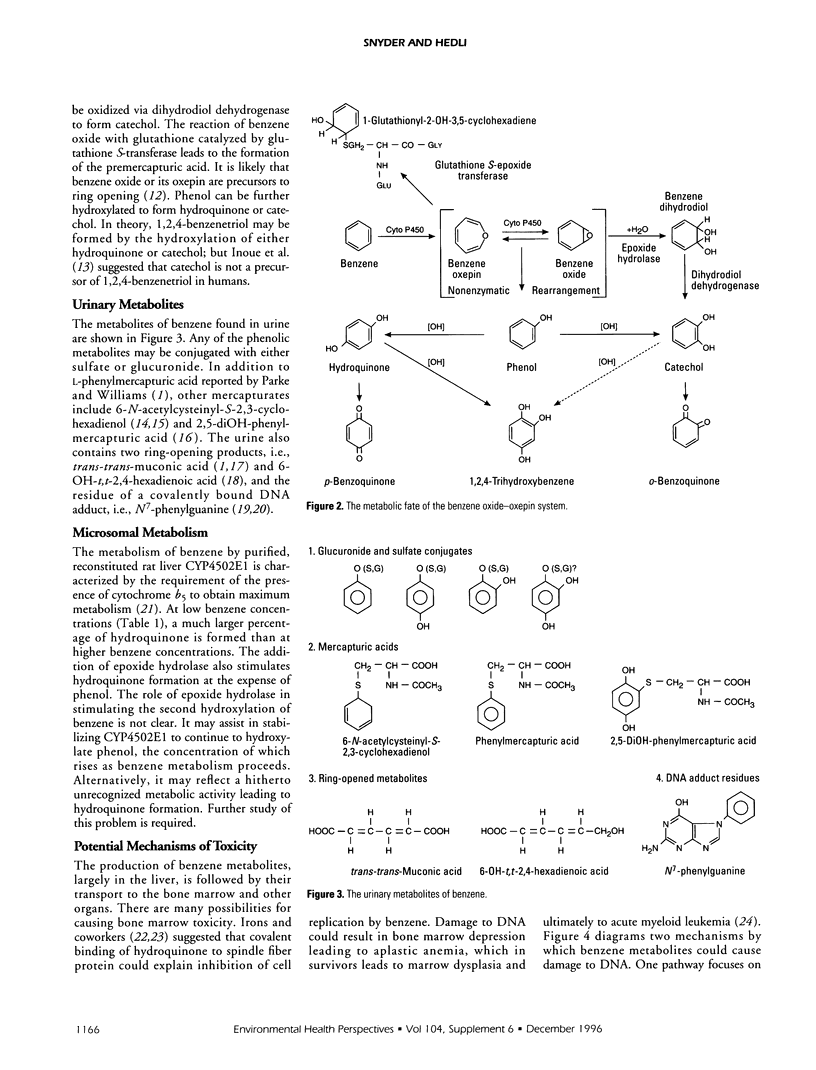
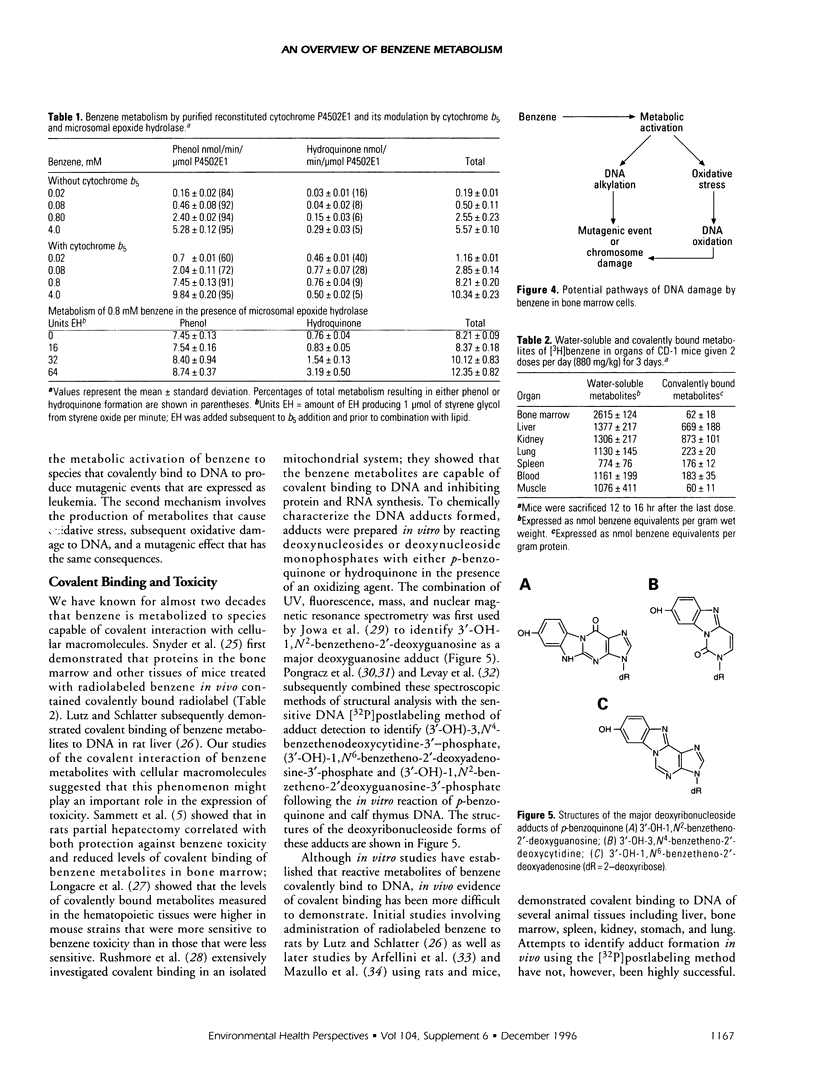
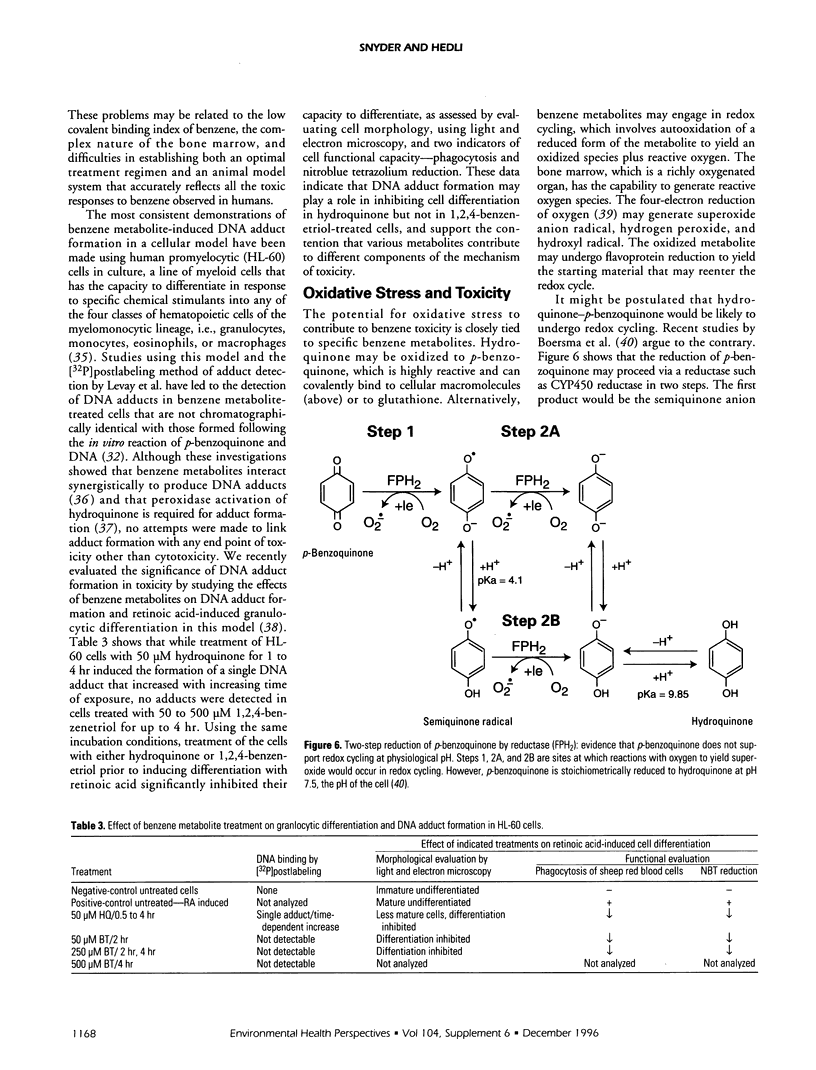
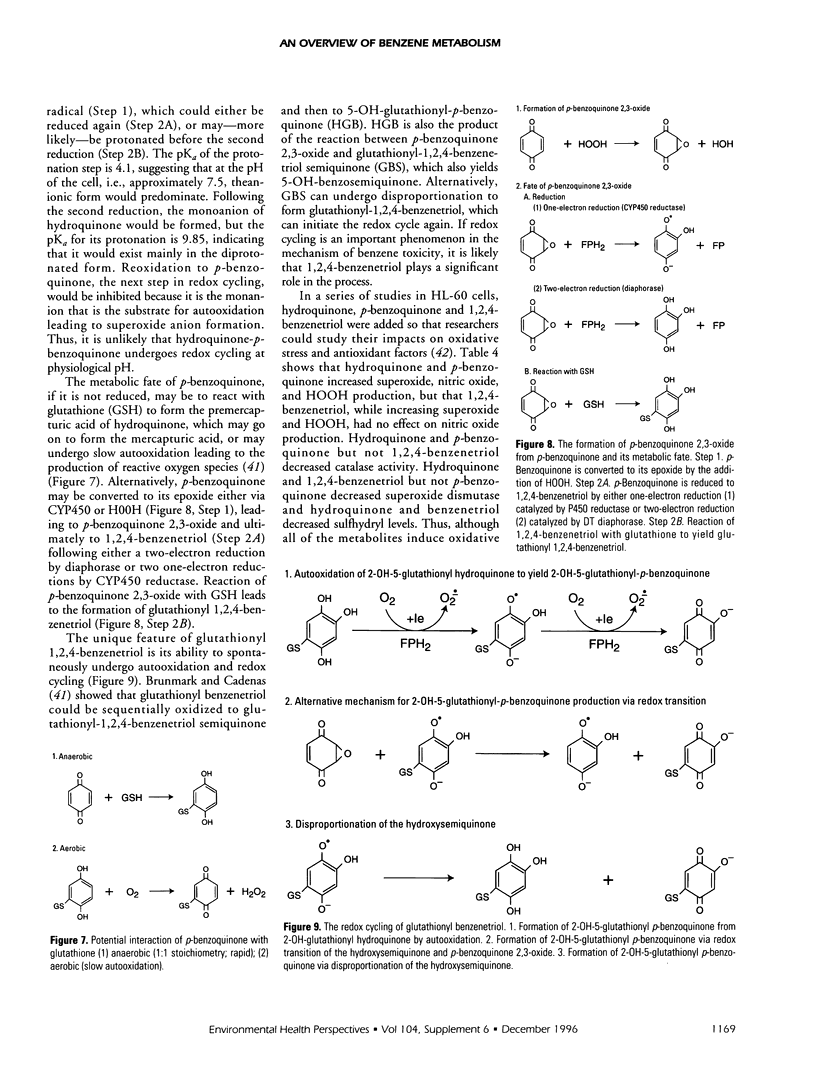
Abstract
Benzene toxicity involves both bone marrow depression and leukemogenesis caused by damage to multiple classes of hematopoietic cells and a variety of hematopoietic cell functions. Study of the relationship between the metabolism and toxicity of benzene indicates that several metabolites of benzene play significant roles in generating benzene toxicity. Benzene is metabolized, primarily in the liver, to a variety of hydroxylated and ring-opened products that are transported to the bone marrow where subsequent secondary metabolism occurs. Two potential mechanisms by which benzene metabolites may damage cellular macromolecules to induce toxicity include the covalent binding of reactive metabolites of benzene and the capacity of benzene metabolites to induce oxidative damage. Although the relative contributions of each of these mechanisms to toxicity remains unestablished, it is clear that different mechanisms contribute to the toxicities associated with different metabolites. As a corollary, it is unlikely that benzene toxicity can be described as the result of the interaction of a single metabolite with a single biological target. Continued investigation of the metabolism of benzene and its metabolites will allow us to determine the specific combination of metabolites as well as the biological target(s) involved in toxicity and will ultimately lead to our understanding of the relationship between the production of benzene metabolites and bone marrow toxicity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Andrews L. S., Lee E. W., Witmer C. M., Kocsis J. J., Snyder R. Effects of toluene on the metabolism, disposition and hemopoietic toxicity of [3H]benzene. Biochem Pharmacol. 1977 Feb 15;26(4):293–300. doi: 10.1016/0006-2952(77)90180-0. [DOI] [PubMed] [Google Scholar]
- Andrews L. S., Sasame H. A., Gillette J. R. 3H-Benzene metabolism in rabbit bone marrow. Life Sci. 1979 Aug 13;25(7):567–572. doi: 10.1016/0024-3205(79)90550-2. [DOI] [PubMed] [Google Scholar]
- Arfellini G., Grilli S., Colacci A., Mazzullo M., Prodi G. In vivo and in vitro binding of benzene to nucleic acids and proteins of various rat and mouse organs. Cancer Lett. 1985 Sep 15;28(2):159–168. doi: 10.1016/0304-3835(85)90071-0. [DOI] [PubMed] [Google Scholar]
- Attwood E. C., Robey E. D., Ross J., Bradley F., Kramer J. J. Determination of platelet and leucocyte vitamin C and the levels found in normal subjects. Clin Chim Acta. 1974 Jul 15;54(1):95–105. doi: 10.1016/0009-8981(74)90047-3. [DOI] [PubMed] [Google Scholar]
- BEUTLER E., DURON O., KELLY B. M. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963 May;61:882–888. [PubMed] [Google Scholar]
- Bass D. A., Parce J. W., Dechatelet L. R., Szejda P., Seeds M. C., Thomas M. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol. 1983 Apr;130(4):1910–1917. [PubMed] [Google Scholar]
- Boersma M. G., Balvers W. G., Boeren S., Vervoort J., Rietjens I. M. NADPH-cytochrome reductase catalysed redox cycling of 1,4-benzoquinone; hampered at physiological conditions, initiated at increased pH values. Biochem Pharmacol. 1994 Jun 1;47(11):1949–1955. doi: 10.1016/0006-2952(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Brunmark A., Cadenas E. Reductive addition of glutathione to p-benzoquinone, 2-hydroxy-p-benzoquinone, and p-benzoquinone epoxides. Effect of the hydroxy- and glutathionyl substituents on p-benzohydroquinone autoxidation. Chem Biol Interact. 1988;68(3-4):273–298. doi: 10.1016/0009-2797(88)90021-x. [DOI] [PubMed] [Google Scholar]
- Collins S. J. The HL-60 promyelocytic leukemia cell line: proliferation, differentiation, and cellular oncogene expression. Blood. 1987 Nov;70(5):1233–1244. [PubMed] [Google Scholar]
- Drummond J. C., Finar I. L. Muconic acid as a metabolic product of benzene. Biochem J. 1938 Jan;32(1):79–84. doi: 10.1042/bj0320079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Hedli C. C., Rao N. R., Reuhl K. R., Witmer C. M., Snyder R. Effects of benzene metabolite treatment on granulocytic differentiation and DNA adduct formation in HL-60 cells. Arch Toxicol. 1996;70(3-4):135–144. doi: 10.1007/s002040050252. [DOI] [PubMed] [Google Scholar]
- Irons R. D., Dent J. G., Baker T. S., Rickert D. E. Benzene is metabolized and covalently bound in bone marrow in situ. Chem Biol Interact. 1980 May;30(2):241–245. doi: 10.1016/0009-2797(80)90130-1. [DOI] [PubMed] [Google Scholar]
- Irons R. D., Neptun D. A. Effects of the principal hydroxy-metabolites of benzene on microtubule polymerization. Arch Toxicol. 1980 Oct;45(4):297–305. doi: 10.1007/BF00293810. [DOI] [PubMed] [Google Scholar]
- Jowa L., Witz G., Snyder R., Winkle S., Kalf G. F. Synthesis and characterization of deoxyguanosine-benzoquinone adducts. J Appl Toxicol. 1990 Feb;10(1):47–54. doi: 10.1002/jat.2550100109. [DOI] [PubMed] [Google Scholar]
- Kline S. A., Robertson J. F., Grotz V. L., Goldstein B. D., Witz G. Identification of 6-hydroxy-trans,trans-2,4-hexadienoic acid, a novel ring-opened urinary metabolite of benzene. Environ Health Perspect. 1993 Sep;101(4):310–312. doi: 10.1289/ehp.93101310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. W., Kocsis J. J., Snyder R. The use of ferrokinetics in the study of experimental anemia. Environ Health Perspect. 1981 Jun;39:29–37. doi: 10.1289/ehp.813929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levay G., Pongracz K., Bodell W. J. Detection of DNA adducts in HL-60 cells treated with hydroquinone and p-benzoquinone by 32P-postlabeling. Carcinogenesis. 1991 Jul;12(7):1181–1186. doi: 10.1093/carcin/12.7.1181. [DOI] [PubMed] [Google Scholar]
- Longacre S. L., Kocsis J. J., Snyder R. Influence of strain differences in mice on the metabolism and toxicity of benzene. Toxicol Appl Pharmacol. 1981 Sep 30;60(3):398–409. doi: 10.1016/0041-008x(81)90324-0. [DOI] [PubMed] [Google Scholar]
- Lutz W. K., Schlatter C. Mechanism of the carcinogenic action of benzene: irreversible binding to rat liver DNA. Chem Biol Interact. 1977 Aug;18(2):241–245. doi: 10.1016/0009-2797(77)90010-2. [DOI] [PubMed] [Google Scholar]
- Lévay G., Bodell W. J. Potentiation of DNA adduct formation in HL-60 cells by combinations of benzene metabolites. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7105–7109. doi: 10.1073/pnas.89.15.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévay G., Ross D., Bodell W. J. Peroxidase activation of hydroquinone results in the formation of DNA adducts in HL-60 cells, mouse bone marrow macrophages and human bone marrow. Carcinogenesis. 1993 Nov;14(11):2329–2334. doi: 10.1093/carcin/14.11.2329. [DOI] [PubMed] [Google Scholar]
- Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974 Sep 16;47(3):469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Mazzullo M., Bartoli S., Bonora B., Colacci A., Grilli S., Lattanzi G., Niero A., Turina M. P., Parodi S. Benzene adducts with rat nucleic acids and proteins: dose-response relationship after treatment in vivo. Environ Health Perspect. 1989 Jul;82:259–266. doi: 10.1289/ehp.8982259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerland D. E., Pierce W. M., Jr Identification of N-acetyl-S-(2,5-dihydroxyphenyl)-L-cysteine as a urinary metabolite of benzene, phenol, and hydroquinone. Drug Metab Dispos. 1990 Nov-Dec;18(6):958–961. [PubMed] [Google Scholar]
- Norpoth K., Stücker W., Krewet E., Müller G. Biomonitoring of benzene exposure by trace analyses of phenylguanine. Int Arch Occup Environ Health. 1988;60(3):163–168. doi: 10.1007/BF00378692. [DOI] [PubMed] [Google Scholar]
- Orzechowski A., Schwarz L. R., Schwegler U., Bock K. W., Snyder R., Schrenk D. Benzene metabolism in rodent hepatocytes: role of sulphate conjugation. Xenobiotica. 1995 Nov;25(10):1093–1102. doi: 10.3109/00498259509061909. [DOI] [PubMed] [Google Scholar]
- PARKE D. V., WILLIAMS R. T. Studies in detoxication. XLIX. The metabolism of benzene containing (14C1) benzene. Biochem J. 1953 May;54(2):231–238. doi: 10.1042/bj0540231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer R. W., Irons R. D. Alteration of lymphocyte function by quinones through a sulfhydryl-dependent disruption of microtubule assembly. Int J Immunopharmacol. 1983;5(5):463–470. doi: 10.1016/0192-0561(83)90023-1. [DOI] [PubMed] [Google Scholar]
- Pongracz K., Bodell W. J. Detection of 3'-hydroxy-1,N6-benzetheno-2'-deoxyadenosine 3'-phosphate by 32P postlabeling of DNA reacted with p-benzoquinone. Chem Res Toxicol. 1991 Mar-Apr;4(2):199–202. doi: 10.1021/tx00020a012. [DOI] [PubMed] [Google Scholar]
- Pongracz K., Kaur S., Burlingame A. L., Bodell W. J. Detection of (3'-hydroxy)-3,N4-benzetheno-2'-deoxycytidine-3'-phosphate by 32P-postlabeling of DNA reacted with p-benzoquinone. Carcinogenesis. 1990 Sep;11(9):1469–1472. doi: 10.1093/carcin/11.9.1469. [DOI] [PubMed] [Google Scholar]
- Post G., Snyder R., Kalf G. F. Metabolism of benzene and phenol in macrophages in vitro and the inhibition of RNA synthesis by benzene metabolites. Cell Biol Toxicol. 1986 Jun;2(2):231–246. doi: 10.1007/BF00122692. [DOI] [PubMed] [Google Scholar]
- Rao N. R., Snyder R. Oxidative modifications produced in HL-60 cells on exposure to benzene metabolites. J Appl Toxicol. 1995 Sep-Oct;15(5):403–409. doi: 10.1002/jat.2550150511. [DOI] [PubMed] [Google Scholar]
- Reid T. M., Loeb L. A. Mutagenic specificity of oxygen radicals produced by human leukemia cells. Cancer Res. 1992 Mar 1;52(5):1082–1086. [PubMed] [Google Scholar]
- Rushmore T., Snyder R., Kalf G. Covalent binding of benzene and its metabolites to DNA in rabbit bone marrow mitochondria in vitro. Chem Biol Interact. 1984 Apr;49(1-2):133–154. doi: 10.1016/0009-2797(84)90057-7. [DOI] [PubMed] [Google Scholar]
- Sabourin P. J., Bechtold W. E., Birnbaum L. S., Lucier G., Henderson R. F. Differences in the metabolism and disposition of inhaled [3H]benzene by F344/N rats and B6C3F1 mice. Toxicol Appl Pharmacol. 1988 Jun 15;94(1):128–140. doi: 10.1016/0041-008x(88)90343-2. [DOI] [PubMed] [Google Scholar]
- Sabourin P. J., Bechtold W. E., Henderson R. F. A high pressure liquid chromatographic method for the separation and quantitation of water-soluble radiolabeled benzene metabolites. Anal Biochem. 1988 May 1;170(2):316–327. doi: 10.1016/0003-2697(88)90637-9. [DOI] [PubMed] [Google Scholar]
- Sammett D., Lee E. W., Kocsis J. J., Snyder R. Partial hepatectomy reduces both metabolism and toxicity of benzene. J Toxicol Environ Health. 1979 Sep;5(5):785–792. doi: 10.1080/15287397909529789. [DOI] [PubMed] [Google Scholar]
- Schlosser M. J., Kalf G. F. Metabolic activation of hydroquinone by macrophage peroxidase. Chem Biol Interact. 1989;72(1-2):191–207. doi: 10.1016/0009-2797(89)90027-6. [DOI] [PubMed] [Google Scholar]
- Snyder R., Chepiga T., Yang C. S., Thomas H., Platt K., Oesch F. Benzene metabolism by reconstituted cytochromes P450 2B1 and 2E1 and its modulation by cytochrome b5, microsomal epoxide hydrolase, and glutathione transferases: evidence for an important role of microsomal epoxide hydrolase in the formation of hydroquinone. Toxicol Appl Pharmacol. 1993 Oct;122(2):172–181. doi: 10.1006/taap.1993.1185. [DOI] [PubMed] [Google Scholar]
- Snyder R., Kalf G. F. A perspective on benzene leukemogenesis. Crit Rev Toxicol. 1994;24(3):177–209. doi: 10.3109/10408449409021605. [DOI] [PubMed] [Google Scholar]
- Snyder R., Kocsis J. J. Current concepts of chronic benzene toxicity. CRC Crit Rev Toxicol. 1975 Jun;3(3):265–288. doi: 10.3109/10408447509079860. [DOI] [PubMed] [Google Scholar]
- Snyder R., Lee E. W., Kocsis J. J. Binding of labeled benzene metabolites to mouse liver and bone marrow. Res Commun Chem Pathol Pharmacol. 1978 Apr;20(1):191–194. [PubMed] [Google Scholar]
- Snyder R., Witz G., Goldstein B. D. The toxicology of benzene. Environ Health Perspect. 1993 Apr;100:293–306. doi: 10.1289/ehp.93100293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subrahmanyam V. V., Doane-Setzer P., Steinmetz K. L., Ross D., Smith M. T. Phenol-induced stimulation of hydroquinone bioactivation in mouse bone marrow in vivo: possible implications in benzene myelotoxicity. Toxicology. 1990 May 14;62(1):107–116. doi: 10.1016/0300-483x(90)90035-f. [DOI] [PubMed] [Google Scholar]
- Subrahmanyam V. V., Kolachana P., Smith M. T. Hydroxylation of phenol to hydroquinone catalyzed by a human myeloperoxidase-superoxide complex: possible implications in benzene-induced myelotoxicity. Free Radic Res Commun. 1991;15(5):285–296. doi: 10.3109/10715769109105224. [DOI] [PubMed] [Google Scholar]
- Witz G., Zhang Z., Goldstein B. D. Reactive ring-opened aldehyde metabolites in benzene hematotoxicity. Environ Health Perspect. 1996 Dec;104 (Suppl 6):1195–1199. doi: 10.1289/ehp.961041195. [DOI] [PMC free article] [PubMed] [Google Scholar]



