Abstract
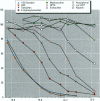
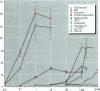
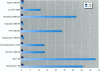
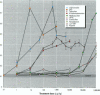
Because of rampant concern that estrogenic chemicals in the environment may be adversely affecting the health of humans and wildlife, reliable methods for detecting and characterizing estrogenic chemicals are needed. It is important that general agreement be reached on which tests to use and that these tests then be applied to the testing of both man-made and naturally occurring chemicals. As a step toward developing a comprehensive approach to screening chemicals for estrogenic activity, three assays for detecting estrogenicity were conducted on 10 chemicals with known or suspected estrogenic activity. The assays were 1) competitive binding with the mouse uterine estrogen receptor, 2) transcriptional activation in HeLa cells transfected with plasmids containing an estrogen receptor and a response element, and 3) the uterotropic assay in mice. The chemicals studied were 17 beta-estradiol, diethylstilbestrol, tamoxifen, 4-hydroxytamoxifen, methoxychlor, the methoxychlor metabolite 2,2-bis(p-hydroxyphenyl)-1,1,1-trichloroethane (HPTE), endosulfan, nonylphenol, o,p'-DDT, and kepone. These studies were conducted to assess the utility of this three-assay combination in the routine screening of chemicals, or combinations of chemicals, for estrogenic activity. Results were consistent among the three assays with respect to what is known about the estrogenic activities of the chemicals tested and their requirements for metabolic activation. By providing information on three levels of hormonal activity (receptor binding, transcriptional activation, and an in vivo effect in an estrogen-responsive tissue), an informative profile of estrogenic activity is obtained with a reasonable investment of resources.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold S. F., Klotz D. M., Collins B. M., Vonier P. M., Guillette L. J., Jr, McLachlan J. A. Synergistic activation of estrogen receptor with combinations of environmental chemicals. Science. 1996 Jun 7;272(5267):1489–1492. doi: 10.1126/science.272.5267.1489. [DOI] [PubMed] [Google Scholar]
- Bitman J., Cecil H. C., Harris S. J., Fries G. F. Estrogenic activity of o,p'-DDT in the mammalian uterus and avian oviduct. Science. 1968 Oct 18;162(3851):371–372. doi: 10.1126/science.162.3851.371. [DOI] [PubMed] [Google Scholar]
- Bitman J., Cecil H. C., Harris S. J., Fries G. F. Estrogenic activity of o,p'-DDT in the mammalian uterus and avian oviduct. Science. 1968 Oct 18;162(3851):371–372. doi: 10.1126/science.162.3851.371. [DOI] [PubMed] [Google Scholar]
- Bulger W. H., Muccitelli R. M., Kupfer D. Studies on the in vivo and in vitro estrogenic activities of methoxychlor and its metabolites. Role of hepatic mono-oxygenase in methoxychlor activation. Biochem Pharmacol. 1978;27(20):2417–2423. doi: 10.1016/0006-2952(78)90354-4. [DOI] [PubMed] [Google Scholar]
- Gorski J., Welshons W., Sakai D. Remodeling the estrogen receptor model. Mol Cell Endocrinol. 1984 Jun;36(1-2):11–15. doi: 10.1016/0303-7207(84)90079-0. [DOI] [PubMed] [Google Scholar]
- Isenhower W. D., Jr, Newbold R. R., Cefalo R. C., Korach K. S., McLachlan J. A. Absence of estrogenic activity in some drugs commonly used during pregnancy. Biol Res Pregnancy Perinatol. 1986;7(1):6–10. [PubMed] [Google Scholar]
- Jordan V. C. Tamoxifen: toxicities and drug resistance during the treatment and prevention of breast cancer. Annu Rev Pharmacol Toxicol. 1995;35:195–211. doi: 10.1146/annurev.pa.35.040195.001211. [DOI] [PubMed] [Google Scholar]
- Korach K. S. Estrogen action in the mouse uterus: characterization of the cytosol and nuclear receptor systems. Endocrinology. 1979 May;104(5):1324–1332. doi: 10.1210/endo-104-5-1324. [DOI] [PubMed] [Google Scholar]
- Kupfer D. Effects of pesticides and related compounds on steroid metabolism and function. CRC Crit Rev Toxicol. 1975 Oct;4(1):83–124. doi: 10.1080/10408447509163835. [DOI] [PubMed] [Google Scholar]
- Lech J. J., Lewis S. K., Ren L. In vivo estrogenic activity of nonylphenol in rainbow trout. Fundam Appl Toxicol. 1996 Apr;30(2):229–232. [PubMed] [Google Scholar]
- Makela S, Davis VL, Tally WC, Korkman J, Salo L, Vihko R, Santti R, Korach KS. Dietary Estrogens Act through Estrogen Receptor-Mediated Processes and Show No Antiestrogenicity in Cultured Breast Cancer Cells. Environ Health Perspect. 1994 Jun;102(6-7):572–578. doi: 10.1289/ehp.94102572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland L. Z., Lacy P. B. Physiologic and endocrinologic effects of the insecticide kepone in the Japanese quail. Toxicol Appl Pharmacol. 1969 Sep;15(2):441–450. doi: 10.1016/0041-008x(69)90042-8. [DOI] [PubMed] [Google Scholar]
- McLachlan J. A. Functional toxicology: a new approach to detect biologically active xenobiotics. Environ Health Perspect. 1993 Oct;101(5):386–387. doi: 10.1289/ehp.93101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio S., Davis V. L., Gibson M. K., Gray T. K., Korach K. S. Estrogens modulate the responsiveness of osteoblast-like cells (ROS 17/2.8) stably transfected with estrogen receptor. Endocrinology. 1992 May;130(5):2617–2624. doi: 10.1210/endo.130.5.1572285. [DOI] [PubMed] [Google Scholar]
- Soto A. M., Chung K. L., Sonnenschein C. The pesticides endosulfan, toxaphene, and dieldrin have estrogenic effects on human estrogen-sensitive cells. Environ Health Perspect. 1994 Apr;102(4):380–383. doi: 10.1289/ehp.94102380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto A. M., Justicia H., Wray J. W., Sonnenschein C. p-Nonyl-phenol: an estrogenic xenobiotic released from "modified" polystyrene. Environ Health Perspect. 1991 May;92:167–173. doi: 10.1289/ehp.9192167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R., Jobling S., Hoare S. A., Sumpter J. P., Parker M. G. Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology. 1994 Jul;135(1):175–182. doi: 10.1210/endo.135.1.8013351. [DOI] [PubMed] [Google Scholar]