Abstract
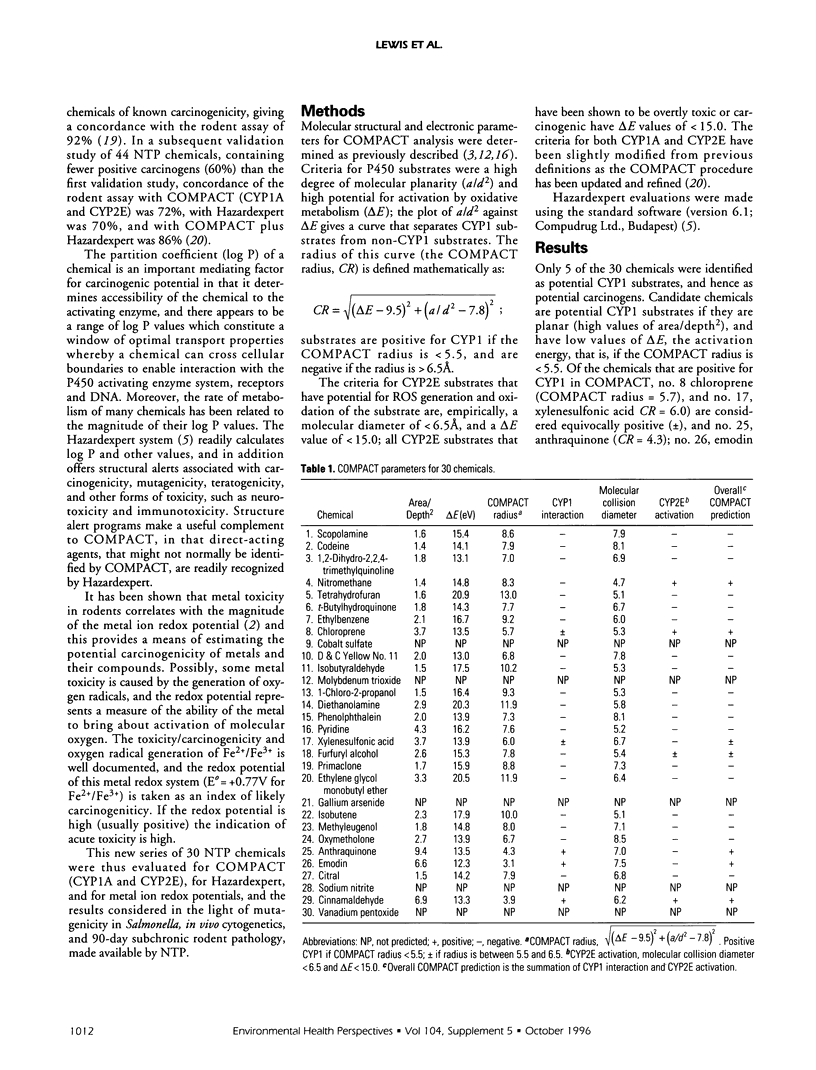
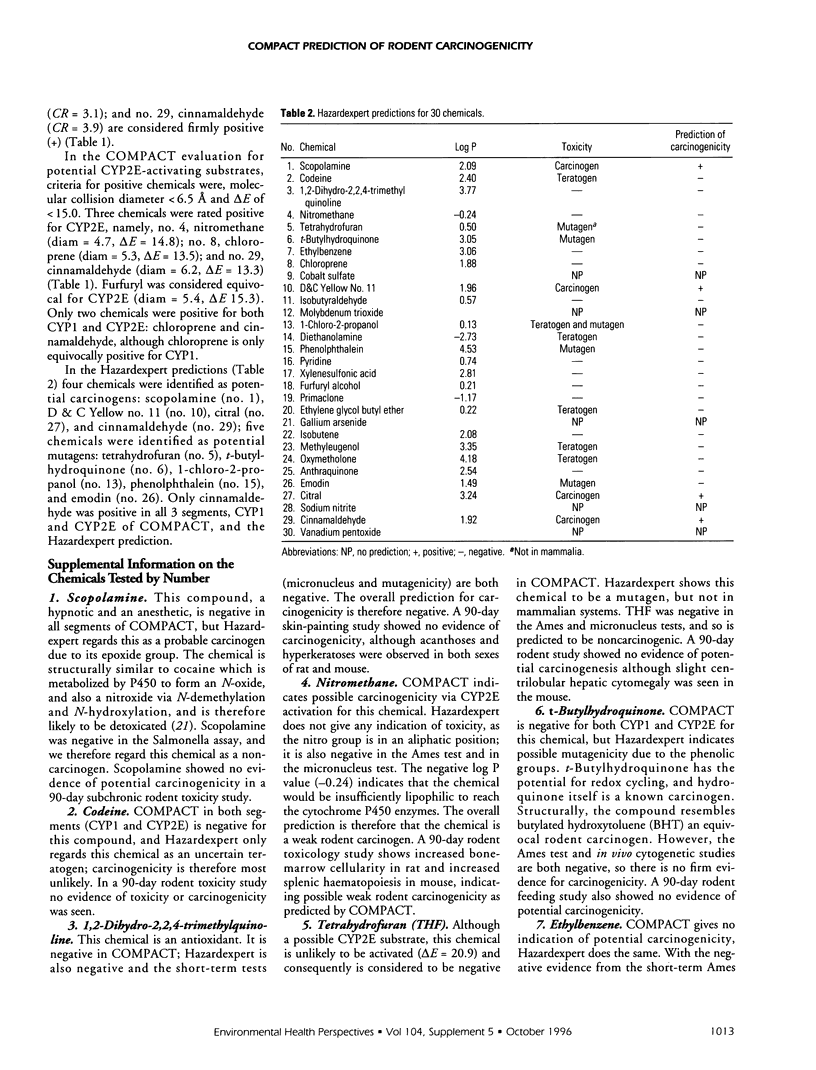
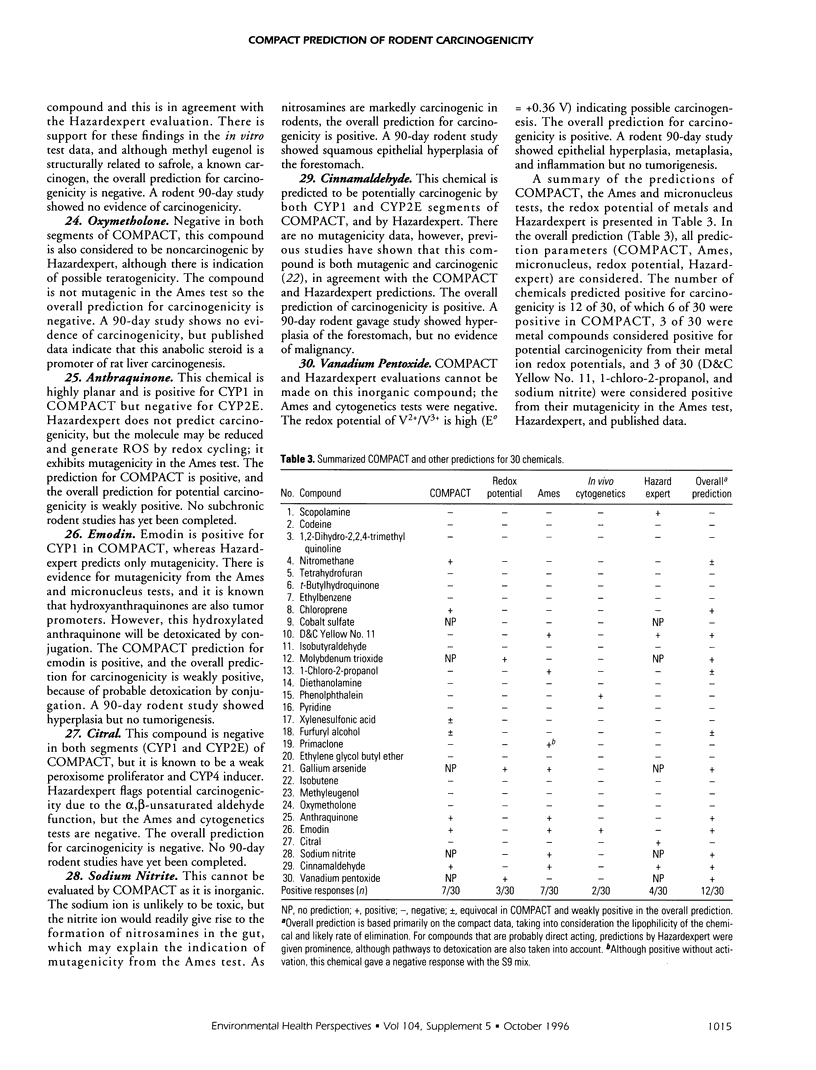
A new series of 30 miscellaneous National Toxicology Program chemicals has been evaluated prospectively for carcinogenicity and overt toxicity by COMPACT (Computer Optimised Molecular Parametric Analysis for Chemical Toxicity. CYP1A and CYP2E1). Evaluations were also made by Hazardexpert, and for metal ion redox potentials; and these, together with COMPACT, were compared with results from the Ames test for mutagenicity in Salmonella, the micronucleus test, and 90-day subchronic rodent pathology. Seven of the 30 chemicals (nitromethane, chloroprene, xylenesulphonic acid, furfuryl alcohol, anthraquinone, emodin, cinnamaldehyde) were positive for potential carcinogenicity in the COMPACT evaluation; xylenesulphonic acid and furfuryl alcohol were only equivocally positive. Four of the 30 chemicals-scopolamine, D&C Yellow No. 11, citral, cinnamaldehyde-were positive by Hazardexpert; 6 of 30-D&C Yellow No. 11, 1-chloro-2-propanol, anthraquinone, emodin, sodium nitrite, cinnamaldehyde-were positive in the Ames test; 2 of 30-phenolphthalein and emodin-were positive in the in vivo cytogenetics test; and 3 of 30-molybdenum trioxide, gallium arsenide, vanadium pentoxide-were metal compounds with redox potentials of the metal/metal ion indicative of possible carcinogenicity. The overall prediction for carcinogenicity was positive for 12 of 30 chemicals: nitromethane, chloroprene, D&C Yellow No. 11, molybdenum trioxide, 1-chloro-2-propanol, furfuryl alcohol, gallium arsenide, anthraquinone, emodin, sodium nitrite, cinnamaldehyde, vanadium pentoxide). This overall prediction has been made on the basis of the results of the computer tests and from consideration of the information from bacterial mutagenicity, together with likely lipid solubility and pathways of metabolism and elimination.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ekström G., Ingelman-Sundberg M. Rat liver microsomal NADPH-supported oxidase activity and lipid peroxidation dependent on ethanol-inducible cytochrome P-450 (P-450IIE1). Biochem Pharmacol. 1989 Apr 15;38(8):1313–1319. doi: 10.1016/0006-2952(89)90338-9. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- Ioannides C., Cheung Y. L., Wilson J., Lewis D. F., Gray T. J. The mutagenicity and interactions of 2- and 4-(acetylamino)fluorene with cytochrome P450 and the aromatic hydrocarbon receptor may explain the difference in their carcinogenic potency. Chem Res Toxicol. 1993 Jul-Aug;6(4):535–541. doi: 10.1021/tx00034a023. [DOI] [PubMed] [Google Scholar]
- Ioannides C., Lewis D. F., Parke D. V. Computer modelling in predicting carcinogenicity. Eur J Cancer Prev. 1993 May;2(3):275–282. doi: 10.1097/00008469-199305000-00015. [DOI] [PubMed] [Google Scholar]
- Ioannides C., Lewis D. F., Parke D. V. The use of computers in the safety evaluation of drugs and other chemicals. Eur J Drug Metab Pharmacokinet. 1994 Jul-Sep;19(3):225–233. doi: 10.1007/BF03188925. [DOI] [PubMed] [Google Scholar]
- Ioannides C., Parke D. V. Induction of cytochrome P4501 as an indicator of potential chemical carcinogenesis. Drug Metab Rev. 1993;25(4):485–501. doi: 10.3109/03602539308993983. [DOI] [PubMed] [Google Scholar]
- Lewis D. F., Ioannides C., Parke D. V. A retrospective evaluation of COMPACT predictions of the outcome of NTP rodent carcinogenicity testing. Environ Health Perspect. 1995 Feb;103(2):178–184. doi: 10.1289/ehp.95103178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. F., Ioannides C., Parke D. V. A retrospective evaluation of COMPACT predictions of the outcome of NTP rodent carcinogenicity testing. Environ Health Perspect. 1995 Feb;103(2):178–184. doi: 10.1289/ehp.95103178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. F., Ioannides C., Parke D. V. Molecular modelling of cytochrome CYP1A1: a putative access channel explains differences in induction potency between the isomers benzo(a)pyrene and benzo(e)pyrene, and 2- and 4-acetylaminofluorene. Toxicol Lett. 1994 May;71(3):235–243. doi: 10.1016/0378-4274(94)90110-4. [DOI] [PubMed] [Google Scholar]
- Lewis D. F., Ioannides C., Parke D. V. Validation of a novel molecular orbital approach (COMPACT) for the prospective safety evaluation of chemicals, by comparison with rodent carcinogenicity and Salmonella mutagenicity data evaluated by the U.S. NCI/NTP. Mutat Res. 1993 Feb;291(1):61–77. doi: 10.1016/0165-1161(93)90018-u. [DOI] [PubMed] [Google Scholar]
- Lewis D. F., Lake B. G. Interaction of some peroxisome proliferators with the mouse liver peroxisome proliferator-activated receptor (PPAR): a molecular modelling and quantitative structure-activity relationship (QSAR) study. Xenobiotica. 1993 Jan;23(1):79–96. doi: 10.3109/00498259309059364. [DOI] [PubMed] [Google Scholar]
- Lewis D. F., Parker M. G., King R. J. Molecular modelling of the human estrogen receptor and ligand interactions based on site-directed mutagenesis and amino acid sequence homology. J Steroid Biochem Mol Biol. 1995 Jan;52(1):55–65. doi: 10.1016/0960-0760(94)00151-b. [DOI] [PubMed] [Google Scholar]
- Parke D. V. Activation mechanisms to chemical toxicity. Arch Toxicol. 1987;60(1-3):5–15. doi: 10.1007/BF00296939. [DOI] [PubMed] [Google Scholar]
- Parke D. V., Ioannides C., Lewis D. F. The 1990 Pharmaceutical Manufacturers Association of Canada keynote lecture. The role of the cytochromes P450 in the detoxication and activation of drugs and other chemicals. Can J Physiol Pharmacol. 1991 May;69(5):537–549. doi: 10.1139/y91-081. [DOI] [PubMed] [Google Scholar]
- Parke D. V. The cytochromes P450 and mechanisms of chemical carcinogenesis. Environ Health Perspect. 1994 Oct;102(10):852–853. doi: 10.1289/ehp.94102852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poet T. S., Brendel K., Halpert J. R. Inactivation of cytochromes P450 2B protects against cocaine-mediated toxicity in rat liver slices. Toxicol Appl Pharmacol. 1994 May;126(1):26–32. doi: 10.1006/taap.1994.1086. [DOI] [PubMed] [Google Scholar]


