Tang C, Capaldi R A. J Biol Chem. 1996;271:28341–28347. doi: 10.1074/jbc.271.45.28341. [DOI] [PubMed] [Google Scholar]
- 42.Laget P P, Smith J B. Arch Biochem Biophys. 1979;197:83–89. doi: 10.1016/0003-9861(79)90222-4. [DOI] [PubMed] [Google Scholar]
- 43.Aggeler R, Capaldi R A. J Biol Chem. 1996;271:13888–13891. doi: 10.1074/jbc.271.23.13888. [DOI] [PubMed] [Google Scholar]
- 44.Kato-Yamada Y, Bald D, Koike M, Motohashi K, Hisabori T, Yoshida M. J Biol Chem. 1999;274:33991–33994. doi: 10.1074/jbc.274.48.33991. [DOI] [PubMed] [Google Scholar]
- 45.Kato Y, Matsui T, Tanaka N, Muneyuki E, Hisabori T, Yoshida M. J Biol Chem. 1997;272:24906–24912. doi: 10.1074/jbc.272.40.24906. [DOI] [PubMed] [Google Scholar]
- 46.Schulenberg B, Capaldi R A. J Biol Chem. 1999;274:28351–28355. doi: 10.1074/jbc.274.40.28351. [DOI] [PubMed] [Google Scholar]
- 47.Yasuda R, Noji H, Kinosita K, Jr, Yoshida M. Cell. 1998;93:1117–1124. doi: 10.1016/s0092-8674(00)81456-7. [DOI] [PubMed] [Google Scholar]
- 48.Adachi K, Yasuda R, Noji H, Itoh H, Harada Y, Yoshida M, Kinosita K., Jr Proc Natl Acad Sci USA. 2000;97:7243–7247. doi: 10.1073/pnas.120174297. . (First Published June 6, 2000; 10.1073/pnas.120174297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wada Y, Sambongi M, Futai M. Biochim Biophys Acta. 2000;1459:499–505. doi: 10.1016/s0005-2728(00)00189-4. [DOI] [PubMed] [Google Scholar]
- 50.Ketchum C J, Nakamoto R K. J Biol Chem. 1998;273:22292–22297. doi: 10.1074/jbc.273.35.22292. [DOI] [PubMed] [Google Scholar]