Abstract
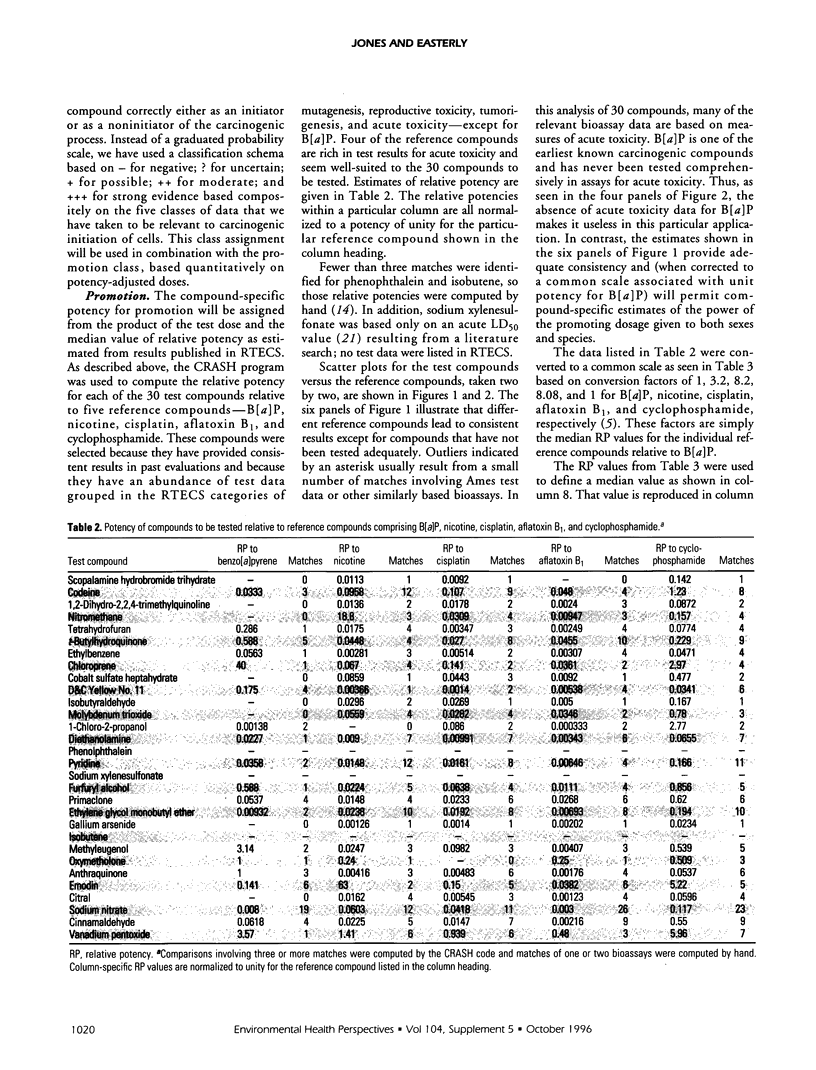
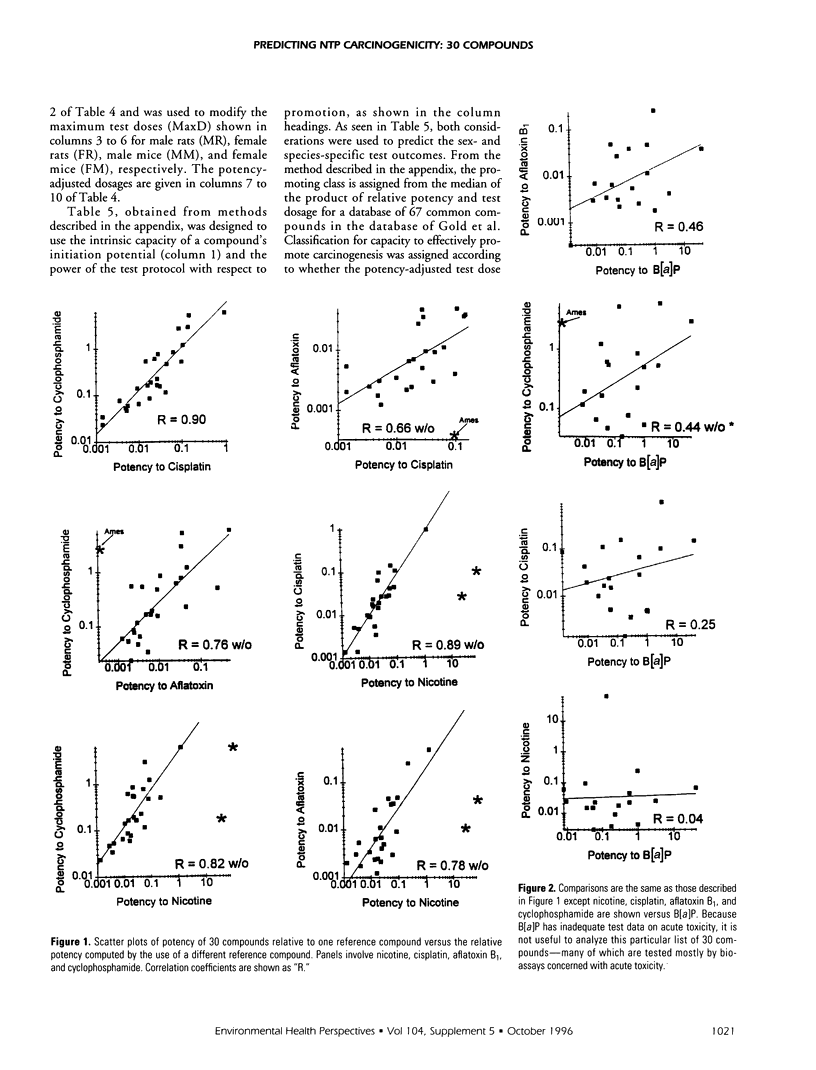
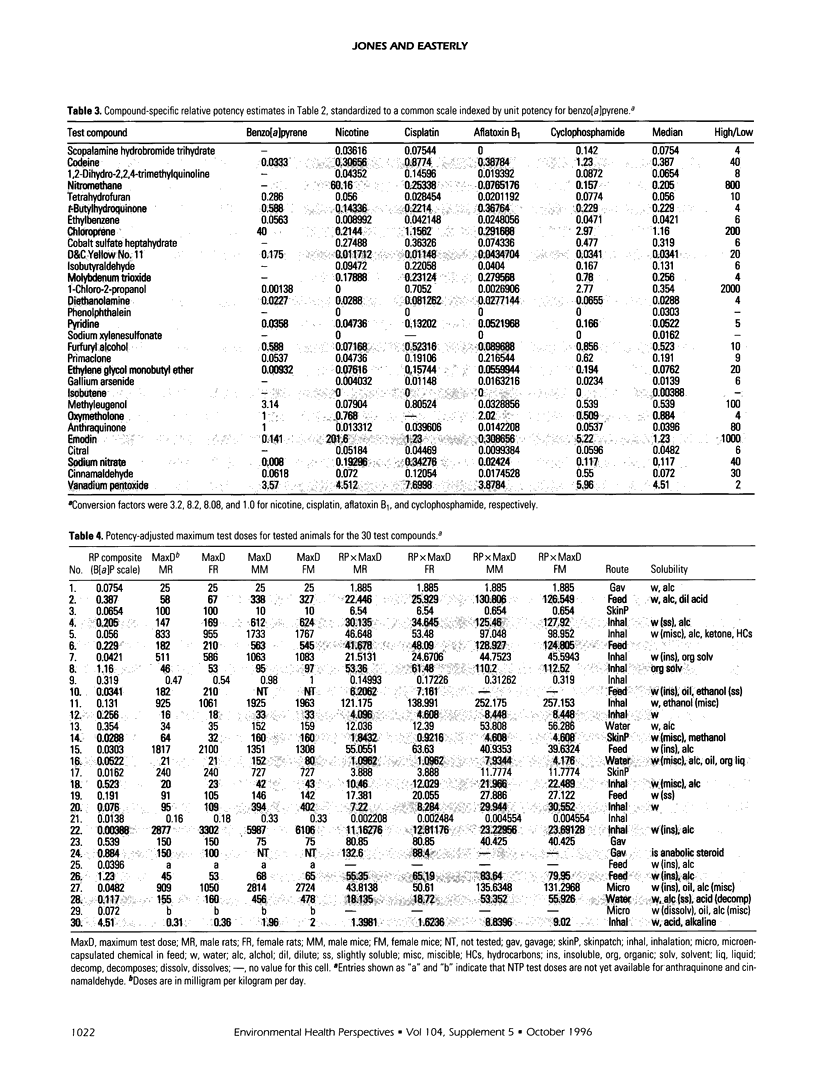
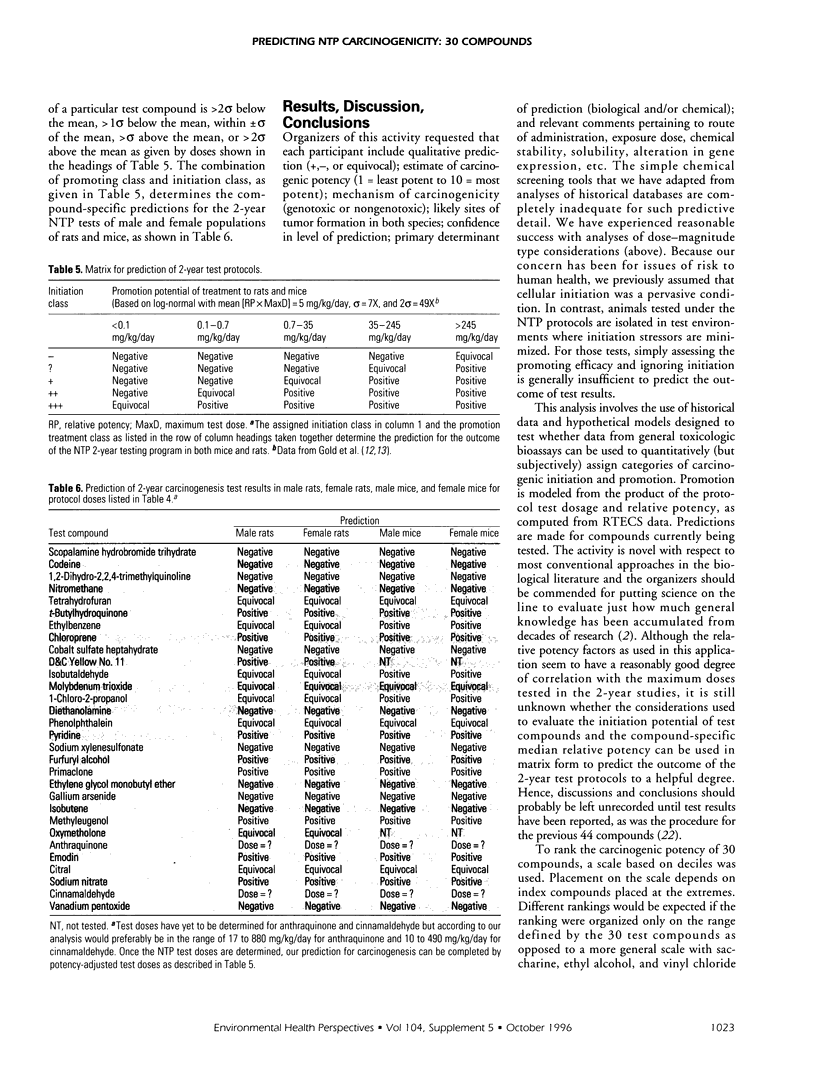
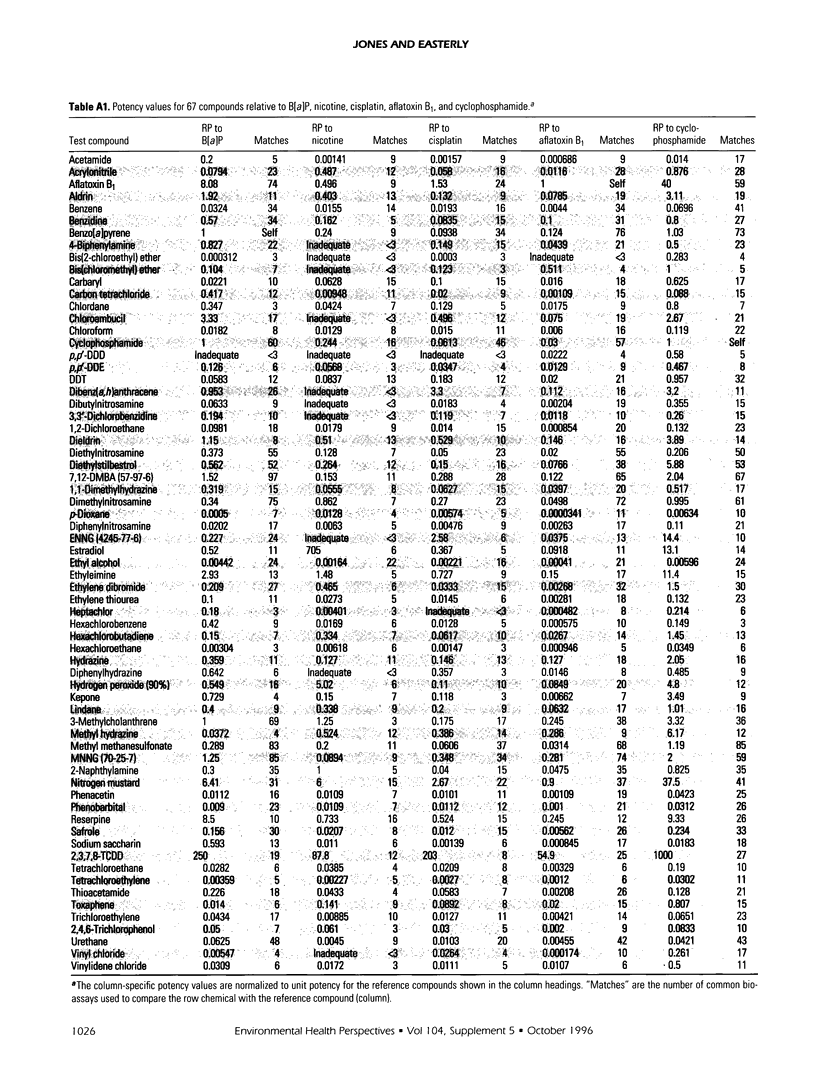
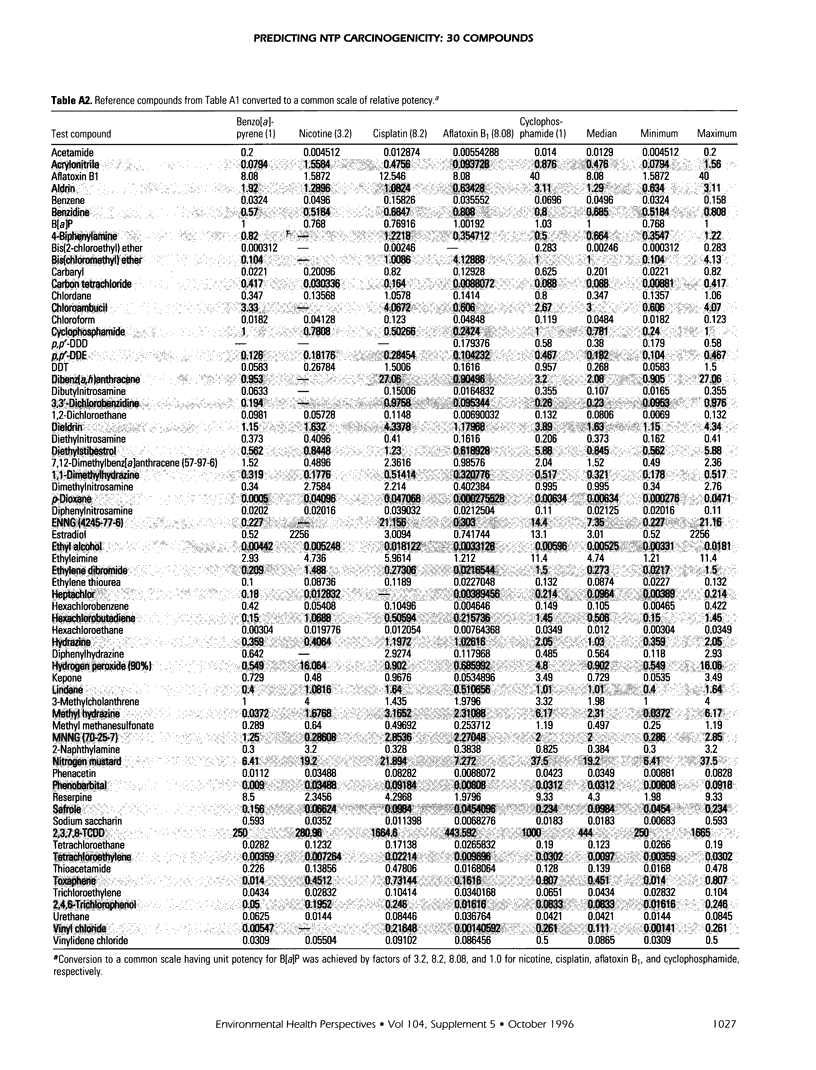
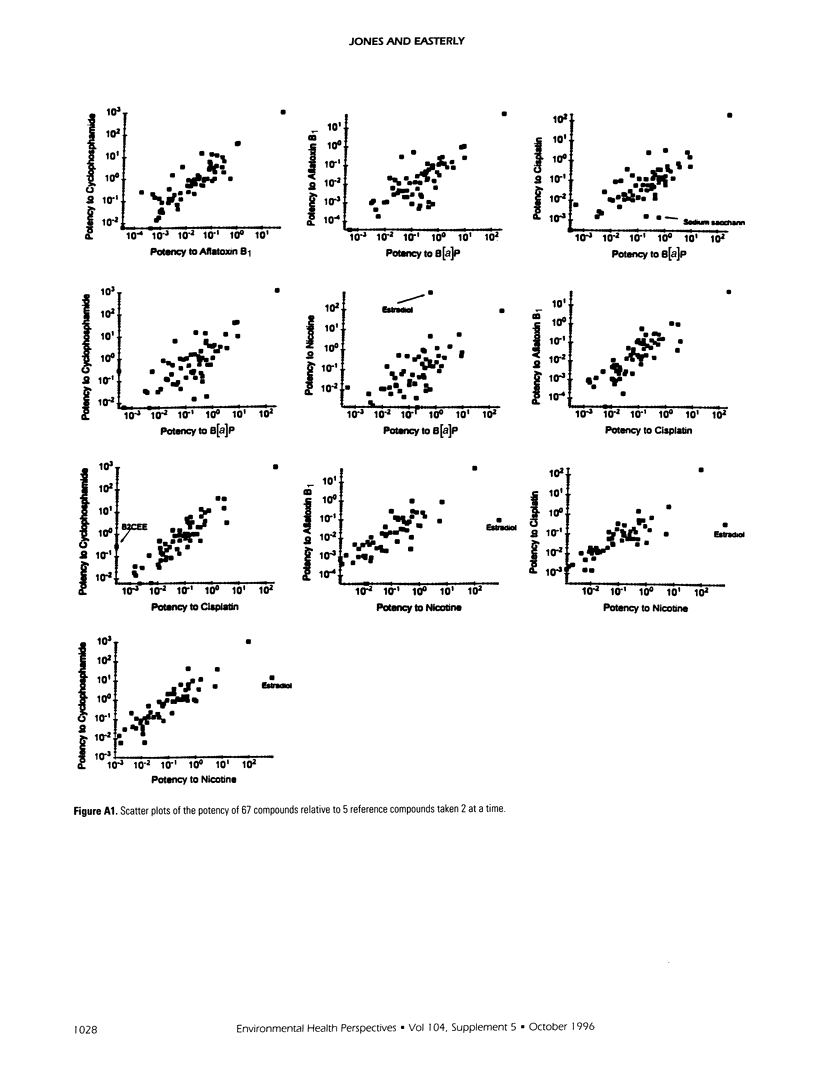
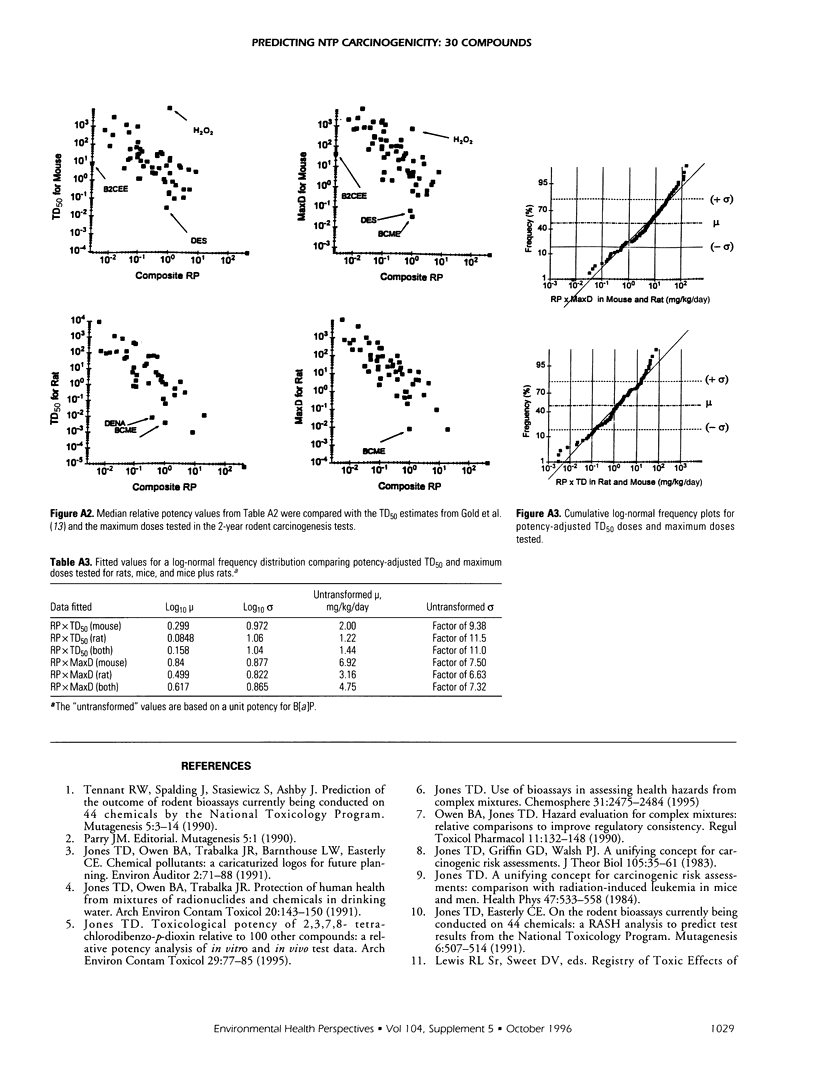
Relative potencies for 30 compounds scheduled for carcinogenic testing in the 2-year rodent bioassays were estimated based on comparisons with a wide variety of bioassay data for benzo[a]pyrene, nicotine, cisplatin, aflatoxin B1, and cyclophosphamide. Potential for oncogenic transformation of each of the compounds was estimated from short-term bioassays. Promoting strength was assigned on the basis of comparisons of the product of relative potency and test dose with the distribution of similar products obtained for 67 common compounds in the data-base of Gold et al. A potency class for promotion was assigned on the basis of whether the potency-adjusted test dosage was > 2 sigma below the mean, > 1 sigma below the mean, within +/- sigma of the mean, > sigma above the mean, or > 2 sigma above the mean, as determined from the 67 compounds. The underlying hypothesis is that a weak test dose may have a low probability of revealing a potential carcinogen, whereas a strong dose may have a high probability of producing false-positive results. Predictions are therefore directed at the central 68% of the log-normal frequency distribution according to the assumption that +/- sigma represents the ideal test dose.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gold L. S., Sawyer C. B., Magaw R., Backman G. M., de Veciana M., Levinson R., Hooper N. K., Havender W. R., Bernstein L., Peto R. A carcinogenic potency database of the standardized results of animal bioassays. Environ Health Perspect. 1984 Dec;58:9–319. doi: 10.1289/ehp.84589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L. S., Slone T. H., Bernstein L. Summary of carcinogenic potency and positivity for 492 rodent carcinogens in the carcinogenic potency database. Environ Health Perspect. 1989 Feb;79:259–272. doi: 10.1289/ehp.8979259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. D. A unifying concept for carcinogenic risk assessments: comparison with radiation-induced leukemia in mice and men. Health Phys. 1984 Oct;47(4):533–558. doi: 10.1097/00004032-198410000-00002. [DOI] [PubMed] [Google Scholar]
- Jones T. D., Easterly C. E. On the rodent bioassays currently being conducted on 44 chemicals: a RASH analysis to predict test results from the National Toxicology Program. Mutagenesis. 1991 Nov;6(6):507–514. doi: 10.1093/mutage/6.6.507. [DOI] [PubMed] [Google Scholar]
- Jones T. D., Griffin G. D., Walsh P. J. A unifying concept for carcinogenic risk assessments. J Theor Biol. 1983 Nov 7;105(1):35–61. doi: 10.1016/0022-5193(83)90423-x. [DOI] [PubMed] [Google Scholar]
- Jones T. D., Owen B. A., Trabalka J. R. Protection of human health from mixtures of radionuclides and chemical in drinking water. Arch Environ Contam Toxicol. 1991 Jan;20(1):143–150. doi: 10.1007/BF01065341. [DOI] [PubMed] [Google Scholar]
- Jones T. D. Toxicological potency of 2,3,7,8-tetrachlorodibenzo-p-dioxin relative to 100 other compounds: a relative potency analysis of in vitro and in vivo test data. Arch Environ Contam Toxicol. 1995 Jul;29(1):77–85. doi: 10.1007/BF00213090. [DOI] [PubMed] [Google Scholar]
- Jones T. D. Use of bioassays in assessing health hazards from complex mixtures: a rash analysis. Chemosphere. 1995 Jul;31(1):2475–2484. doi: 10.1016/0045-6535(95)00117-q. [DOI] [PubMed] [Google Scholar]
- Jones T. D., Walsh P. J., Watson A. P., Owen B. A., Barnthouse L. W., Sanders D. A. Chemical scoring by a rapid screening of hazard (RASH) method. Risk Anal. 1988 Mar;8(1):99–118. doi: 10.1111/j.1539-6924.1988.tb01157.x. [DOI] [PubMed] [Google Scholar]
- Jones T. D., Walsh P. J., Zeighami E. A. Permissible concentrations of chemicals in air and water derived from RTECS entries: a "rash" chemical scoring system. Toxicol Ind Health. 1985 Dec;1(4):213–234. doi: 10.1177/074823378500100414. [DOI] [PubMed] [Google Scholar]
- Owen B. A., Jones T. D. Hazard evaluation for complex mixtures: relative comparisons to improve regulatory consistency. Regul Toxicol Pharmacol. 1990 Apr;11(2):132–148. doi: 10.1016/0273-2300(90)90017-6. [DOI] [PubMed] [Google Scholar]
- Tennant R. W., Spalding J., Stasiewicz S., Ashby J. Prediction of the outcome of rodent carcinogenicity bioassays currently being conducted on 44 chemicals by the National Toxicology Program. Mutagenesis. 1990 Jan;5(1):3–14. doi: 10.1093/mutage/5.1.3. [DOI] [PubMed] [Google Scholar]
- The third UKEMS collaborative trial. Mutagenesis. 1990;5 (Suppl):1–88. [PubMed] [Google Scholar]


