Abstract
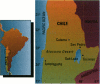
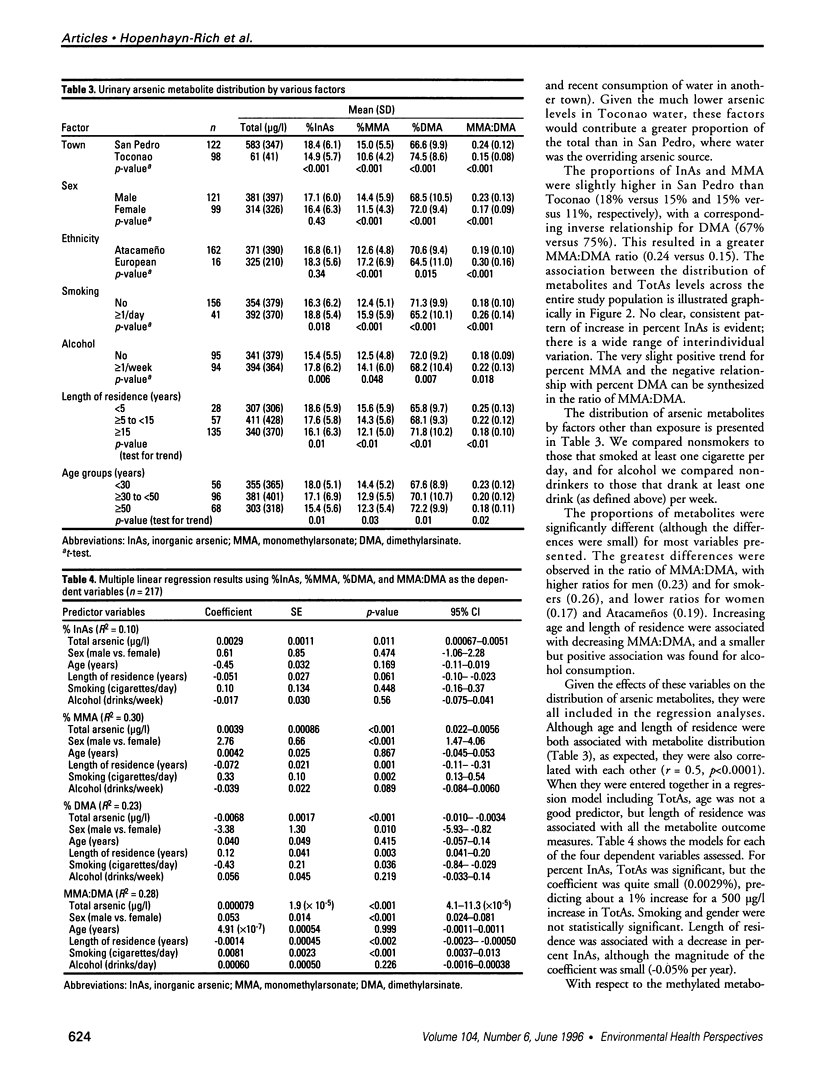
Methylation is considered the detoxification pathway for inorganic arsenic (InAs), an established human carcinogen. Urinary speciation analysis is used to assess the distribution of metabolites [monomethylarsonate (MMA), dimethylarsinate (DMA), and unmethylated arsenic (InAs)], as indicators of methylation capacity. We conducted a large biomarker study in northern Chile of a population chronically exposed to high levels of arsenic in drinking water. We report the results of the methylation study, which focused on the effects of exposure and other variables on the percent InAs, MMA, DMA, and the ratio of MMA to DMA in urine. The study consisted of 122 people in a town with arsenic water levels around 600 micrograms/l and 98 participants in a neighboring town with arsenic levels in water of about 15 micrograms/l. The corresponding mean urinary arsenic levels were 580 micrograms/l and 60 micrograms/l, of which 18.4% and 14.9% were InAs, respectively. The main differences were found for MMA:DMA; exposure, smoking, and being male were associated with higher MMA:DMA, while longer residence, Atacameño ethnicity, and being female were associated with lower MMA:DMA. Together, these variables explained about 30% of the variability in MMA:DMA. Overall, there was no evidence of a threshold for methylation capacity, even at very high exposures, and the interindividual differences were within a much wider range than those attributed to the variables investigated. The differences in percent InAs were small and within the ranges of other studies of background exposure levels. The biological significance of MMA:DMA, which was more than 1.5 times greater in the exposed group, and its relationship to sex, length of exposure, and ethnicity need further investigation because its relevance to health risk is not clear.
Full text
PDF

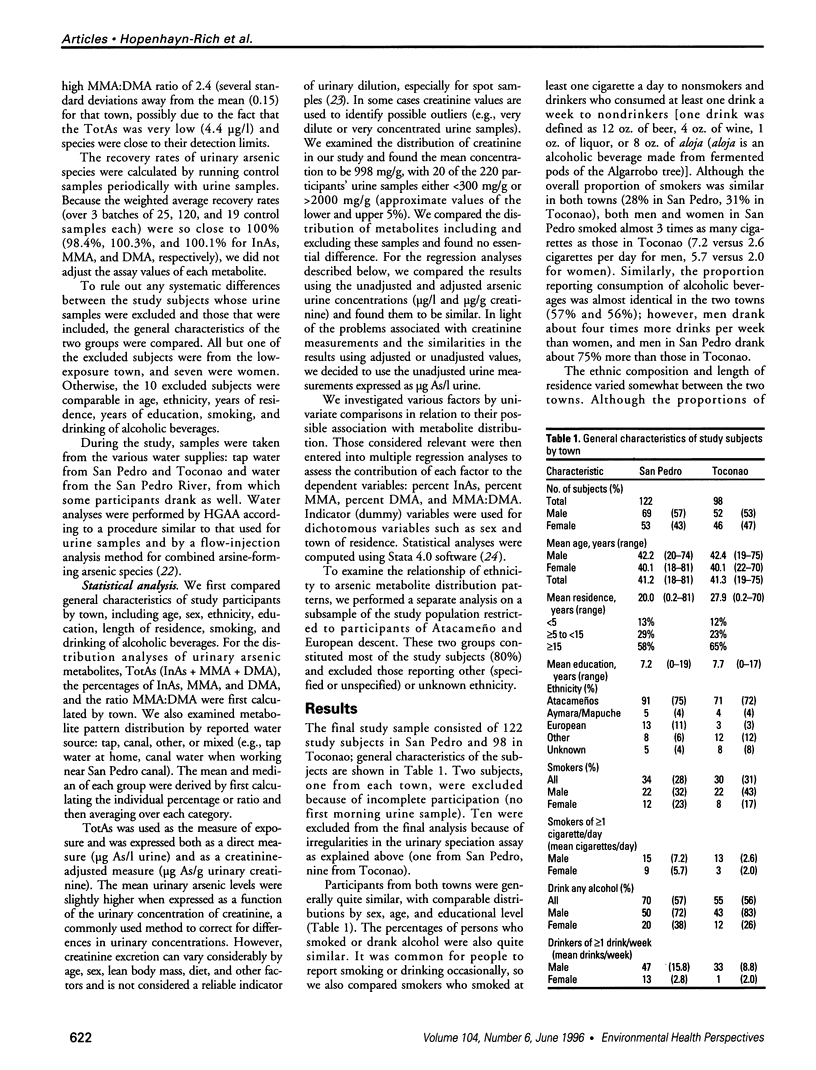
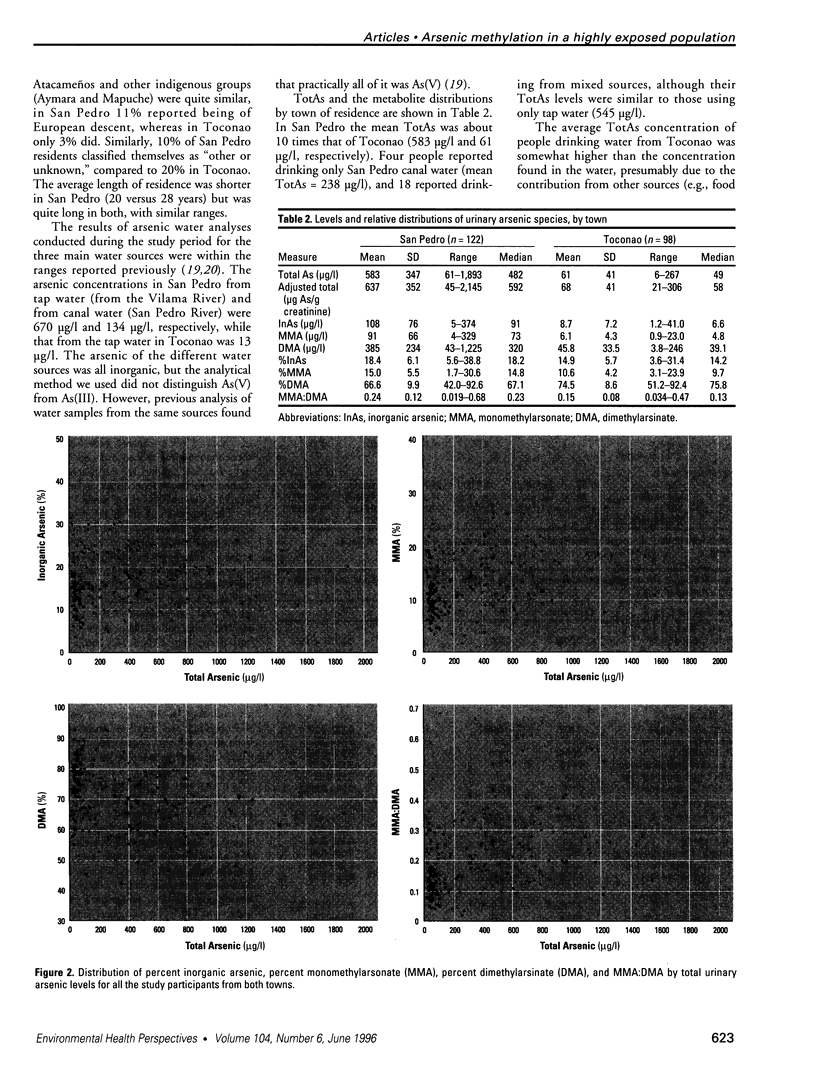




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bates M. N., Smith A. H., Hopenhayn-Rich C. Arsenic ingestion and internal cancers: a review. Am J Epidemiol. 1992 Mar 1;135(5):462–476. doi: 10.1093/oxfordjournals.aje.a116313. [DOI] [PubMed] [Google Scholar]
- Bell D. A., Taylor J. A., Paulson D. F., Robertson C. N., Mohler J. L., Lucier G. W. Genetic risk and carcinogen exposure: a common inherited defect of the carcinogen-metabolism gene glutathione S-transferase M1 (GSTM1) that increases susceptibility to bladder cancer. J Natl Cancer Inst. 1993 Jul 21;85(14):1159–1164. doi: 10.1093/jnci/85.14.1159. [DOI] [PubMed] [Google Scholar]
- Boeniger M. F., Lowry L. K., Rosenberg J. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J. 1993 Oct;54(10):615–627. doi: 10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- Bogdan G. M., Sampayo-Reyes A., Aposhian H. V. Arsenic binding proteins of mammalian systems: I. Isolation of three arsenite-binding proteins of rabbit liver. Toxicology. 1994 Nov 11;93(2-3):175–193. doi: 10.1016/0300-483x(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Borgoño J. M., Vicent P., Venturino H., Infante A. Arsenic in the drinking water of the city of Antofagasta: epidemiological and clinical study before and after the installation of a treatment plant. Environ Health Perspect. 1977 Aug;19:103–105. doi: 10.1289/ehp.19-1637404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchet J. P., Lauwerys R., Roels H. Urinary excretion of inorganic arsenic and its metabolites after repeated ingestion of sodium metaarsenite by volunteers. Int Arch Occup Environ Health. 1981;48(2):111–118. doi: 10.1007/BF00378431. [DOI] [PubMed] [Google Scholar]
- Buchet J. P., Lauwerys R. Role of thiols in the in-vitro methylation of inorganic arsenic by rat liver cytosol. Biochem Pharmacol. 1988 Aug 15;37(16):3149–3153. doi: 10.1016/0006-2952(88)90313-9. [DOI] [PubMed] [Google Scholar]
- Buchet J. P., Lauwerys R. Study of factors influencing the in vivo methylation of inorganic arsenic in rats. Toxicol Appl Pharmacol. 1987 Oct;91(1):65–74. doi: 10.1016/0041-008x(87)90194-3. [DOI] [PubMed] [Google Scholar]
- Caporaso N., Landi M. T., Vineis P. Relevance of metabolic polymorphisms to human carcinogenesis: evaluation of epidemiologic evidence. Pharmacogenetics. 1991 Oct;1(1):4–19. doi: 10.1097/00008571-199110000-00003. [DOI] [PubMed] [Google Scholar]
- Carlson-Lynch H., Beck B. D., Boardman P. D. Arsenic risk assessment. Environ Health Perspect. 1994 Apr;102(4):354–356. doi: 10.1289/ehp.94102354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. J., Chen C. W., Wu M. M., Kuo T. L. Cancer potential in liver, lung, bladder and kidney due to ingested inorganic arsenic in drinking water. Br J Cancer. 1992 Nov;66(5):888–892. doi: 10.1038/bjc.1992.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo G., Kuroda K., Okamoto A., Horiguchi S. Dimethylarsenic acid induces tetraploids in Chinese hamster cells. Bull Environ Contam Toxicol. 1992 Jan;48(1):131–137. doi: 10.1007/BF00197495. [DOI] [PubMed] [Google Scholar]
- Georis B., Cardenas A., Buchet J. P., Lauwerys R. Inorganic arsenic methylation by rat tissue slices. Toxicology. 1990 Jul;63(1):73–84. doi: 10.1016/0300-483x(90)90070-w. [DOI] [PubMed] [Google Scholar]
- Giralt M., Lafuente A., Pujol F., Mallol J. Enhanced glutathione S-transferase activity and glutathione content in human bladder cancer. Followup study: influence of smoking. J Urol. 1993 Jun;149(6):1452–1454. doi: 10.1016/s0022-5347(17)36413-3. [DOI] [PubMed] [Google Scholar]
- Gonsebatt M. E., Vega L., Montero R., Garcia-Vargas G., Del Razo L. M., Albores A., Cebrian M. E., Ostrosky-Wegman P. Lymphocyte replicating ability in individuals exposed to arsenic via drinking water. Mutat Res. 1994 Oct-Dec;313(2-3):293–299. doi: 10.1016/0165-1161(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Hewitt D. J., Millner G. C., Nye A. C., Simmons H. F. Investigation of arsenic exposure from soil at a superfund site. Environ Res. 1995 Feb;68(2):73–81. doi: 10.1006/enrs.1995.1010. [DOI] [PubMed] [Google Scholar]
- Hirata M., Tanaka A., Hisanaga A., Ishinishi N. Effects of glutathione depletion on the acute nephrotoxic potential of arsenite and on arsenic metabolism in hamsters. Toxicol Appl Pharmacol. 1990 Dec;106(3):469–481. doi: 10.1016/0041-008x(90)90342-r. [DOI] [PubMed] [Google Scholar]
- Hopenhayn-Rich C., Smith A. H., Goeden H. M. Human studies do not support the methylation threshold hypothesis for the toxicity of inorganic arsenic. Environ Res. 1993 Feb;60(2):161–177. doi: 10.1006/enrs.1993.1024. [DOI] [PubMed] [Google Scholar]
- Smith A. H., Hopenhayn-Rich C., Bates M. N., Goeden H. M., Hertz-Picciotto I., Duggan H. M., Wood R., Kosnett M. J., Smith M. T. Cancer risks from arsenic in drinking water. Environ Health Perspect. 1992 Jul;97:259–267. doi: 10.1289/ehp.9297259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam G. K., Charbonneau S. M., Bryce F., Pomroy C., Sandi E. Metabolism of inorganic arsenic (74As) in humans following oral ingestion. Toxicol Appl Pharmacol. 1979 Sep 15;50(2):319–322. doi: 10.1016/0041-008x(79)90157-1. [DOI] [PubMed] [Google Scholar]
- Thompson D. J. A chemical hypothesis for arsenic methylation in mammals. Chem Biol Interact. 1993 Sep;88(2-3):89–14. doi: 10.1016/0009-2797(93)90086-e. [DOI] [PubMed] [Google Scholar]
- Tu S. H., Shirachi D. Y. Cytotoxicity of inorganic and organic arsenics in cell culture. Proc West Pharmacol Soc. 1992;35:61–63. [PubMed] [Google Scholar]
- Warner M. L., Moore L. E., Smith M. T., Kalman D. A., Fanning E., Smith A. H. Increased micronuclei in exfoliated bladder cells of individuals who chronically ingest arsenic-contaminated water in Nevada. Cancer Epidemiol Biomarkers Prev. 1994 Oct-Nov;3(7):583–590. [PubMed] [Google Scholar]
- Weinshilboum R. Pharmacogenetics of methylation: relationship to drug metabolism. Clin Biochem. 1988 Aug;21(4):201–210. doi: 10.1016/s0009-9120(88)80002-x. [DOI] [PubMed] [Google Scholar]
- Wu M. M., Kuo T. L., Hwang Y. H., Chen C. J. Dose-response relation between arsenic concentration in well water and mortality from cancers and vascular diseases. Am J Epidemiol. 1989 Dec;130(6):1123–1132. doi: 10.1093/oxfordjournals.aje.a115439. [DOI] [PubMed] [Google Scholar]
- Yamanaka K., Ohba H., Hasegawa A., Sawamura R., Okada S. Mutagenicity of dimethylated metabolites of inorganic arsenics. Chem Pharm Bull (Tokyo) 1989 Oct;37(10):2753–2756. doi: 10.1248/cpb.37.2753. [DOI] [PubMed] [Google Scholar]
- Zhong S., Wyllie A. H., Barnes D., Wolf C. R., Spurr N. K. Relationship between the GSTM1 genetic polymorphism and susceptibility to bladder, breast and colon cancer. Carcinogenesis. 1993 Sep;14(9):1821–1824. doi: 10.1093/carcin/14.9.1821. [DOI] [PubMed] [Google Scholar]