Abstract
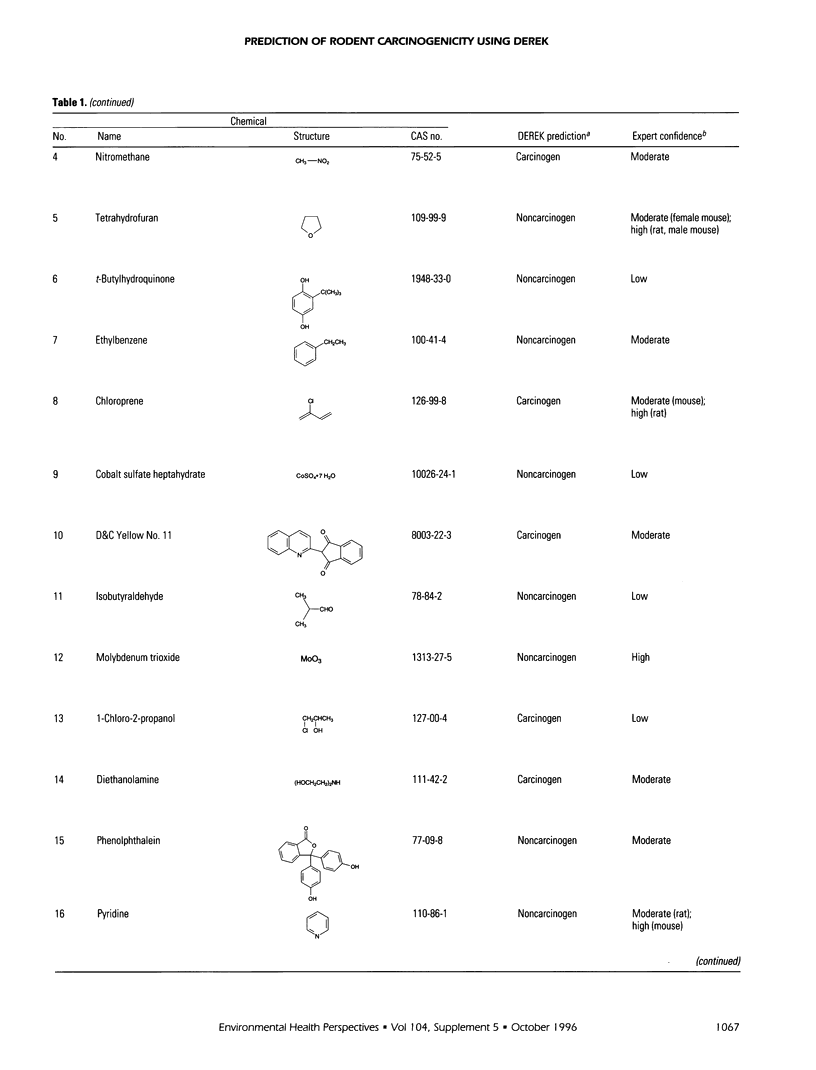
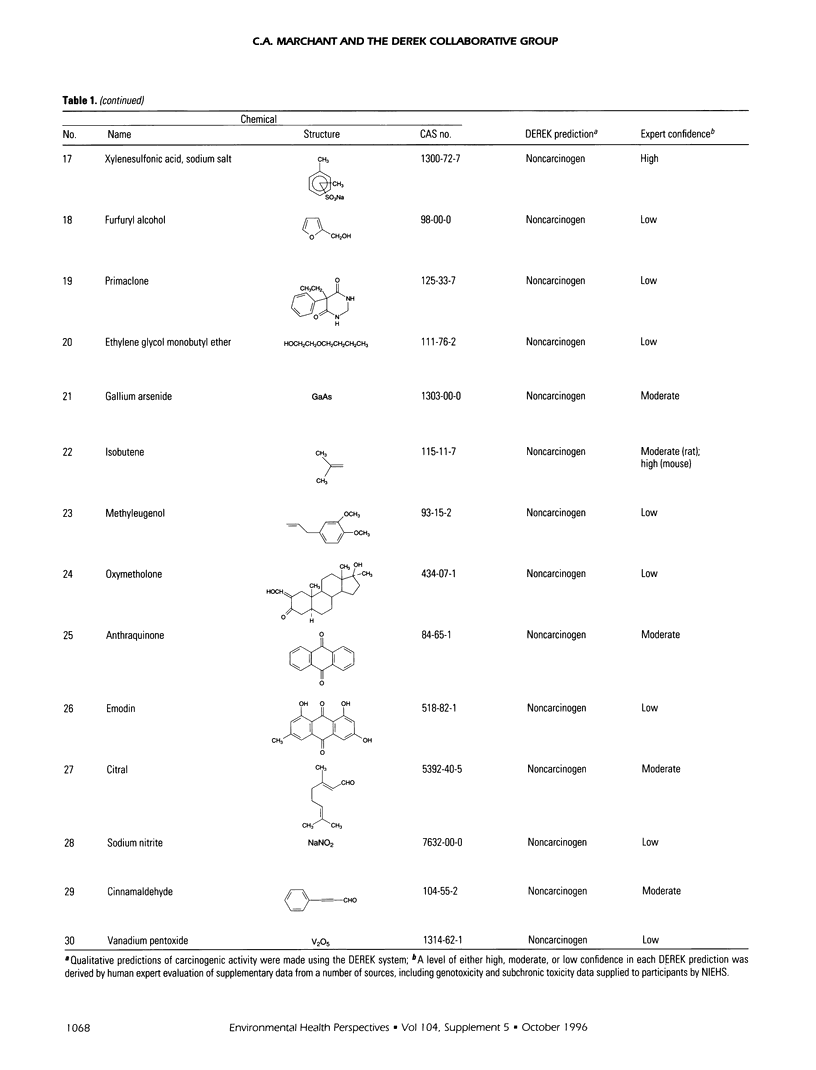
DEREK is a knowledge-based expert system for the qualitative prediction of toxicity. The DEREK system has been used to predict the carcinogenicity in rodents of the 30 chemicals in the second National Toxicology Program (NTP) carcinogenicity prediction exercise. Seven of the chemicals were predicted to be carcinogens. For 23 chemicals, there was no evidence in the DEREK knowledge base to suggest carcinogenic activity. Supplementary data from a variety of sources have been evaluated by human experts to assess confidence in each DEREK prediction. These sources included standard toxicology reference texts, genotoxicity and subchronic toxicity assay results for each chemical, as well as Salmonella mutagenicity and carcinogenicity data for close structural analogues. This process has led to the proposal of a number of improvements to the DEREK carcinogenicity knowledge base.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allavena A., Martelli A., Robbiano L., Brambilla G. Evaluation in a battery of in vivo assays of four in vitro genotoxins proved to be noncarcinogens in rodents. Teratog Carcinog Mutagen. 1992;12(1):31–41. doi: 10.1002/tcm.1770120105. [DOI] [PubMed] [Google Scholar]
- Ashby J., Paton D. The influence of chemical structure on the extent and sites of carcinogenesis for 522 rodent carcinogens and 55 different human carcinogen exposures. Mutat Res. 1993 Mar;286(1):3–74. doi: 10.1016/0027-5107(93)90003-x. [DOI] [PubMed] [Google Scholar]
- Ashby J., Tennant R. W. Prediction of rodent carcinogenicity for 44 chemicals: results. Mutagenesis. 1994 Jan;9(1):7–15. doi: 10.1093/mutage/9.1.7. [DOI] [PubMed] [Google Scholar]
- Ashby J., Tennant R. W., Zeiger E., Stasiewicz S. Classification according to chemical structure, mutagenicity to Salmonella and level of carcinogenicity of a further 42 chemicals tested for carcinogenicity by the U.S. National Toxicology Program. Mutat Res. 1989 Jun;223(2):73–103. doi: 10.1016/0165-1218(89)90037-2. [DOI] [PubMed] [Google Scholar]
- Bösch R., Friederich U., Lutz W. K., Brocker E., Bachmann M., Schlatter C. Investigations on DNA binding in rat liver and in Salmonella and on mutagenicity in the Ames test by emodin, a natural anthraquinone. Mutat Res. 1987 Jul;188(3):161–168. doi: 10.1016/0165-1218(87)90085-1. [DOI] [PubMed] [Google Scholar]
- Cornet M., Castelain P., Vercruysse A., Laib R., Kirsch-Volders M., Rogiers V. Mutagenicity of 2-methylpropene (isobutene) and its epoxide in a modified Salmonella assay for volatile compounds. Mutat Res. 1992 Jun;271(3):213–221. doi: 10.1016/0165-1161(92)90016-f. [DOI] [PubMed] [Google Scholar]
- Eder E., Deininger C., Neudecker T., Deininger D. Mutagenicity of beta-alkyl substituted acrolein congeners in the Salmonella typhimurium strain TA100 and genotoxicity testing in the SOS chromotest. Environ Mol Mutagen. 1992;19(4):338–345. doi: 10.1002/em.2850190413. [DOI] [PubMed] [Google Scholar]
- Fiala E. S., Sodum R. S., Hussain N. S., Rivenson A., Dolan L. Secondary nitroalkanes: induction of DNA repair in rat hepatocytes, activation by aryl sulfotransferase and hepatocarcinogenicity of 2-nitrobutane and 3-nitropentane in male F344 rats. Toxicology. 1995 May 5;99(1-2):89–97. doi: 10.1016/0300-483x(94)03004-l. [DOI] [PubMed] [Google Scholar]
- Geldof A. A., Engel C., Rao B. R. Estrogenic action of commonly used fragrant agent citral induces prostatic hyperplasia. Urol Res. 1992;20(2):139–144. doi: 10.1007/BF00296526. [DOI] [PubMed] [Google Scholar]
- Hartwig A. Current aspects in metal genotoxicity. Biometals. 1995 Jan;8(1):3–11. doi: 10.1007/BF00156151. [DOI] [PubMed] [Google Scholar]
- Haworth S., Lawlor T., Mortelmans K., Speck W., Zeiger E. Salmonella mutagenicity test results for 250 chemicals. Environ Mutagen. 1983;5 (Suppl 1):1–142. [PubMed] [Google Scholar]
- Howes A. J., Chan V. S., Caldwell J. Structure-specificity of the genotoxicity of some naturally occurring alkenylbenzenes determined by the unscheduled DNA synthesis assay in rat hepatocytes. Food Chem Toxicol. 1990 Aug;28(8):537–542. doi: 10.1016/0278-6915(90)90152-d. [DOI] [PubMed] [Google Scholar]
- Hébert C. D., Yuan J., Dieter M. P. Comparison of the toxicity of cinnamaldehyde when administered by microencapsulation in feed or by corn oil gavage. Food Chem Toxicol. 1994 Dec;32(12):1107–1115. doi: 10.1016/0278-6915(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Ito N., Fukushima S., Hagiwara A., Shibata M., Ogiso T. Carcinogenicity of butylated hydroxyanisole in F344 rats. J Natl Cancer Inst. 1983 Feb;70(2):343–352. [PubMed] [Google Scholar]
- Jönsson A. K., Steen G. n-Butoxyacetic acid, a urinary metabolite from inhaled n-butoxyethanol (Butylcellosolve). Acta Pharmacol Toxicol (Copenh) 1978 May;42(5):354–356. doi: 10.1111/j.1600-0773.1978.tb02216.x. [DOI] [PubMed] [Google Scholar]
- Kari F. W., Bucher J., Eustis S. L., Haseman J. K., Huff J. E. Toxicity and carcinogenicity of hydroquinone in F344/N rats and B6C3F1 mice. Food Chem Toxicol. 1992 Sep;30(9):737–747. doi: 10.1016/0278-6915(92)90075-v. [DOI] [PubMed] [Google Scholar]
- Lijinsky W., Kovatch R., Riggs C. W. Altered incidences of hepatic and hemopoietic neoplasms in F344 rats fed sodium nitrite. Carcinogenesis. 1983 Sep;4(9):1189–1191. doi: 10.1093/carcin/4.9.1189. [DOI] [PubMed] [Google Scholar]
- Léonard A., Gerber G. B. Mutagenicity, carcinogenicity and teratogenicity of vanadium compounds. Mutat Res. 1994 Feb;317(1):81–88. doi: 10.1016/0165-1110(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Maltoni C., Conti B., Cotti G., Belpoggi F. Experimental studies on benzene carcinogenicity at the Bologna Institute of Oncology: current results and ongoing research. Am J Ind Med. 1985;7(5-6):415–446. doi: 10.1002/ajim.4700070508. [DOI] [PubMed] [Google Scholar]
- McConnell E. E. NTP carcinogens--interpretational problems. Mutat Res. 1991 Jun;248(2):233–237. doi: 10.1016/0027-5107(91)90059-w. [DOI] [PubMed] [Google Scholar]
- McElhatton P. R., Sullivan F. M., Toseland P. A. Teratogenic activity and metabolism of primidone in the mouse. Epilepsia. 1977 Mar;18(1):1–11. doi: 10.1111/j.1528-1157.1977.tb05581.x. [DOI] [PubMed] [Google Scholar]
- Miller E. C., Swanson A. B., Phillips D. H., Fletcher T. L., Liem A., Miller J. A. Structure-activity studies of the carcinogenicities in the mouse and rat of some naturally occurring and synthetic alkenylbenzene derivatives related to safrole and estragole. Cancer Res. 1983 Mar;43(3):1124–1134. [PubMed] [Google Scholar]
- Mirvish S. S., Bulay O., Runge R. G., Patil K. Study of the carcinogenicity of large doses of dimethylnitramine, N-nitroso-L-proline, and sodium nitrite administered in drinking water to rats. J Natl Cancer Inst. 1980 Jun;64(6):1435–1442. doi: 10.1093/jnci/64.6.1435. [DOI] [PubMed] [Google Scholar]
- Mori H., Yoshimi N., Iwata H., Mori Y., Hara A., Tanaka T., Kawai K. Carcinogenicity of naturally occurring 1-hydroxyanthraquinone in rats: induction of large bowel, liver and stomach neoplasms. Carcinogenesis. 1990 May;11(5):799–802. doi: 10.1093/carcin/11.5.799. [DOI] [PubMed] [Google Scholar]
- Mortelmans K., Haworth S., Lawlor T., Speck W., Tainer B., Zeiger E. Salmonella mutagenicity tests: II. Results from the testing of 270 chemicals. Environ Mutagen. 1986;8 (Suppl 7):1–119. [PubMed] [Google Scholar]
- Mukherjee A., Giri A. K., Talukder G., Sharma A. Sister chromatid exchanges and micronuclei formations induced by sorbic acid and sorbic acid-nitrite in vivo in mice. Toxicol Lett. 1988 Jul;42(1):47–53. doi: 10.1016/0378-4274(88)90101-4. [DOI] [PubMed] [Google Scholar]
- Nomeir A. A., Silveira D. M., McComish M. F., Chadwick M. Comparative metabolism and disposition of furfural and furfuryl alcohol in rats. Drug Metab Dispos. 1992 Mar-Apr;20(2):198–204. [PubMed] [Google Scholar]
- Ridings J. E., Barratt M. D., Cary R., Earnshaw C. G., Eggington C. E., Ellis M. K., Judson P. N., Langowski J. J., Marchant C. A., Payne M. P. Computer prediction of possible toxic action from chemical structure: an update on the DEREK system. Toxicology. 1996 Jan 8;106(1-3):267–279. doi: 10.1016/0300-483x(95)03190-q. [DOI] [PubMed] [Google Scholar]
- Rosner M. H., Carter D. E. Metabolism and excretion of gallium arsenide and arsenic oxides by hamsters following intratracheal instillation. Fundam Appl Toxicol. 1987 Nov;9(4):730–737. doi: 10.1016/0272-0590(87)90180-1. [DOI] [PubMed] [Google Scholar]
- Sanderson D. M., Earnshaw C. G. Computer prediction of possible toxic action from chemical structure; the DEREK system. Hum Exp Toxicol. 1991 Jul;10(4):261–273. doi: 10.1177/096032719101000405. [DOI] [PubMed] [Google Scholar]
- Shaw I. C., Jones H. B. Mechanisms of non-genotoxic carcinogenesis. Trends Pharmacol Sci. 1994 Mar;15(3):89–93. doi: 10.1016/0165-6147(94)90284-4. [DOI] [PubMed] [Google Scholar]
- Shimoji N., Imaida K., Hasegawa R., Matsuoka C., Uneyama C. T., Takahashi M., Hayashi Y. Enhancing effect of oxymetholone, an anabolic steroid, on development of liver cell foci in rats initiated with N-diethylnitrosamine. Cancer Lett. 1990 Feb;49(2):165–168. doi: 10.1016/0304-3835(90)90153-o. [DOI] [PubMed] [Google Scholar]
- Wachsman J. T., Bristol D. W., Spalding J., Shelby M., Tennant R. W. Predicting chemical carcinogenesis in rodents. Environ Health Perspect. 1993 Oct;101(5):444–445. doi: 10.1289/ehp.93101444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölfle D., Schmutte C., Westendorf J., Marquardt H. Hydroxyanthraquinones as tumor promoters: enhancement of malignant transformation of C3H mouse fibroblasts and growth stimulation of primary rat hepatocytes. Cancer Res. 1990 Oct 15;50(20):6540–6544. [PubMed] [Google Scholar]
- Zeiger E., Anderson B., Haworth S., Lawlor T., Mortelmans K. Salmonella mutagenicity tests: IV. Results from the testing of 300 chemicals. Environ Mol Mutagen. 1988;11 (Suppl 12):1–157. [PubMed] [Google Scholar]
- Zeiger E., Anderson B., Haworth S., Lawlor T., Mortelmans K. Salmonella mutagenicity tests: V. Results from the testing of 311 chemicals. Environ Mol Mutagen. 1992;19 (Suppl 21):2–141. doi: 10.1002/em.2850190603. [DOI] [PubMed] [Google Scholar]
- Zeiger E., Anderson B., Haworth S., Lawlor T., Mortelmans K., Speck W. Salmonella mutagenicity tests: III. Results from the testing of 255 chemicals. Environ Mutagen. 1987;9 (Suppl 9):1–109. [PubMed] [Google Scholar]


