Abstract
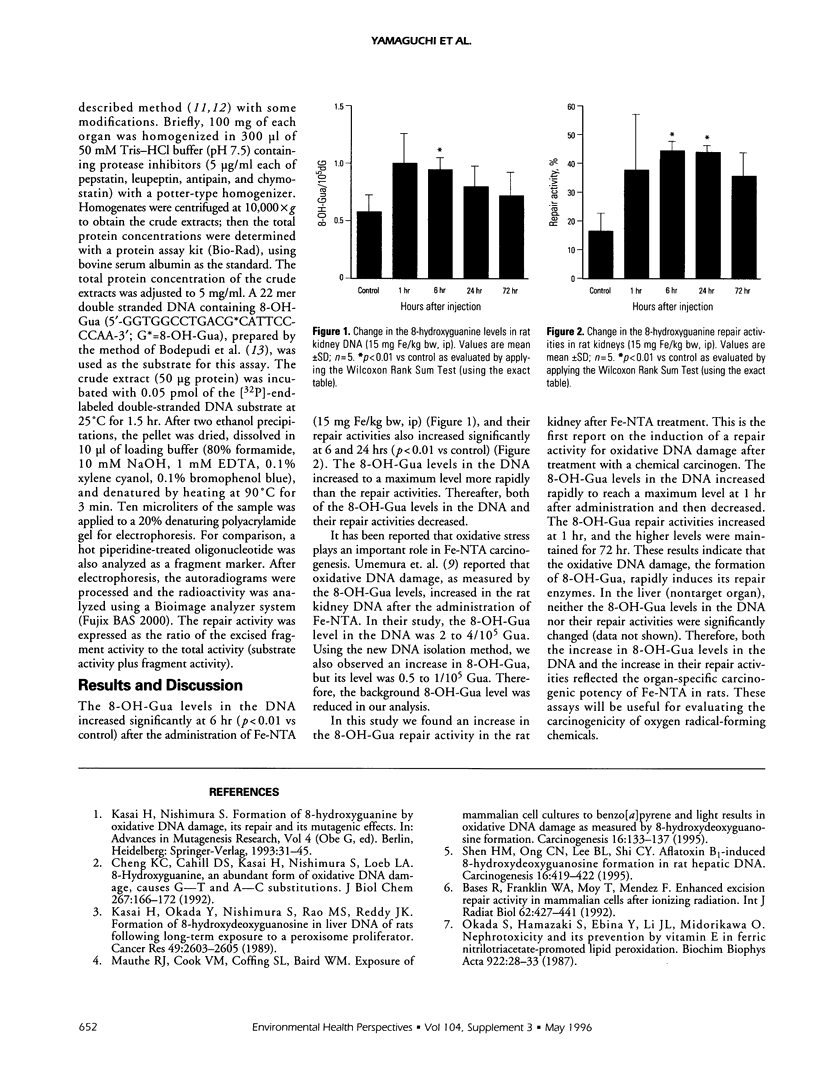
One type of oxidative DNA damage, 8-hydroxyguanine (8-OH-Gua), is known to increase in rat kidney DNA after the administration of a renal carcinogen, ferric nitrilotriacetate (Fe-NTA). To determine the involvement of oxygen radicals in Fe-NTA carcinogenesis, we examined whether the 8-OH-Gua repair enzymes are induced in the rat kidney after Fe-NTA administration, in addition to our analysis of the 8-OH-Gua levels in the DNA, because the 8-OH-Gua repair activity is known to be induced in mammalian cells by oxidative stress due to ionizing radiation. The 8-OH-Gua repair enzyme activity was determined with an endonuclease assay using a 22-mer double strand DNA, which contains 8-OH-Gua at a specific position. A significant increase in the 8-OH-Gua repair activity was observed in the rat kidney after a single intraperitoneal injection of Fe-NTA (p < 0.01). This is the first report on the induction of the repair activity for 8-OH-Gua after treatment with a chemical carcinogen. This assay will be useful for evaluating the carcinogenicity of oxygen radical-forming chemicals.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bases R., Franklin W. A., Moy T., Mendez F. Enhanced excision repair activity in mammalian cells after ionizing radiation. Int J Radiat Biol. 1992 Oct;62(4):427–441. doi: 10.1080/09553009214552311. [DOI] [PubMed] [Google Scholar]
- Bodepudi V., Shibutani S., Johnson F. Synthesis of 2'-deoxy-7,8-dihydro-8-oxoguanosine and 2'-deoxy-7,8-dihydro-8-oxoadenosine and their incorporation into oligomeric DNA. Chem Res Toxicol. 1992 Sep-Oct;5(5):608–617. doi: 10.1021/tx00029a004. [DOI] [PubMed] [Google Scholar]
- Cheng K. C., Cahill D. S., Kasai H., Nishimura S., Loeb L. A. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G----T and A----C substitutions. J Biol Chem. 1992 Jan 5;267(1):166–172. [PubMed] [Google Scholar]
- Chung M. H., Kasai H., Jones D. S., Inoue H., Ishikawa H., Ohtsuka E., Nishimura S. An endonuclease activity of Escherichia coli that specifically removes 8-hydroxyguanine residues from DNA. Mutat Res. 1991 Jan;254(1):1–12. doi: 10.1016/0921-8777(91)90035-n. [DOI] [PubMed] [Google Scholar]
- Ebina Y., Okada S., Hamazaki S., Ogino F., Li J. L., Midorikawa O. Nephrotoxicity and renal cell carcinoma after use of iron- and aluminum-nitrilotriacetate complexes in rats. J Natl Cancer Inst. 1986 Jan;76(1):107–113. [PubMed] [Google Scholar]
- Kasai H., Okada Y., Nishimura S., Rao M. S., Reddy J. K. Formation of 8-hydroxydeoxyguanosine in liver DNA of rats following long-term exposure to a peroxisome proliferator. Cancer Res. 1989 May 15;49(10):2603–2605. [PubMed] [Google Scholar]
- Mauthe R. J., Cook V. M., Coffing S. L., Baird W. M. Exposure of mammalian cell cultures to benzo[a]pyrene and light results in oxidative DNA damage as measured by 8-hydroxydeoxyguanosine formation. Carcinogenesis. 1995 Jan;16(1):133–137. doi: 10.1093/carcin/16.1.133. [DOI] [PubMed] [Google Scholar]
- Nakae D., Mizumoto Y., Kobayashi E., Noguchi O., Konishi Y. Improved genomic/nuclear DNA extraction for 8-hydroxydeoxyguanosine analysis of small amounts of rat liver tissue. Cancer Lett. 1995 Nov 6;97(2):233–239. doi: 10.1016/0304-3835(95)03980-b. [DOI] [PubMed] [Google Scholar]
- Okada S., Hamazaki S., Ebina Y., Li J. L., Midorikawa O. Nephrotoxicity and its prevention by vitamin E in ferric nitrilotriacetate-promoted lipid peroxidation. Biochim Biophys Acta. 1987 Oct 31;922(1):28–33. doi: 10.1016/0005-2760(87)90241-4. [DOI] [PubMed] [Google Scholar]
- Shen H. M., Ong C. N., Lee B. L., Shi C. Y. Aflatoxin B1-induced 8-hydroxydeoxyguanosine formation in rat hepatic DNA. Carcinogenesis. 1995 Feb;16(2):419–422. doi: 10.1093/carcin/16.2.419. [DOI] [PubMed] [Google Scholar]
- Umemura T., Sai K., Takagi A., Hasegawa R., Kurokawa Y. Formation of 8-hydroxydeoxyguanosine (8-OH-dG) in rat kidney DNA after intraperitoneal administration of ferric nitrilotriacetate (Fe-NTA). Carcinogenesis. 1990 Feb;11(2):345–347. doi: 10.1093/carcin/11.2.345. [DOI] [PubMed] [Google Scholar]
- Yamamoto F., Kasai H., Bessho T., Chung M. H., Inoue H., Ohtsuka E., Hori T., Nishimura S. Ubiquitous presence in mammalian cells of enzymatic activity specifically cleaving 8-hydroxyguanine-containing DNA. Jpn J Cancer Res. 1992 Apr;83(4):351–357. doi: 10.1111/j.1349-7006.1992.tb00114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



