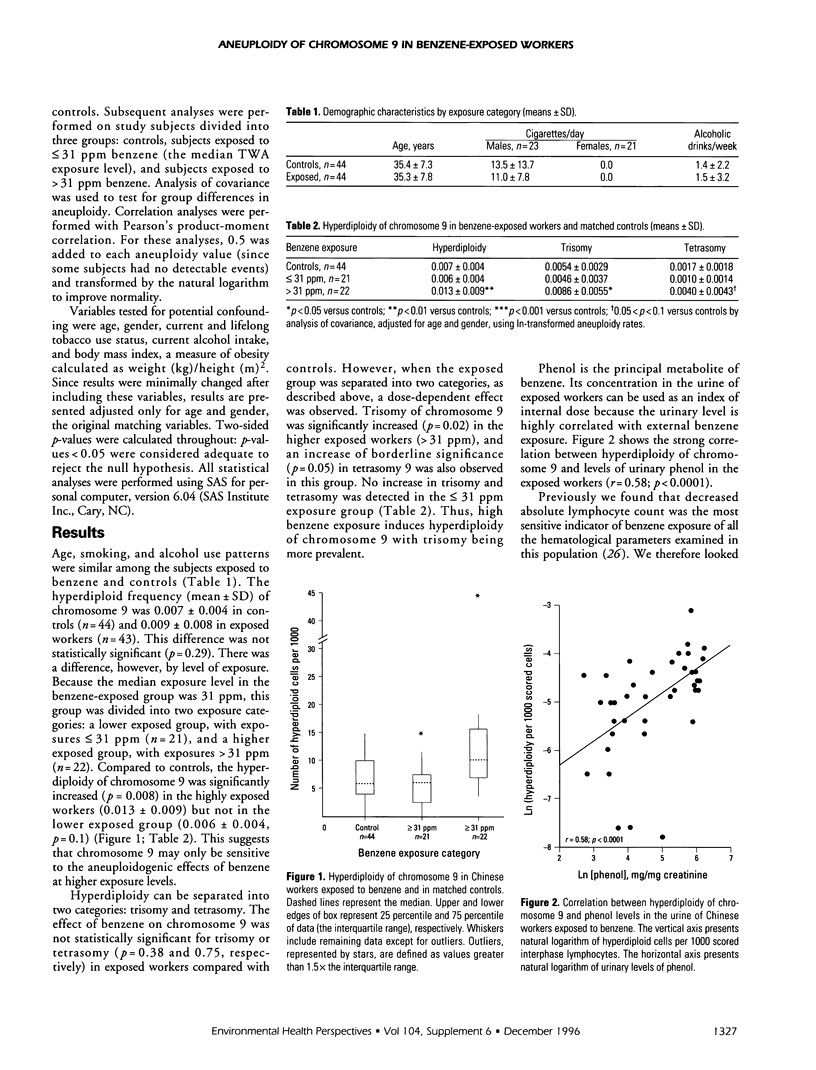
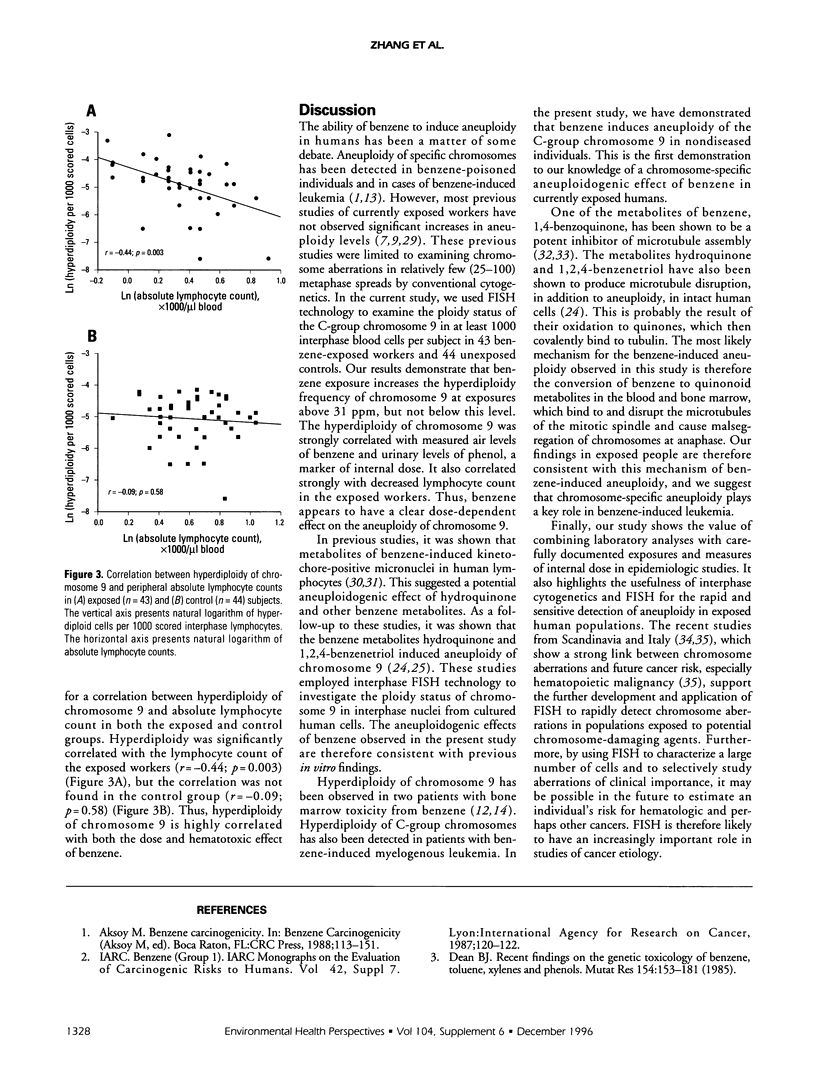
Abstract
Fluorescence in situ hybridization (FISH) is a powerful new technique that allows numerical chromosome aberrations (aneuploidy) to be detected in interphase cells. In previous studies, FISH has been used to demonstrate that the benzene metabolites hydroquinone and 1,2,4-benzenetriol induce aneuploidy of chromosomes 7 and 9 in cultures of human cells. In the present study, we used an interphase FISH procedure to perform cytogenetic analyses on the blood cells of 43 workers exposed to benzene (median = 31 ppm, 8-hr time-weighted average) and 44 matched controls from Shanghai, China. High benzene exposure (> 31 ppm, n = 22) increased the hyperdiploid frequency of chromosome 9 (p < 0.01), but lower exposure (< or = 31 ppm, n = 21) did not. Trisomy 9 was the major form of benzene-induced hyperdiploidy. The level of hyperploidy in exposed workers correlated with their urinary phenol level (r = 0.58, p < 0.0001), a measure of internal benzene dose. A significant correlation was also found between hyperdiploidy and decreased absolute lymphocyte count, an indicator of benzene hematotoxicity, in the exposed group (r = -0.44, p = 0.003) but not in controls (r = -0.09, p = 0.58). These results show that high benzene exposure induces aneuploidy of chromosome 9 in nondiseased individuals, with trisomy being the most prevalent form. They further highlight the usefulness of interphase cytogenetics and FISH for the rapid and sensitive detection of aneuploidy in exposed human populations.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aksoy M., Erdem S., Erdogan G., Dinçol G. Acute leukaemia in two generations following chronic exposure to benzene. Hum Hered. 1974;24(1):70–74. doi: 10.1159/000152639. [DOI] [PubMed] [Google Scholar]
- Bechtold W. E., Willis J. K., Sun J. D., Griffith W. C., Reddy T. V. Biological markers of exposure to benzene: S-phenylcysteine in albumin. Carcinogenesis. 1992 Jul;13(7):1217–1220. doi: 10.1093/carcin/13.7.1217. [DOI] [PubMed] [Google Scholar]
- Bonassi S., Abbondandolo A., Camurri L., Dal Prá L., De Ferrari M., Degrassi F., Forni A., Lamberti L., Lando C., Padovani P. Are chromosome aberrations in circulating lymphocytes predictive of future cancer onset in humans? Preliminary results of an Italian cohort study. Cancer Genet Cytogenet. 1995 Feb;79(2):133–135. doi: 10.1016/0165-4608(94)00131-t. [DOI] [PubMed] [Google Scholar]
- Dean B. J. Recent findings on the genetic toxicology of benzene, toluene, xylenes and phenols. Mutat Res. 1985 Nov;154(3):153–181. doi: 10.1016/0165-1110(85)90016-8. [DOI] [PubMed] [Google Scholar]
- Eastmond D. A., Pinkel D. Detection of aneuploidy and aneuploidy-inducing agents in human lymphocytes using fluorescence in situ hybridization with chromosome-specific DNA probes. Mutat Res. 1990 Oct;234(5):303–318. doi: 10.1016/0165-1161(90)90041-l. [DOI] [PubMed] [Google Scholar]
- Eastmond D. A., Rupa D. S., Hasegawa L. S. Detection of hyperdiploidy and chromosome breakage in interphase human lymphocytes following exposure to the benzene metabolite hydroquinone using multicolor fluorescence in situ hybridization with DNA probes. Mutat Res. 1994 Jul;322(1):9–20. doi: 10.1016/0165-1218(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Erdoğan G., Aksoy M. Cytogenetic studies in thirteen patients with pancytopenia and leukaemia associated with long-term exposure to benzene. New Istanbul Contrib Clin Sci. 1973 Oct;10(4):230–247. [PubMed] [Google Scholar]
- Forni A. Chromosome changes and benzene exposure. A review. Rev Environ Health. 1979;3(1):5–17. [PubMed] [Google Scholar]
- Forni A., Moreo L. Cytogenetic studies in a case of benzene leukaemia. Eur J Cancer. 1967 Nov;3(4):251–255. doi: 10.1016/0014-2964(67)90005-9. [DOI] [PubMed] [Google Scholar]
- Gray J. W., Pinkel D. Molecular cytogenetics in human cancer diagnosis. Cancer. 1992 Mar 15;69(6 Suppl):1536–1542. doi: 10.1002/1097-0142(19920315)69:6+<1536::aid-cncr2820691306>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Hagmar L., Brøgger A., Hansteen I. L., Heim S., Högstedt B., Knudsen L., Lambert B., Linnainmaa K., Mitelman F., Nordenson I. Cancer risk in humans predicted by increased levels of chromosomal aberrations in lymphocytes: Nordic study group on the health risk of chromosome damage. Cancer Res. 1994 Jun 1;54(11):2919–2922. [PubMed] [Google Scholar]
- Irons R. D., Neptun D. A. Effects of the principal hydroxy-metabolites of benzene on microtubule polymerization. Arch Toxicol. 1980 Oct;45(4):297–305. doi: 10.1007/BF00293810. [DOI] [PubMed] [Google Scholar]
- Irons R. D., Pfeifer R. W., Aune T. M., Pierce C. W. Soluble immune response suppressor (SIRS) inhibits microtubule function in vivo and microtubule assembly in vitro. J Immunol. 1984 Oct;133(4):2032–2036. [PubMed] [Google Scholar]
- Major J., Jakab M., Kiss G., Tompa A. Chromosome aberration, sister-chromatid exchange, proliferative rate index, and serum thiocyanate concentration in smokers exposed to low-dose benzene. Environ Mol Mutagen. 1994;23(2):137–142. doi: 10.1002/em.2850230211. [DOI] [PubMed] [Google Scholar]
- Pinkel D., Landegent J., Collins C., Fuscoe J., Segraves R., Lucas J., Gray J. Fluorescence in situ hybridization with human chromosome-specific libraries: detection of trisomy 21 and translocations of chromosome 4. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9138–9142. doi: 10.1073/pnas.85.23.9138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy M. V., Schultz S. C., Blackburn G. R., Mackerer C. R. Lack of DNA adduct formation in mice treated with benzene. Mutat Res. 1994 Dec;325(4):149–155. doi: 10.1016/0165-7992(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Rothman N., Haas R., Hayes R. B., Li G. L., Wiemels J., Campleman S., Quintana P. J., Xi L. J., Dosemeci M., Titenko-Holland N. Benzene induces gene-duplicating but not gene-inactivating mutations at the glycophorin A locus in exposed humans. Proc Natl Acad Sci U S A. 1995 Apr 25;92(9):4069–4073. doi: 10.1073/pnas.92.9.4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman N., Li G. L., Dosemeci M., Bechtold W. E., Marti G. E., Wang Y. Z., Linet M., Xi L. Q., Lu W., Smith M. T. Hematotoxicity among Chinese workers heavily exposed to benzene. Am J Ind Med. 1996 Mar;29(3):236–246. doi: 10.1002/(SICI)1097-0274(199603)29:3<236::AID-AJIM3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Rowley J. D., Blaisdell R. K., Jacobson L. O. Chromosome studies in preleukemia. I. Aneuploidy of group C chromosomes in three patients. Blood. 1966 Jun;27(6):782–799. [PubMed] [Google Scholar]
- SANDBERG A. A., ISHIHARA T., CROSSWHITE L. H. GROUP-C TRISOMY IN MYELOID METAPLASIA WITH POSSIBLE LEUKEMIA. Blood. 1964 Dec;24:716–725. [PubMed] [Google Scholar]
- Sasiadek M., Jagielski J. Genotoxic effects observed in workers occupationally exposed to organic solvents. Pol J Occup Med. 1990;3(1):103–108. [PubMed] [Google Scholar]
- Sasiadek M. Nonrandom distribution of breakpoints in the karyotypes of workers occupationally exposed to benzene. Environ Health Perspect. 1992 Jul;97:255–257. doi: 10.1289/ehp.9297255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellyei M., Keleman E. Chromosome study in a case of granulocytic leukaemia with 'Pelgerisation' 7 years after benzene pancytopenia. Eur J Cancer. 1971 Feb;7(1):83–85. doi: 10.1016/0014-2964(71)90099-5. [DOI] [PubMed] [Google Scholar]
- Tompa A., Major J., Jakab M. G. Monitoring of benzene-exposed workers for genotoxic effects of benzene: improved-working-condition-related decrease in the frequencies of chromosomal aberrations in peripheral blood lymphocytes. Mutat Res. 1994 Jan 16;304(2):159–165. doi: 10.1016/0027-5107(94)90207-0. [DOI] [PubMed] [Google Scholar]
- Türkel B., Egeli U. Analysis of chromosomal aberrations in shoe workers exposed long term to benzene. Occup Environ Med. 1994 Jan;51(1):50–53. doi: 10.1136/oem.51.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters M. D., Stack H. F., Brady A. L., Lohman P. H., Haroun L., Vainio H. Use of computerized data listings and activity profiles of genetic and related effects in the review of 195 compounds. Mutat Res. 1988 May-Aug;205(1-4):295–312. doi: 10.1016/0165-1218(88)90024-9. [DOI] [PubMed] [Google Scholar]
- Winkelstein A., Sparkes R. S., Craddock C. G. Trisomy of group C in a myeloproliferative disorder. Report of case. Blood. 1966 May;27(5):722–733. [PubMed] [Google Scholar]
- Yager J. W., Eastmond D. A., Robertson M. L., Paradisin W. M., Smith M. T. Characterization of micronuclei induced in human lymphocytes by benzene metabolites. Cancer Res. 1990 Jan 15;50(2):393–399. [PubMed] [Google Scholar]
- Yardley-Jones A., Anderson D., Parke D. V. The toxicity of benzene and its metabolism and molecular pathology in human risk assessment. Br J Ind Med. 1991 Jul;48(7):437–444. doi: 10.1136/oem.48.7.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Robertson M. L., Kolachana P., Davison A. J., Smith M. T. Benzene metabolite, 1,2,4-benzenetriol, induces micronuclei and oxidative DNA damage in human lymphocytes and HL60 cells. Environ Mol Mutagen. 1993;21(4):339–348. doi: 10.1002/em.2850210405. [DOI] [PubMed] [Google Scholar]
- Zhang L., Venkatesh P., Creek M. L., Smith M. T. Detection of 1,2,4-benzenetriol induced aneuploidy and microtubule disruption by fluorescence in situ hybridization and immunocytochemistry. Mutat Res. 1994 Mar;320(4):315–327. doi: 10.1016/0165-1218(94)90084-1. [DOI] [PubMed] [Google Scholar]



