Abstract
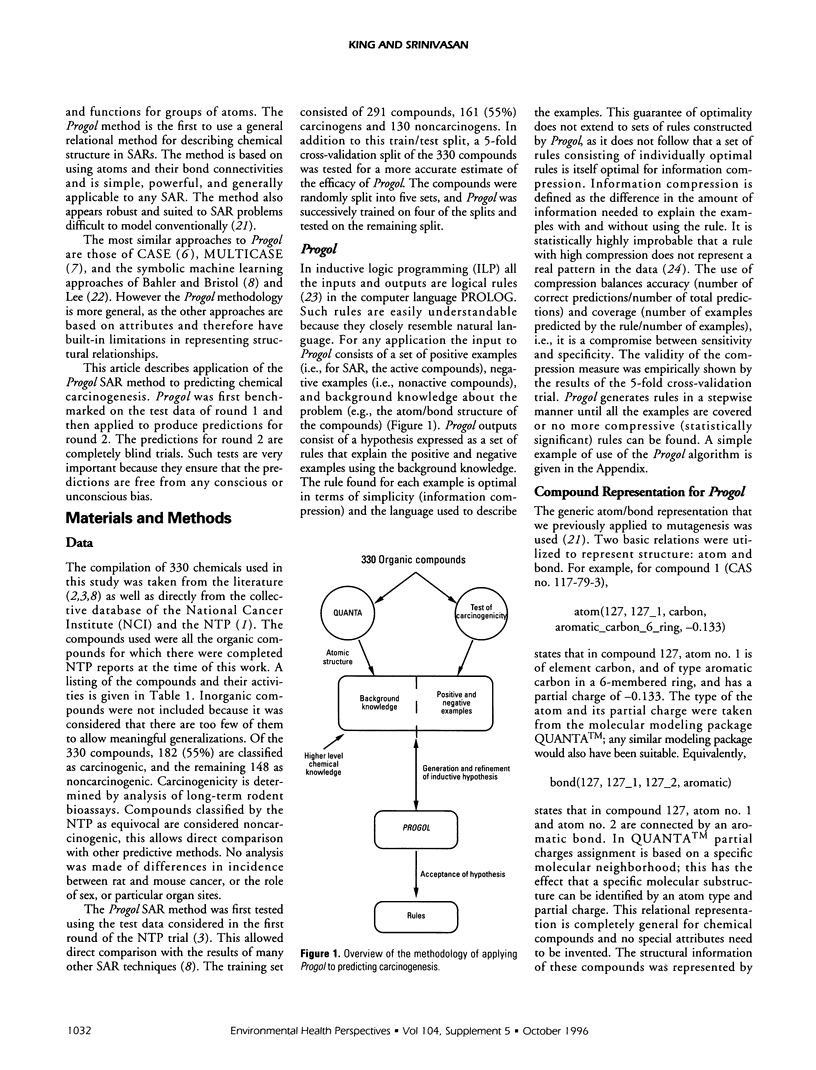
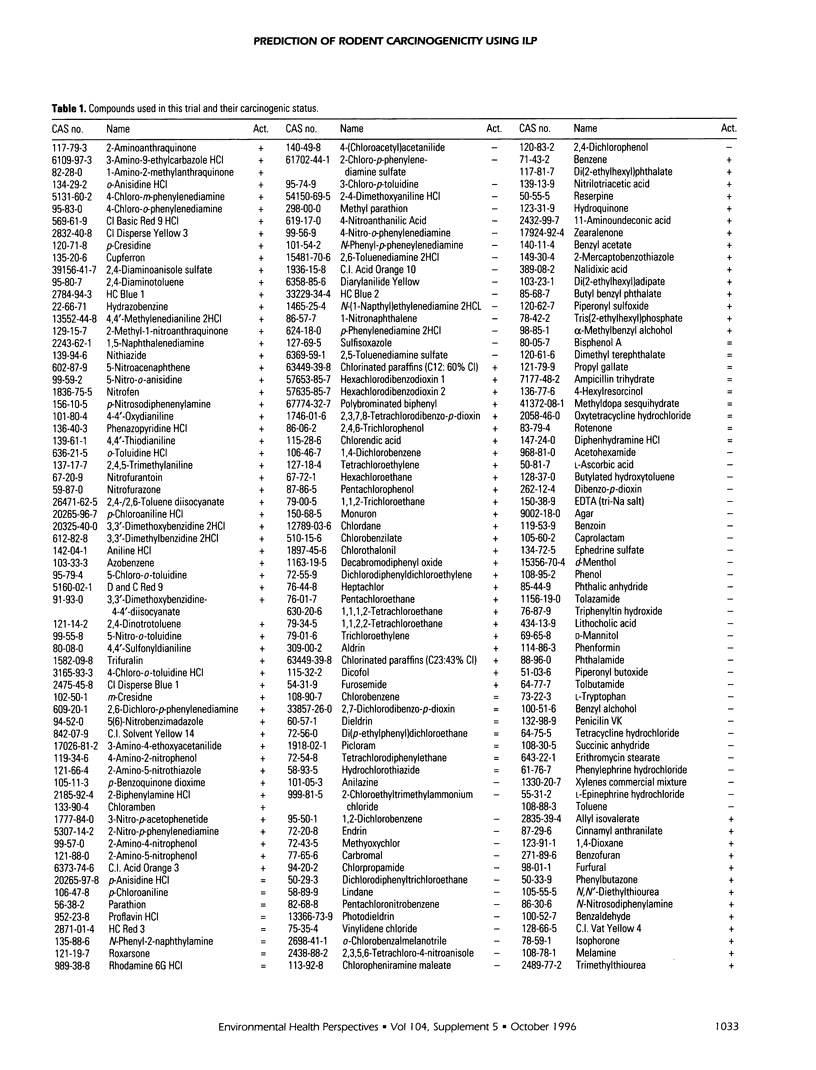
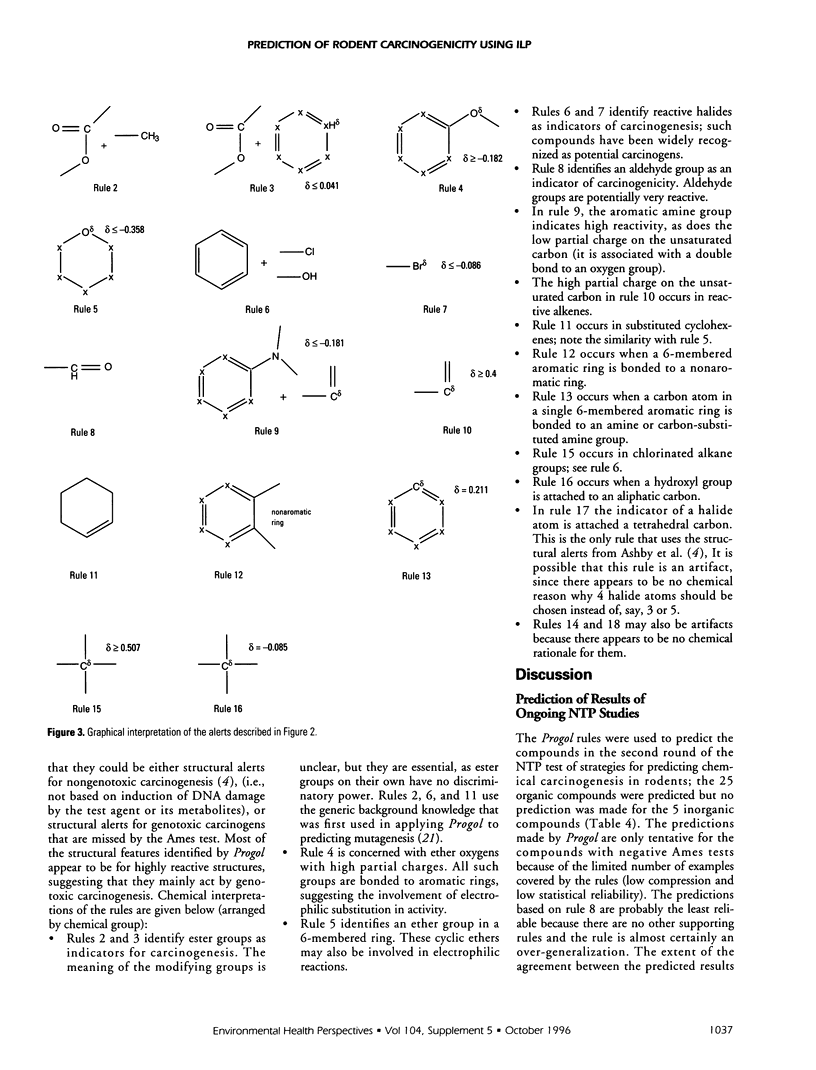
The machine learning program Progol was applied to the problem of forming the structure-activity relationship (SAR) for a set of compounds tested for carcinogenicity in rodent bioassays by the U.S. National Toxicology Program (NTP). Progol is the first inductive logic programming (ILP) algorithm to use a fully relational method for describing chemical structure in SARs, based on using atoms and their bond connectivities. Progol is well suited to forming SARs for carcinogenicity as it is designed to produce easily understandable rules (structural alerts) for sets of noncongeneric compounds. The Progol SAR method was tested by prediction of a set of compounds that have been widely predicted by other SAR methods (the compounds used in the NTP's first round of carcinogenesis predictions). For these compounds no method (human or machine) was significantly more accurate than Progol. Progol was the most accurate method that did not use data from biological tests on rodents (however, the difference in accuracy is not significant). The Progol predictions were based solely on chemical structure and the results of tests for Salmonella mutagenicity. Using the full NTP database, the prediction accuracy of Progol was estimated to be 63% (+/- 3%) using 5-fold cross validation. A set of structural alerts for carcinogenesis was automatically generated and the chemical rationale for them investigated- these structural alerts are statistically independent of the Salmonella mutagenicity. Carcinogenicity is predicted for the compounds used in the NTP's second round of carcinogenesis predictions. The results for prediction of carcinogenesis, taken together with the previous successful applications of predicting mutagenicity in nitroaromatic compounds, and inhibition of angiogenesis by suramin analogues, show that Progol has a role to play in understanding the SARs of cancer-related compounds.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashby J. International Commission for Protection Against Environmental Mutagens and Carcinogens. Two million rodent carcinogens? The role of SAR and QSAR in their detection. Mutat Res. 1994 Feb 1;305(1):3–12. doi: 10.1016/0027-5107(94)90122-8. [DOI] [PubMed] [Google Scholar]
- Ashby J., Paton D. The influence of chemical structure on the extent and sites of carcinogenesis for 522 rodent carcinogens and 55 different human carcinogen exposures. Mutat Res. 1993 Mar;286(1):3–74. doi: 10.1016/0027-5107(93)90003-x. [DOI] [PubMed] [Google Scholar]
- Ashby J., Tennant R. W. Definitive relationships among chemical structure, carcinogenicity and mutagenicity for 301 chemicals tested by the U.S. NTP. Mutat Res. 1991 May;257(3):229–306. doi: 10.1016/0165-1110(91)90003-e. [DOI] [PubMed] [Google Scholar]
- Ashby J., Tennant R. W. Prediction of rodent carcinogenicity for 44 chemicals: results. Mutagenesis. 1994 Jan;9(1):7–15. doi: 10.1093/mutage/9.1.7. [DOI] [PubMed] [Google Scholar]
- Ashby J., Tennant R. W., Zeiger E., Stasiewicz S. Classification according to chemical structure, mutagenicity to Salmonella and level of carcinogenicity of a further 42 chemicals tested for carcinogenicity by the U.S. National Toxicology Program. Mutat Res. 1989 Jun;223(2):73–103. doi: 10.1016/0165-1218(89)90037-2. [DOI] [PubMed] [Google Scholar]
- Bahler D., Bristol D. W. The induction of rules for predicting chemical carcinogenesis in rodents. Proc Int Conf Intell Syst Mol Biol. 1993;1:29–37. [PubMed] [Google Scholar]
- Bakale G., McCreary R. D. Prospective ke screening of potential carcinogens being tested in rodent bioassays by the US National Toxicology Program. Mutagenesis. 1992 Mar;7(2):91–94. doi: 10.1093/mutage/7.2.91. [DOI] [PubMed] [Google Scholar]
- Benigni R. Predicting chemical carcinogenesis in rodents: the state of the art in light of a comparative exercise. Mutat Res. 1995 Feb;334(1):103–113. doi: 10.1016/0165-1161(95)90036-5. [DOI] [PubMed] [Google Scholar]
- Benigni R. QSAR prediction of rodent carcinogenicity for a set of chemicals currently bioassayed by the US National Toxicology Program. Mutagenesis. 1991 Sep;6(5):423–425. doi: 10.1093/mutage/6.5.423. [DOI] [PubMed] [Google Scholar]
- Enslein K., Blake B. W., Borgstedt H. H. Prediction of probability of carcinogenicity for a set of ongoing NTP bioassays. Mutagenesis. 1990 Jul;5(4):305–306. doi: 10.1093/mutage/5.4.305. [DOI] [PubMed] [Google Scholar]
- Hirst J. D., King R. D., Sternberg M. J. Quantitative structure-activity relationships by neural networks and inductive logic programming. I. The inhibition of dihydrofolate reductase by pyrimidines. J Comput Aided Mol Des. 1994 Aug;8(4):405–420. doi: 10.1007/BF00125375. [DOI] [PubMed] [Google Scholar]
- Hirst J. D., King R. D., Sternberg M. J. Quantitative structure-activity relationships by neural networks and inductive logic programming. II. The inhibition of dihydrofolate reductase by triazines. J Comput Aided Mol Des. 1994 Aug;8(4):421–432. doi: 10.1007/BF00125376. [DOI] [PubMed] [Google Scholar]
- Huff J., Haseman J. Long-term chemical carcinogenesis experiments for identifying potential human cancer hazards: collective database of the National Cancer Institute and National Toxicology Program (1976-1991). Environ Health Perspect. 1991 Dec;96:23–31. doi: 10.1289/ehp.919623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. D., Easterly C. E. On the rodent bioassays currently being conducted on 44 chemicals: a RASH analysis to predict test results from the National Toxicology Program. Mutagenesis. 1991 Nov;6(6):507–514. doi: 10.1093/mutage/6.6.507. [DOI] [PubMed] [Google Scholar]
- King R. D., Muggleton S. H., Srinivasan A., Sternberg M. J. Structure-activity relationships derived by machine learning: the use of atoms and their bond connectivities to predict mutagenicity by inductive logic programming. Proc Natl Acad Sci U S A. 1996 Jan 9;93(1):438–442. doi: 10.1073/pnas.93.1.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. D., Muggleton S., Lewis R. A., Sternberg M. J. Drug design by machine learning: the use of inductive logic programming to model the structure-activity relationships of trimethoprim analogues binding to dihydrofolate reductase. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11322–11326. doi: 10.1073/pnas.89.23.11322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Buchanan B. G., Mattison D. M., Klopman G., Rosenkranz H. S. Learning rules to predict rodent carcinogenicity of non-genotoxic chemicals. Mutat Res. 1995 May;328(2):127–149. doi: 10.1016/0027-5107(94)00202-g. [DOI] [PubMed] [Google Scholar]
- Lewis D. F., Ioannides C., Parke D. V. A prospective toxicity evaluation (COMPACT) on 40 chemicals currently being tested by the National Toxicology Program. Mutagenesis. 1990 Sep;5(5):433–435. doi: 10.1093/mutage/5.5.433. [DOI] [PubMed] [Google Scholar]
- Richard A. M. International Commission for Protection Against Environmental Mutagens and Carcinogens. Application of SAR methods to non-congeneric data bases associated with carcinogenicity and mutagenicity: issues and approaches. Mutat Res. 1994 Feb 1;305(1):73–97. doi: 10.1016/0027-5107(94)90127-9. [DOI] [PubMed] [Google Scholar]
- Rosenkranz H. S., Klopman G. Prediction of the carcinogenicity in rodents of chemicals currently being tested by the US National Toxicology Program: structure-activity correlations. Mutagenesis. 1990 Sep;5(5):425–432. doi: 10.1093/mutage/5.5.425. [DOI] [PubMed] [Google Scholar]
- Sanderson D. M., Earnshaw C. G. Computer prediction of possible toxic action from chemical structure; the DEREK system. Hum Exp Toxicol. 1991 Jul;10(4):261–273. doi: 10.1177/096032719101000405. [DOI] [PubMed] [Google Scholar]
- Tennant R. W., Spalding J., Stasiewicz S., Ashby J. Prediction of the outcome of rodent carcinogenicity bioassays currently being conducted on 44 chemicals by the National Toxicology Program. Mutagenesis. 1990 Jan;5(1):3–14. doi: 10.1093/mutage/5.1.3. [DOI] [PubMed] [Google Scholar]


