Abstract
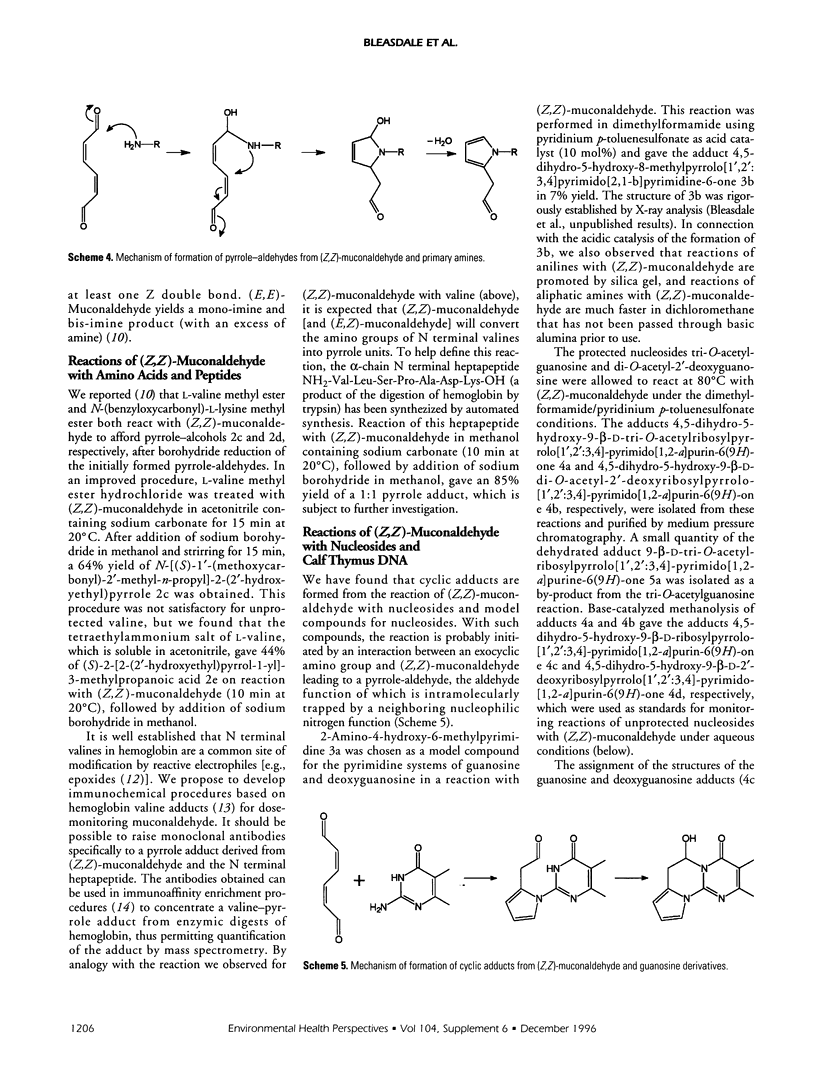
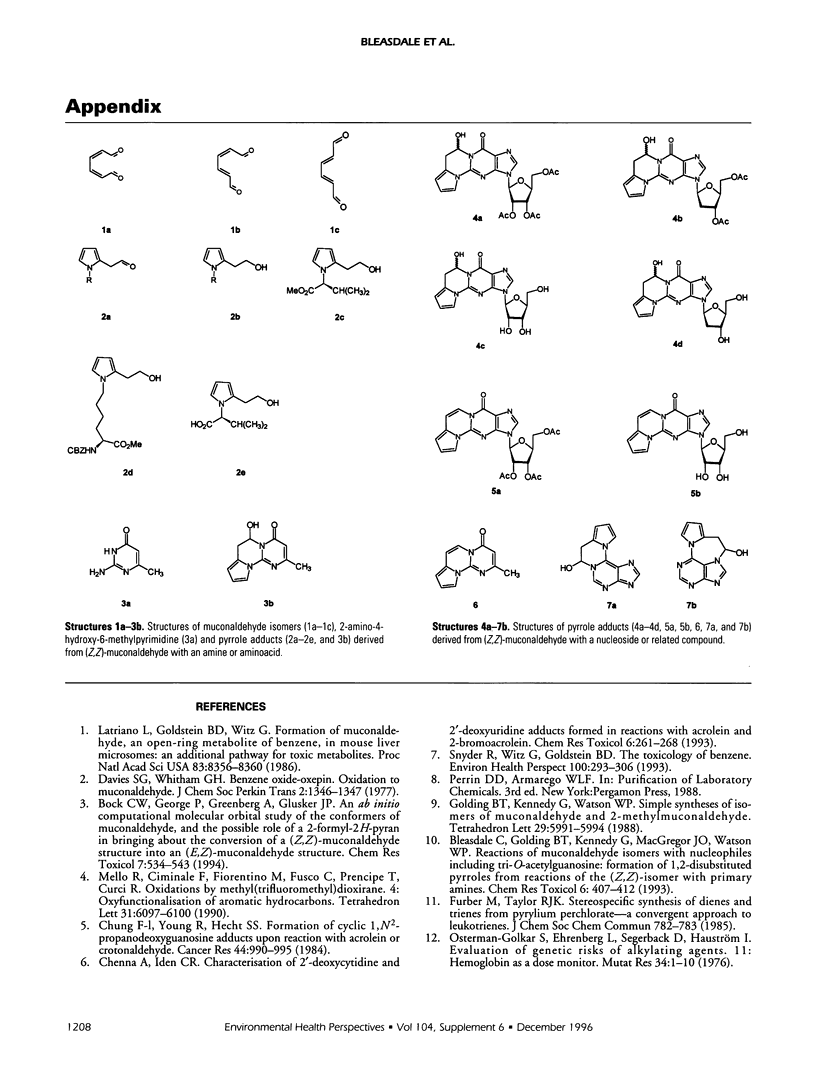
(Z,Z)-Muconaldehyde reacted with primary amines to give N-substituted-2(2'-oxoethyl)-pyrroles, which were reduced to N-substituted-2-(2'-hydroxyethyl)-pyrroles by sodium borohydride. The pyrrole-forming reaction is exhibited by valine and its methyl ester, and is being developed with terminal valine in hemoglobin as a means of dose monitoring (Z,Z)-muconaldehyde, a putative metabolite of benzene. Reactions in aqueous solution between (Z,Z)-muconaldehyde and adenosine, deoxyadenosine, guanosine, or deoxyguanosine leading to pyrrole-containing adducts are described. The elucidation of the structures of the adducts was assisted by the study of reactions between (Z,Z)-muconaldehyde and both nucleoside derivatives and a model compound for guanosine. Reactions of (Z,Z)-muconaldehyde are complicated by its isomerization to (E,Z)- and (E-E)-muconaldehyde. The kinetics of this process have been studied in benzene, acetonitrile, and dimethylsulfoxide.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bleasdale C., Golding B. T., Kennedy G., MacGregor J. O., Watson W. P. Reactions of muconaldehyde isomers with nucleophiles including tri-O-acetylguanosine: formation of 1,2-disubstituted pyrroles from reactions of the (Z,Z)-isomer with primary amines. Chem Res Toxicol. 1993 Jul-Aug;6(4):407–412. doi: 10.1021/tx00034a002. [DOI] [PubMed] [Google Scholar]
- Bock C. W., George P., Greenberg A., Glusker J. P. An ab initio computational molecular orbital study of the conformers of muconaldehyde, and the possible role of 2-formyl-2H-pyran in bringing about the conversion of a (Z,Z)-muconaldehyde structure into an (E,Z)-muconaldehyde structure. Chem Res Toxicol. 1994 Jul-Aug;7(4):534–543. doi: 10.1021/tx00040a009. [DOI] [PubMed] [Google Scholar]
- Booth E. D., Aston J. P., van den Berg P. T., Baan R. A., Riddick D. A., Wade L. T., Wright A. S., Watson W. P. Class-specific immunoadsorption purification for polycyclic aromatic hydrocarbon-DNA adducts. Carcinogenesis. 1994 Oct;15(10):2099–2106. doi: 10.1093/carcin/15.10.2099. [DOI] [PubMed] [Google Scholar]
- Chenna A., Iden C. R. Characterization of 2'-deoxycytidine and 2'-deoxyuridine adducts formed in reactions with acrolein and 2-bromoacrolein. Chem Res Toxicol. 1993 May-Jun;6(3):261–268. doi: 10.1021/tx00033a003. [DOI] [PubMed] [Google Scholar]
- Chung F. L., Young R., Hecht S. S. Formation of cyclic 1,N2-propanodeoxyguanosine adducts in DNA upon reaction with acrolein or crotonaldehyde. Cancer Res. 1984 Mar;44(3):990–995. [PubMed] [Google Scholar]
- Green T., Hathway D. E. Interactions of vinyl chloride with rat-liver DNA in vivo. Chem Biol Interact. 1978 Sep;22(2-3):211–224. doi: 10.1016/0009-2797(78)90126-6. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P., Kim D. H. Enzymatic oxidation of ethyl carbamate to vinyl carbamate and its role as an intermediate in the formation of 1,N6-ethenoadenosine. Chem Res Toxicol. 1991 Jul-Aug;4(4):413–421. doi: 10.1021/tx00022a003. [DOI] [PubMed] [Google Scholar]
- Laib R. J., Gwinner L. M., Bolt H. M. DNA alkylation by vinyl chloride metabolites: etheno derivatives or 7-alkylation of guanine? Chem Biol Interact. 1981 Oct;37(1-2):219–231. doi: 10.1016/0009-2797(81)90179-4. [DOI] [PubMed] [Google Scholar]
- Latriano L., Goldstein B. D., Witz G. Formation of muconaldehyde, an open-ring metabolite of benzene, in mouse liver microsomes: an additional pathway for toxic metabolites. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8356–8360. doi: 10.1073/pnas.83.21.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterman-Golkar S., Ehrenberg L., Segerbäck D., Hällström I. Evaluation of genetic risks of alkylating agents. II. Haemoglobin as a dose monitor. Mutat Res. 1976 Jan;34(1):1–10. doi: 10.1016/0027-5107(76)90256-6. [DOI] [PubMed] [Google Scholar]
- Snyder R., Witz G., Goldstein B. D. The toxicology of benzene. Environ Health Perspect. 1993 Apr;100:293–306. doi: 10.1289/ehp.93100293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgeon R. J., Flanagan C., Naik D. V., Schulman S. G. In vitro adsorption of doxorubicin hydrochloride on insoluble calcium phosphate. J Pharm Sci. 1977 Sep;66(9):1346–1347. doi: 10.1002/jps.2600660940. [DOI] [PubMed] [Google Scholar]



