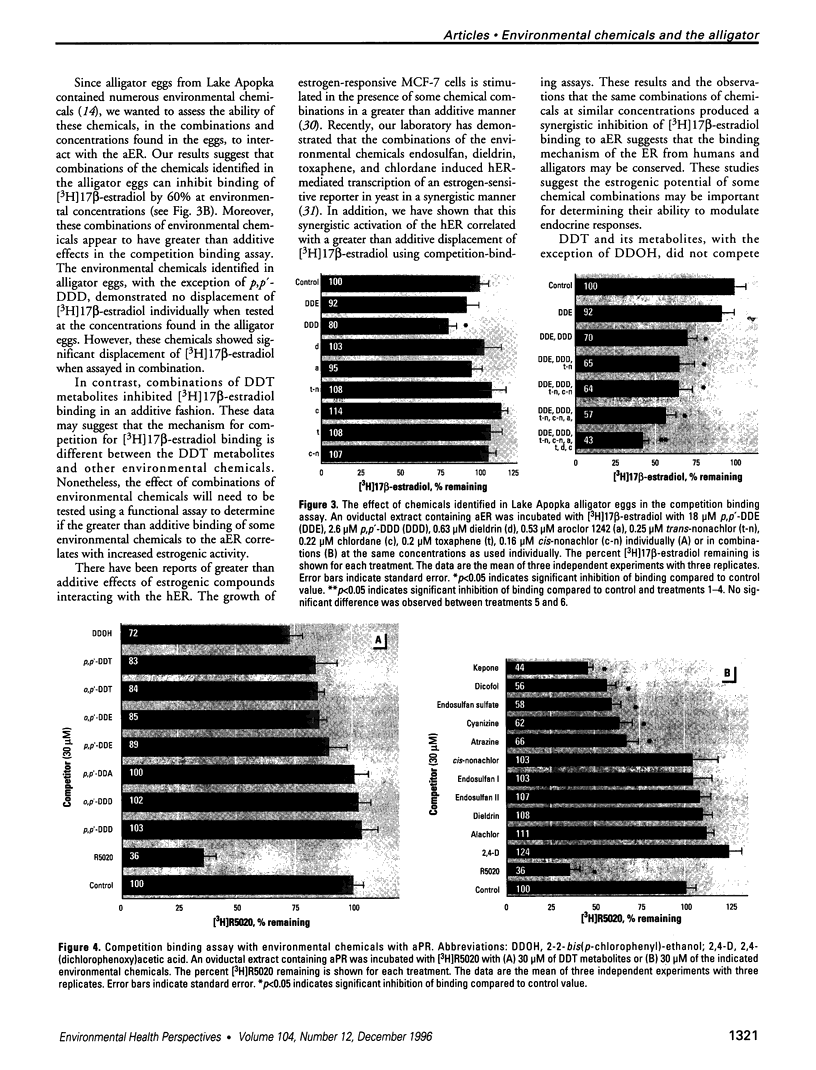
Abstract
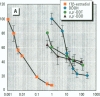
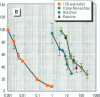
Reports of reproductive abnormalities in the American alligator from Lake Apopka, Florida, have been linked to a spill of DDT and other pesticides suspected of having hormonelike activity. To determine whether environmental chemicals had the potential to function as exogenous hormones in the American alligator, we examined the ability of chemicals to bind the estrogen receptor (aER) and progesterone receptor (aPR) in a protein extract prepared from the oviduct of the alligator. In competition binding assays with [3H]17 beta-estradiol, some DDT metabolites showed inhibition of [3H]17 beta-estradiol binding to aER. A combination of DDTs demonstrated an additive decrease in [3H]17 beta-estradiol binding to aER. Modern-use chemicals such as alachlor, trans-nonachlor, endosulfan, and atrazine also competed with [3H]17 beta-estradiol for binding to the aER. To test the effect of chemicals identified in alligator eggs from Lake Apopka on [3H]17 beta-estradiol binding, we mixed these chemicals at concentrations measured in eggs in the competition binding assay. 2,2-bis(4-chlorophenyl)-N-(methoxymethyl)acetamide (p,p'-DDD) and trans-nonachlor, both found in Lake Apopka, interacted with aER, whereas others such as chlordane and toxaphene did not. Surprisingly, combinations of these chemicals decreased [3H]17 beta-estradiol binding in a greater than additive manner. To assess the ability of chemicals to interact with aPR, we performed commpetition binding assays with the synthetic progestin [3H]R5020. Most of the chemicals tested did not reduce [3H]R5020 binding to aPR, whereas endosulfan, alachlor, and kepone inhibited binding. These results provide the first evidence that environmental chemicals bind the aER and aPR from the American alligator, supporting the hypothesis that the reported reproductive abnormalities may be related to the modulation of endocrine-related responses. The findings that combinations of chemicals demonstrated a greater than additive interaction with the aER and some chemicals bind to the aPR in the competition binding assay are novel. This suggests that interactions of these chemicals with the endocrine system are complex.
Full text
PDF

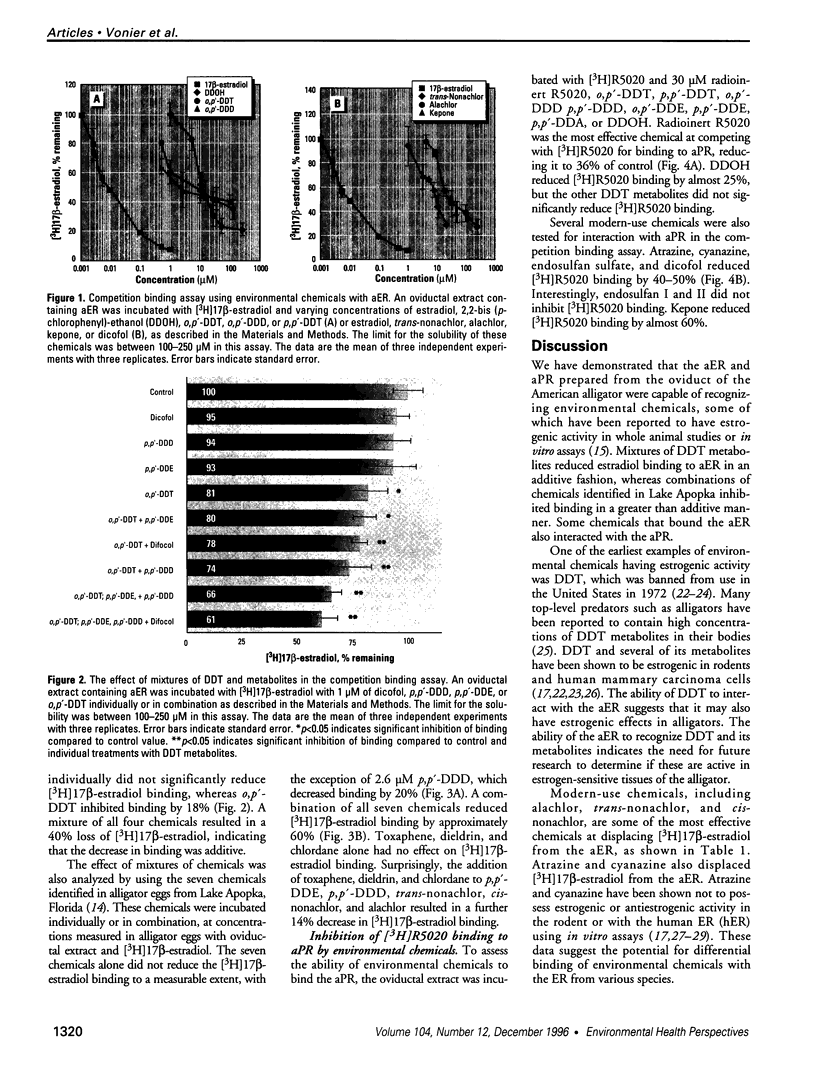

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews G. K., Teng C. S. Studies on sex-organ development. Prenatal effect of oestrogenic hormone on tubular-gland cell morphogenesis and ovalbumin-gene expression in the chick Müllerian duct. Biochem J. 1979 Aug 15;182(2):271–286. doi: 10.1042/bj1820271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold S. F., Klotz D. M., Collins B. M., Vonier P. M., Guillette L. J., Jr, McLachlan J. A. Synergistic activation of estrogen receptor with combinations of environmental chemicals. Science. 1996 Jun 7;272(5267):1489–1492. doi: 10.1126/science.272.5267.1489. [DOI] [PubMed] [Google Scholar]
- Arnold S. F., Robinson M. K., Notides A. C., Guillette L. J., Jr, McLachlan J. A. A yeast estrogen screen for examining the relative exposure of cells to natural and xenoestrogens. Environ Health Perspect. 1996 May;104(5):544–548. doi: 10.1289/ehp.96104544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitman J., Cecil H. C., Harris S. J., Fries G. F. Estrogenic activity of o,p'-DDT in the mammalian uterus and avian oviduct. Science. 1968 Oct 18;162(3851):371–372. doi: 10.1126/science.162.3851.371. [DOI] [PubMed] [Google Scholar]
- Bulger W. H., Muccitelli R. M., Kupfer D. Studies on the in vivo and in vitro estrogenic activities of methoxychlor and its metabolites. Role of hepatic mono-oxygenase in methoxychlor activation. Biochem Pharmacol. 1978;27(20):2417–2423. doi: 10.1016/0006-2952(78)90354-4. [DOI] [PubMed] [Google Scholar]
- Cecil H. C., Bitman J., Harris S. J. Estrogenicity of o,p'-DDT in rats. J Agric Food Chem. 1971 Jan-Feb;19(1):61–65. doi: 10.1021/jf60173a049. [DOI] [PubMed] [Google Scholar]
- Connor K., Howell J., Chen I., Liu H., Berhane K., Sciarretta C., Safe S., Zacharewski T. Failure of chloro-S-triazine-derived compounds to induce estrogen receptor-mediated responses in vivo and in vitro. Fundam Appl Toxicol. 1996 Mar;30(1):93–101. [PubMed] [Google Scholar]
- Greco T. L., Duello T. M., Gorski J. Estrogen receptors, estradiol, and diethylstilbestrol in early development: the mouse as a model for the study of estrogen receptors and estrogen sensitivity in embryonic development of male and female reproductive tracts. Endocr Rev. 1993 Feb;14(1):59–71. doi: 10.1210/edrv-14-1-59. [DOI] [PubMed] [Google Scholar]
- Guillette L. J., Jr, Crain D. A., Rooney A. A., Pickford D. B. Organization versus activation: the role of endocrine-disrupting contaminants (EDCs) during embryonic development in wildlife. Environ Health Perspect. 1995 Oct;103 (Suppl 7):157–164. doi: 10.1289/ehp.95103s7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillette L. J., Jr, Gross T. S., Masson G. R., Matter J. M., Percival H. F., Woodward A. R. Developmental abnormalities of the gonad and abnormal sex hormone concentrations in juvenile alligators from contaminated and control lakes in Florida. Environ Health Perspect. 1994 Aug;102(8):680–688. doi: 10.1289/ehp.94102680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillette L. J., Jr, Pickford D. B., Crain D. A., Rooney A. A., Percival H. F. Reduction in penis size and plasma testosterone concentrations in juvenile alligators living in a contaminated environment. Gen Comp Endocrinol. 1996 Jan;101(1):32–42. doi: 10.1006/gcen.1996.0005. [DOI] [PubMed] [Google Scholar]
- Haney A. F., Newbold R. R., Fetter B. F., McLachlan J. A. Paraovarian cysts associated with prenatal diethylstilbestrol exposure. Comparison of the human with a mouse model. Am J Pathol. 1986 Sep;124(3):405–411. [PMC free article] [PubMed] [Google Scholar]
- Kelce W. R., Stone C. R., Laws S. C., Gray L. E., Kemppainen J. A., Wilson E. M. Persistent DDT metabolite p,p'-DDE is a potent androgen receptor antagonist. Nature. 1995 Jun 15;375(6532):581–585. doi: 10.1038/375581a0. [DOI] [PubMed] [Google Scholar]
- Klotz D. M., Beckman B. S., Hill S. M., McLachlan J. A., Walters M. R., Arnold S. F. Identification of environmental chemicals with estrogenic activity using a combination of in vitro assays. Environ Health Perspect. 1996 Oct;104(10):1084–1089. doi: 10.1289/ehp.961041084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer D., Bulger W. H. Interaction of o, p'DDT with the estrogen-binding protein (EBP) in human mammary and uterine tumors. Res Commun Chem Pathol Pharmacol. 1977 Mar;16(3):451–462. [PubMed] [Google Scholar]
- Kupfer D., Bulger W. H. Interaction of o, p'DDT with the estrogen-binding protein (EBP) in human mammary and uterine tumors. Res Commun Chem Pathol Pharmacol. 1977 Mar;16(3):451–462. [PubMed] [Google Scholar]
- McLachlan J. A., Newbold R. R., Bullock B. C. Long-term effects on the female mouse genital tract associated with prenatal exposure to diethylstilbestrol. Cancer Res. 1980 Nov;40(11):3988–3999. [PubMed] [Google Scholar]
- Nelson J. A. Effects of dichlorodiphenyltrichloroethane (DDT) analogs and polychlorinated biphenyl (PCB) mixtures on 17beta-(3H)estradiol binding to rat uterine receptor. Biochem Pharmacol. 1974 Jan 15;23(2):447–451. doi: 10.1016/0006-2952(74)90436-5. [DOI] [PubMed] [Google Scholar]
- Newbold R. R., Bullock B. C., Mc Lachlan J. A. Exposure to diethylstilbestrol during pregnancy permanently alters the ovary and oviduct. Biol Reprod. 1983 Apr;28(3):735–744. doi: 10.1095/biolreprod28.3.735. [DOI] [PubMed] [Google Scholar]
- Newbold R. R., Pentecost B. T., Yamashita S., Lum K., Miller J. V., Nelson P., Blair J., Kong H., Teng C., McLachlan J. A. Female gene expression in the seminal vesicle of mice after prenatal exposure to diethylstilbestrol. Endocrinology. 1989 May;124(5):2568–2576. doi: 10.1210/endo-124-5-2568. [DOI] [PubMed] [Google Scholar]
- Soto A. M., Chung K. L., Sonnenschein C. The pesticides endosulfan, toxaphene, and dieldrin have estrogenic effects on human estrogen-sensitive cells. Environ Health Perspect. 1994 Apr;102(4):380–383. doi: 10.1289/ehp.94102380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto A. M., Sonnenschein C., Chung K. L., Fernandez M. F., Olea N., Serrano F. O. The E-SCREEN assay as a tool to identify estrogens: an update on estrogenic environmental pollutants. Environ Health Perspect. 1995 Oct;103 (Suppl 7):113–122. doi: 10.1289/ehp.95103s7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKASUGI N., BERN H. A., DEOME K. B. Persistent vaginal cornification in mice. Science. 1962 Oct 19;138(3538):438–439. doi: 10.1126/science.138.3538.438. [DOI] [PubMed] [Google Scholar]
- Tennant M. K., Hill D. S., Eldridge J. C., Wetzel L. T., Breckenridge C. B., Stevens J. T. Possible antiestrogenic properties of chloro-s-triazines in rat uterus. J Toxicol Environ Health. 1994 Oct;43(2):183–196. doi: 10.1080/15287399409531914. [DOI] [PubMed] [Google Scholar]
- Tezak Z., Simić B., Kniewald J. Effect of pesticides on oestradiol-receptor complex formation in rat uterus cytosol. Food Chem Toxicol. 1992 Oct;30(10):879–885. doi: 10.1016/0278-6915(92)90054-o. [DOI] [PubMed] [Google Scholar]
- Welch R. M., Levin W., Conney A. H. Estrogenic action of DDT and its analogs. Toxicol Appl Pharmacol. 1969 Mar;14(2):358–367. doi: 10.1016/0041-008x(69)90117-3. [DOI] [PubMed] [Google Scholar]