Abstract
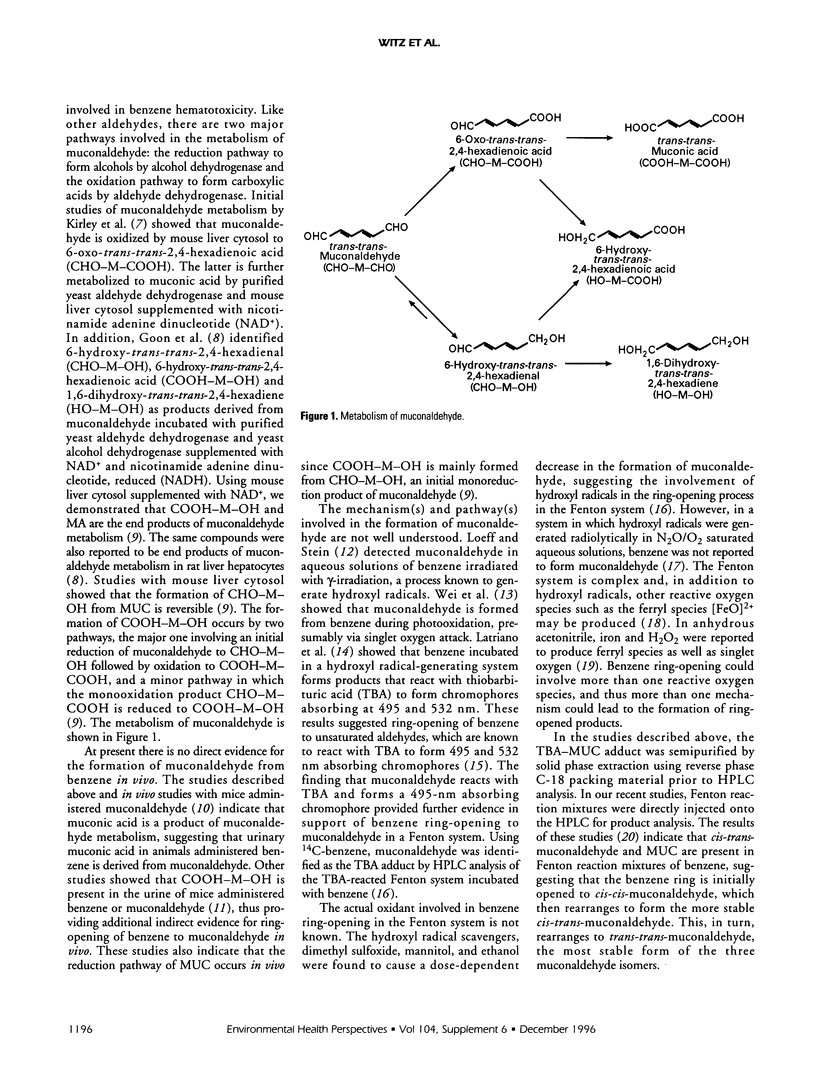
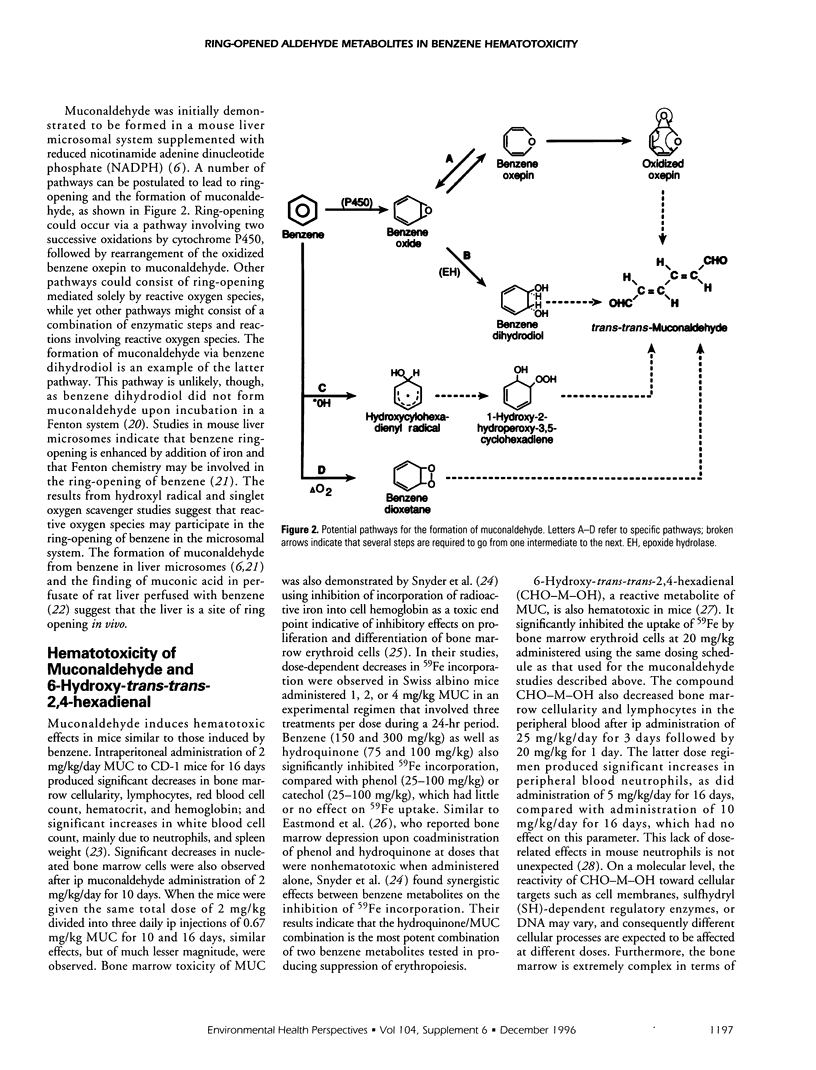
The hematotoxicity of benzene is mediated by reactive benzene metabolites and possibly by other intermediates including reactive oxygen species. We previously hypothesized that ring-opened metabolites may significantly contribute to benzene hematotoxicity. Consistent with this hypothesis, our studies initially demonstrated that benzene is metabolized in vitro to trans-trans-muconaldehyde (MUC), a reactive six-carbon diene dialdehyde, and that MUC is toxic to the bone marrow in a manner similar to benzene. Benzene toxicity most likely involves interactions among several metabolites that operate by different mechanisms to produce more than one biological effect. Our studies indicate that MUC coadministered with hydroquinone is a particularly potent metabolite combination that causes bone marrow damage, suggesting that the involvement of ring-opened metabolites in benzene toxicity may be related to their biological effects in combination with other benzene metabolites. Studies in our laboratory and by others indicate that MUC is metabolized to a variety of compounds by oxidation or reduction of the aldehyde groups. The aldehydic MUC metabolite 6-hydroxy-trans-trans-2,4-hexadienal (CHO-M-OH), similar to MUC but to a lesser extent, is reactive toward glutathione, mutagenic in V79 cells, and hematotoxic in mice. It is formed by monoreduction of MUC, a process that is reversible and could be of biological significance in benzene bone marrow toxicity. The MUC metabolite 6-hydroxy-trans-trans-2,4-hexadienoic (COOH-M-OH) is an end product of MUC metabolism in vitro. Our studies indicate that COOH-M-OH is a urinary metabolite of benzene in mice, a finding that provides further indirect evidence for the in vivo formation of MUC from benzene. Mechanistic studies showed the formation of cis-trans-muconaldehyde in addition to MUC from benzene incubated in a hydroxyl radical-generating Fenton system. These results suggest that the benzene ring is initially opened to cis,cis-muconaldehyde, an unstable isomer that rearranges to cis-trans-muconaldehyde, which further rearranges to trans-trans-muconaldehyde. The latter is not formed from benzene dihydrodiol by reactive oxygen species in a Fenton system that contains reactive oxygen species.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang R. L., Wong C. Q., Kline S. A., Conney A. H., Goldstein B. D., Witz G. Mutagenicity of trans,trans-muconaldehyde and its metabolites in V79 cells. Environ Mol Mutagen. 1994;24(2):112–115. doi: 10.1002/em.2850240206. [DOI] [PubMed] [Google Scholar]
- Drummond J. C., Finar I. L. Muconic acid as a metabolic product of benzene. Biochem J. 1938 Jan;32(1):79–84. doi: 10.1042/bj0320079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatt H., Witz G. Studies on the induction of gene mutations in bacterial and mammalian cells by the ring-opened benzene metabolites trans,trans-muconaldehyde and trans,trans-muconic acid. Mutagenesis. 1990 May;5(3):263–266. doi: 10.1093/mutage/5.3.263. [DOI] [PubMed] [Google Scholar]
- Goon D., Cheng X., Ruth J. A., Petersen D. R., Ross D. Metabolism of trans,trans-muconaldehyde by aldehyde and alcohol dehydrogenases: identification of a novel metabolite. Toxicol Appl Pharmacol. 1992 May;114(1):147–155. doi: 10.1016/0041-008x(92)90107-4. [DOI] [PubMed] [Google Scholar]
- Grotz V. L., Ji S., Kline S. A., Goldstein B. D., Witz G. Metabolism of benzene and trans,trans-muconaldehyde in the isolated perfused rat liver. Toxicol Lett. 1994 Feb 15;70(3):281–290. doi: 10.1016/0378-4274(94)90122-8. [DOI] [PubMed] [Google Scholar]
- HORIGUCHI M., KANDATSU M. Isolation of 2-aminoethane phosphonic acid from rumen protozoa. Nature. 1959 Sep 19;184(Suppl 12):901–902. doi: 10.1038/184901b0. [DOI] [PubMed] [Google Scholar]
- Henschler D., Eder E., Epe B., Schiffmann D. Genotoxic and cell-transforming properties of (trans,trans)-muconaldehyde. Mutat Res. 1991 May;248(1):35–43. doi: 10.1016/0027-5107(91)90085-3. [DOI] [PubMed] [Google Scholar]
- Kirley T. A., Goldstein B. D., Maniara W. M., Witz G. Metabolism of trans, trans-muconaldehyde, a microsomal hematotoxic metabolite of benzene, by purified yeast aldehyde dehydrogenase and a mouse liver soluble fraction. Toxicol Appl Pharmacol. 1989 Sep 1;100(2):360–367. doi: 10.1016/0041-008x(89)90322-0. [DOI] [PubMed] [Google Scholar]
- Kline S. A., Robertson J. F., Grotz V. L., Goldstein B. D., Witz G. Identification of 6-hydroxy-trans,trans-2,4-hexadienoic acid, a novel ring-opened urinary metabolite of benzene. Environ Health Perspect. 1993 Sep;101(4):310–312. doi: 10.1289/ehp.93101310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline S. A., Xiang Q., Goldstein B. D., Witz G. Reaction of (E,E)-muconaldehyde and its aldehydic metabolites, (E,E)-6-oxohexadienoic acid and (E,E)-6-hydroxyhexa-2,4-dienal, with glutathione. Chem Res Toxicol. 1993 Jul-Aug;6(4):578–583. doi: 10.1021/tx00034a029. [DOI] [PubMed] [Google Scholar]
- Latriano L., Goldstein B. D., Witz G. Formation of muconaldehyde, an open-ring metabolite of benzene, in mouse liver microsomes: an additional pathway for toxic metabolites. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8356–8360. doi: 10.1073/pnas.83.21.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latriano L., Zaccaria A., Goldstein B. D., Witz G. Muconaldehyde formation from 14C-benzene in a hydroxyl radical generating system. J Free Radic Biol Med. 1985;1(5-6):363–371. doi: 10.1016/0748-5514(85)90148-5. [DOI] [PubMed] [Google Scholar]
- PARKE D. V., WILLIAMS R. T. Studies in detoxication. XLIX. The metabolism of benzene containing (14C1) benzene. Biochem J. 1953 May;54(2):231–238. doi: 10.1042/bj0540231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser M. J., Shurina R. D., Kalf G. F. Metabolism of phenol and hydroquinone to reactive products by macrophage peroxidase or purified prostaglandin H synthase. Environ Health Perspect. 1989 Jul;82:229–237. doi: 10.1289/ehp.8982229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. T., Yager J. W., Steinmetz K. L., Eastmond D. A. Peroxidase-dependent metabolism of benzene's phenolic metabolites and its potential role in benzene toxicity and carcinogenicity. Environ Health Perspect. 1989 Jul;82:23–29. doi: 10.1289/ehp.898223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder R., Kalf G. F. A perspective on benzene leukemogenesis. Crit Rev Toxicol. 1994;24(3):177–209. doi: 10.3109/10408449409021605. [DOI] [PubMed] [Google Scholar]
- Subrahmanyam V. V., Ross D., Eastmond D. A., Smith M. T. Potential role of free radicals in benzene-induced myelotoxicity and leukemia. Free Radic Biol Med. 1991;11(5):495–515. doi: 10.1016/0891-5849(91)90063-9. [DOI] [PubMed] [Google Scholar]
- Sutton H. C., Winterbourn C. C. On the participation of higher oxidation states of iron and copper in Fenton reactions. Free Radic Biol Med. 1989;6(1):53–60. doi: 10.1016/0891-5849(89)90160-3. [DOI] [PubMed] [Google Scholar]
- Witz G. Biological interactions of alpha,beta-unsaturated aldehydes. Free Radic Biol Med. 1989;7(3):333–349. doi: 10.1016/0891-5849(89)90137-8. [DOI] [PubMed] [Google Scholar]
- Witz G., Gad S. C., Tice R. R., Oshiro Y., Piper C. E., Goldstein B. D. Genetic toxicity of the benzene metabolite trans, trans-muconaldehyde in mammalian and bacterial cells. Mutat Res. 1990 Apr;240(4):295–306. doi: 10.1016/0165-1218(90)90080-l. [DOI] [PubMed] [Google Scholar]
- Witz G., Lawrie N. J., Amoruso M. A., Goldstein B. D. Inhibition by reactive aldehydes of superoxide anion radical production from stimulated polymorphonuclear leukocytes and pulmonary alveolar macrophages. Effects on cellular sulfhydryl groups and NADPH oxidase activity. Biochem Pharmacol. 1987 Mar 1;36(5):721–726. doi: 10.1016/0006-2952(87)90725-8. [DOI] [PubMed] [Google Scholar]
- Witz G., Lawrie N. J., Amoruso M. A., Goldstein B. D. Inhibition by reactive aldehydes of superoxide anion radical production in stimulated human neutrophils. Chem Biol Interact. 1985 Feb-Apr;53(1-2):13–23. doi: 10.1016/s0009-2797(85)80080-6. [DOI] [PubMed] [Google Scholar]
- Witz G., Lawrie N. J., Zaccaria A., Ferran H. E., Jr, Goldstein B. D. The reaction of 2-thiobarbituric acid with biologically active alpha,beta-unsaturated aldehydes. J Free Radic Biol Med. 1986;2(1):33–39. doi: 10.1016/0748-5514(86)90121-2. [DOI] [PubMed] [Google Scholar]
- Witz G., Maniara W., Mylavarapu V., Goldstein B. D. Comparative metabolism of benzene and trans,trans-muconaldehyde to trans,trans-muconic acid in DBA/2N and C57BL/6 mice. Biochem Pharmacol. 1990 Sep 15;40(6):1275–1280. doi: 10.1016/0006-2952(90)90393-y. [DOI] [PubMed] [Google Scholar]
- Witz G., Rao G. S., Goldstein B. D. Short-term toxicity of trans,trans-muconaldehyde. Toxicol Appl Pharmacol. 1985 Sep 30;80(3):511–516. doi: 10.1016/0041-008x(85)90396-5. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Goldstein B. D., Witz G. Iron-stimulated ring-opening of benzene in a mouse liver microsomal system. Mechanistic studies and formation of a new metabolite. Biochem Pharmacol. 1995 Nov 9;50(10):1607–1617. doi: 10.1016/0006-2952(95)02043-8. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Kline S. A., Kirley T. A., Goldstein B. D., Witz G. Pathways of trans,trans-muconaldehyde metabolism in mouse liver cytosol: reversibility of monoreductive metabolism and formation of end products. Arch Toxicol. 1993;67(7):461–467. doi: 10.1007/BF01969916. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Schafer F., Schoenfeld H., Cooper K., Snyder R., Goldstein B. D., Witz G. The hematotoxic effects of 6-hydroxy-trans,trans-2,4-hexadienal, a reactive metabolite of trans,trans-muconaldehyde, in CD-1 mice. Toxicol Appl Pharmacol. 1995 Jun;132(2):213–219. doi: 10.1006/taap.1995.1101. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Xiang Q., Glatt H., Platt K. L., Goldstein B. D., Witz G. Studies on pathways of ring opening of benzene in a Fenton system. Free Radic Biol Med. 1995 Mar;18(3):411–419. doi: 10.1016/0891-5849(94)00148-d. [DOI] [PubMed] [Google Scholar]



