Abstract
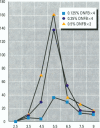
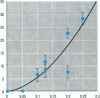
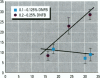
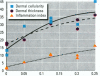
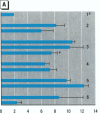
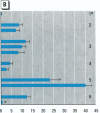
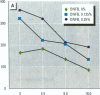
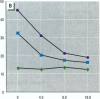
The effect of the corticosteroid fluocinolone acetonide (FA) on skin tumor induction and inflammation by the contact sensitizer dinitrofluorobenzene (DNFB) was examined. This study broadly relates to the question of whether contact sensitizers, as electrophilic chemicals that produce protein adduction, may constitute an environmental cancer hazard. The specific aim of this study was to evaluate the extent to which the immunogenic inflammatory response to DNFB, in contrast to DNFB cytotoxicity, might be responsible for tumor induction. Experiments were conducted on a transgenic (TG.AC) mouse, incorporating a mutated ras oncogene (v-Ha-ras) that responds rapidly and profusely with skin papillomas to tumor promoters as if it were genetically initiated. Various doses and patterns of DNFB and FA were applied to the skin in a 2-week period; DNFB was given four times and FA was given either with the DNFB or daily. The tumor response to DNFB was completed by 8 weeks from the first dose and was consistent with a dose-squared relationship. FA was not tumorigenic alone; when given with DNFB, it caused only a small reduction in inflammation and tumor yield. When given daily, FA increased ulcerative skin damage, inflammation, and the yield tumors. The results suggest that tumorigenesis by DNFB, in the high-dose short-term regimen used here, is mainly due to its cytotoxicity and not contact sensitization.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert R. E. Carcinogen risk assessment in the U.S. Environmental Protection Agency. Crit Rev Toxicol. 1994;24(1):75–85. doi: 10.3109/10408449409017920. [DOI] [PubMed] [Google Scholar]
- Albert R. E., Miller M. L., Cody T. E., Talaska G., Underwood P., Andringa A. Epidermal cytokinetics, DNA adducts, and dermal inflammation in the mouse skin in response to repeated benzo[a]pyrene exposures. Toxicol Appl Pharmacol. 1996 Jan;136(1):67–74. doi: 10.1006/taap.1996.0007. [DOI] [PubMed] [Google Scholar]
- Argyris T. S. Tumor promotion by abrasion induced epidermal hyperplasia in the skin of mice. J Invest Dermatol. 1980 Oct;75(4):360–362. doi: 10.1111/1523-1747.ep12531153. [DOI] [PubMed] [Google Scholar]
- Ashby J., Hilton J., Dearman R. J., Callander R. D., Kimber I. Mechanistic relationship among mutagenicity, skin sensitization, and skin carcinogenicity. Environ Health Perspect. 1993 Apr 22;101(1):62–67. doi: 10.1289/ehp.9310162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby J., Tennant R. W. Prediction of rodent carcinogenicity for 44 chemicals: results. Mutagenesis. 1994 Jan;9(1):7–15. doi: 10.1093/mutage/9.1.7. [DOI] [PubMed] [Google Scholar]
- Belman S., Troll W. The inhibition of croton oil-promoted mouse skin tumorigenesis by steroid hormones. Cancer Res. 1972 Mar;32(3):450–454. [PubMed] [Google Scholar]
- Bock F. G., Fjelde A., Fox H. W., Klein E. Tumor promotion by 1-fluoro-2,4-dinitrobenzene, a potent skin sensitizer. Cancer Res. 1969 Jan;29(1):179–182. [PubMed] [Google Scholar]
- CREECH H. J. Chemical and immunological properties of carcinogen-protein conjugates. Cancer Res. 1952 Aug;12(8):557–564. [PubMed] [Google Scholar]
- Cavey D., Bouclier M., Burg G., Delamadeleine F., Hensby C. N. The pharmacological modulation of delayed type hypersensitivity (DTH) reactions to topical oxazolone in mouse skin. Agents Actions. 1990 Jan;29(1-2):65–67. doi: 10.1007/BF01964723. [DOI] [PubMed] [Google Scholar]
- Cerutti P. A., Trump B. F. Inflammation and oxidative stress in carcinogenesis. Cancer Cells. 1991 Jan;3(1):1–7. [PubMed] [Google Scholar]
- GHADIALLY F. N., GREEN H. N. The effect of cortisone on chemical carcinogenesis in the mouse skin. Br J Cancer. 1954 Jun;8(2):291–295. doi: 10.1038/bjc.1954.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L. S., Slone T. H., Stern B. R., Manley N. B., Ames B. N. Rodent carcinogens: setting priorities. Science. 1992 Oct 9;258(5080):261–265. doi: 10.1126/science.1411524. [DOI] [PubMed] [Google Scholar]
- Goldstein B. D., Witz G., Amoruso M., Stone D. S., Troll W. Stimulation of human polymorphonuclear leukocyte superoxide anion radical production by tumor promoters. Cancer Lett. 1981 Jan;11(3):257–262. doi: 10.1016/0304-3835(81)90117-8. [DOI] [PubMed] [Google Scholar]
- Inagaki N., Miura T., Nakajima T., Yoshida K., Nagai H., Koda A. Studies on the anti-allergic mechanism of glucocorticoids in mice. J Pharmacobiodyn. 1992 Oct;15(10):581–587. doi: 10.1248/bpb1978.15.581. [DOI] [PubMed] [Google Scholar]
- Kraus A. L., Munro I. C., Orr J. C., Binder R. L., LeBoeuf R. A., Williams G. M. Benzoyl peroxide: an integrated human safety assessment for carcinogenicity. Regul Toxicol Pharmacol. 1995 Feb;21(1):87–107. doi: 10.1006/rtph.1995.1014. [DOI] [PubMed] [Google Scholar]
- Leder A., Kuo A., Cardiff R. D., Sinn E., Leder P. v-Ha-ras transgene abrogates the initiation step in mouse skin tumorigenesis: effects of phorbol esters and retinoic acid. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9178–9182. doi: 10.1073/pnas.87.23.9178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldve R. E., Fischer S. M. Tumor-promoting activity of 2,4-dinitrofluorobenzene. Int J Cancer. 1995 Feb 8;60(4):545–553. doi: 10.1002/ijc.2910600420. [DOI] [PubMed] [Google Scholar]
- Miyauchi H., Horio T. Ultraviolet B-induced local immunosuppression of contact hypersensitivity is modulated by ultraviolet irradiation and hapten application. J Invest Dermatol. 1995 Mar;104(3):364–369. doi: 10.1111/1523-1747.ep12665832. [DOI] [PubMed] [Google Scholar]
- OLD L. J., BENACERRAF B., CARSWELL E. Contact reactivity to carcinogenic polycyclic hydrocarbons. Nature. 1963 Jun 22;198:1215–1216. doi: 10.1038/1981215a0. [DOI] [PubMed] [Google Scholar]
- SANGER F., TUPPY H. The amino-acid sequence in the phenylalanyl chain of insulin. 2. The investigation of peptides from enzymic hydrolysates. Biochem J. 1951 Sep;49(4):481–490. doi: 10.1042/bj0490481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleimer R. P. An overview of glucocorticoid anti-inflammatory actions. Eur J Clin Pharmacol. 1993;45 (Suppl 1):S3–S44. doi: 10.1007/BF01844196. [DOI] [PubMed] [Google Scholar]
- Scribner J. D., Scribner N. K., McKnight B., Mottet N. K. Evidence for a new model of tumor progression from carcinogenesis and tumor promotion studies with 7-bromomethylbenz[a]anthracene. Cancer Res. 1983 May;43(5):2034–2041. [PubMed] [Google Scholar]
- Slaga T. J., Scribner J. D. Inhibition of tumor initiation and promotion by anti-inflammatory agents. J Natl Cancer Inst. 1973 Nov;51(5):1723–1725. doi: 10.1093/jnci/51.5.1723. [DOI] [PubMed] [Google Scholar]
- Spalding J. W., Momma J., Elwell M. R., Tennant R. W. Chemically induced skin carcinogenesis in a transgenic mouse line (TG.AC) carrying a v-Ha-ras gene. Carcinogenesis. 1993 Jul;14(7):1335–1341. doi: 10.1093/carcin/14.7.1335. [DOI] [PubMed] [Google Scholar]
- TRIOLO V. A. NINETEENTH CENTURY FOUNDATIONS OF CANCER RESEARCH ADVANCES IN TUMOR PATHOLOGY, NOMENCLATURE, AND THEORIES OF ONCOGENESIS. Cancer Res. 1965 Feb;25:75–106. [PubMed] [Google Scholar]
- Troll W., Wiesner R., Frenkel K. Anticarcinogenic action of protease inhibitors. Adv Cancer Res. 1987;49:265–283. doi: 10.1016/s0065-230x(08)60800-3. [DOI] [PubMed] [Google Scholar]
- Warren S. T., Doolittle D. J., Chang C. C., Goodmann J. I., Trosko J. E. Evaluation of the carcinogenic potential of 2,4-dinitrofluorobenzene and its implications regarding mutagenicity testing. Carcinogenesis. 1982;3(2):139–145. doi: 10.1093/carcin/3.2.139. [DOI] [PubMed] [Google Scholar]
- Wei H., Frenkel K. Relationship of oxidative events and DNA oxidation in SENCAR mice to in vivo promoting activity of phorbol ester-type tumor promoters. Carcinogenesis. 1993 Jun;14(6):1195–1201. doi: 10.1093/carcin/14.6.1195. [DOI] [PubMed] [Google Scholar]
- Weitzman S. A., Gordon L. I. Inflammation and cancer: role of phagocyte-generated oxidants in carcinogenesis. Blood. 1990 Aug 15;76(4):655–663. [PubMed] [Google Scholar]
- Weitzman S. A., Weitberg A. B., Clark E. P., Stossel T. P. Phagocytes as carcinogens: malignant transformation produced by human neutrophils. Science. 1985 Mar 8;227(4691):1231–1233. doi: 10.1126/science.3975611. [DOI] [PubMed] [Google Scholar]
- Woodworth B. A., Scribner J. D., Scribner N. K. Enhancement of beta-propiolactone tumorigenesis in mouse skin by pretreatment with the anti-inflammatory steroid fluocinolone acetonide. Cancer Lett. 1986 Jun;31(3):293–297. doi: 10.1016/0304-3835(86)90150-3. [DOI] [PubMed] [Google Scholar]