Abstract
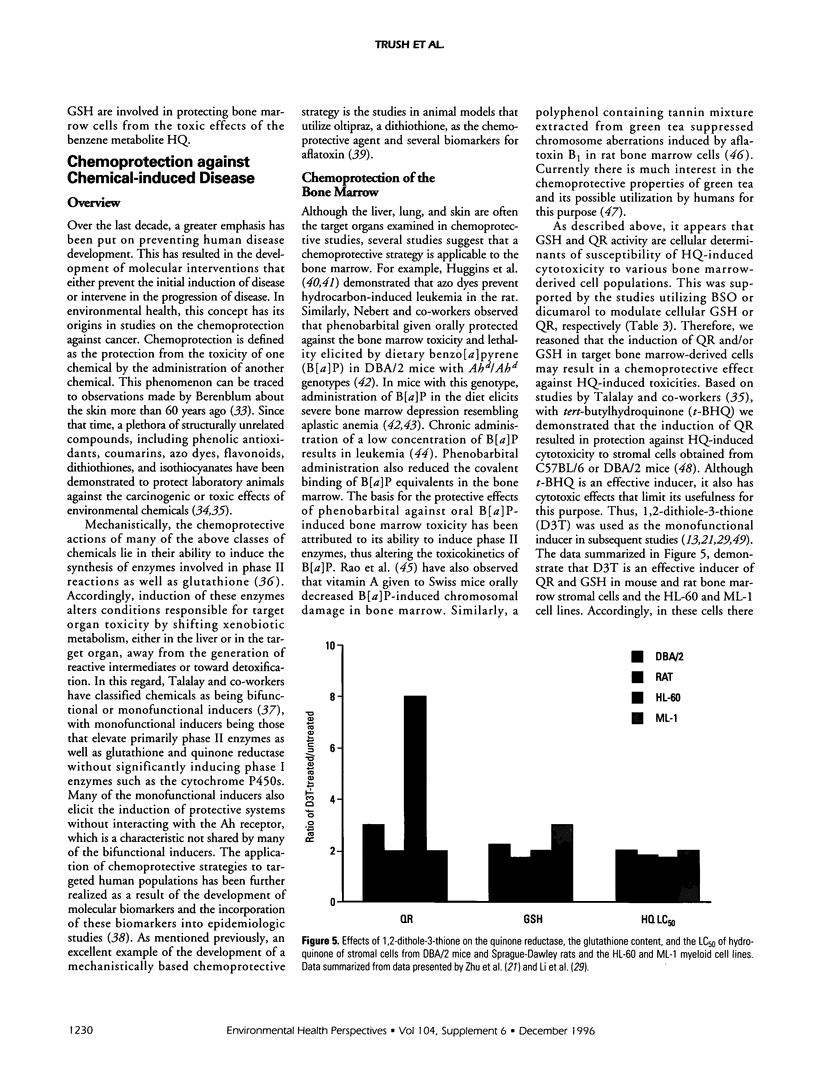
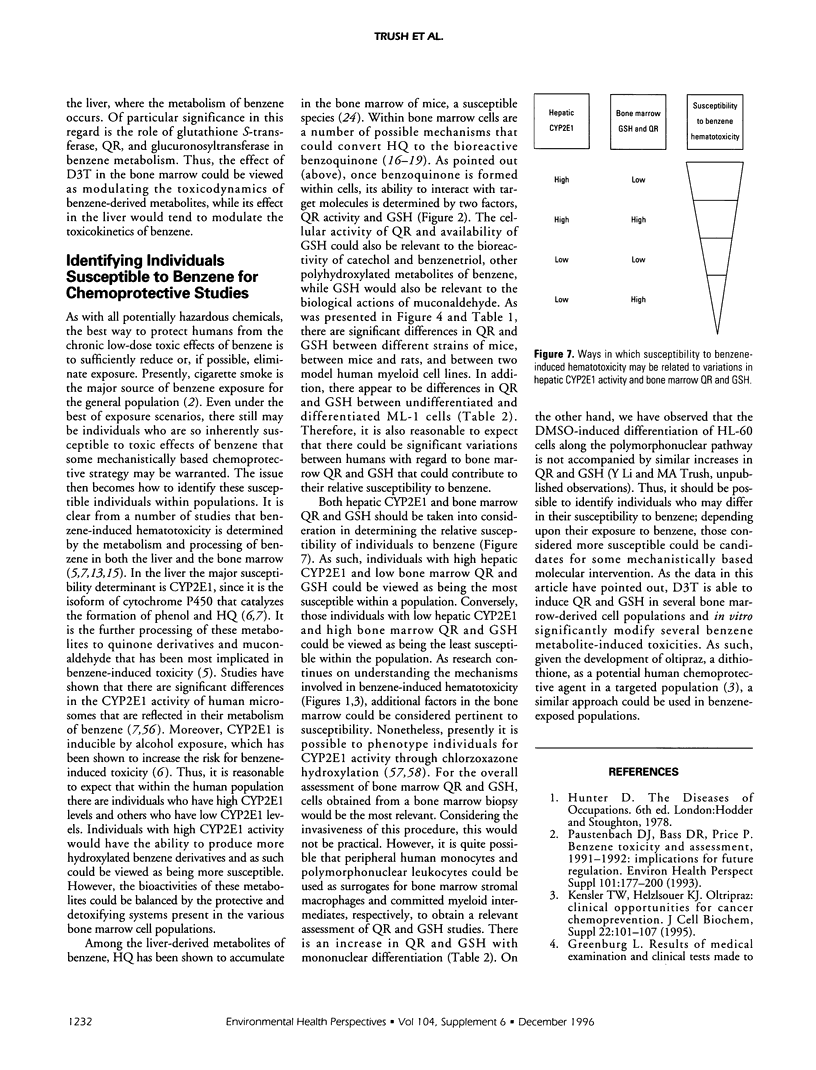
A goal of our research is to identify biochemical factors that underlie the susceptibility of bone marrow cell populations to benzene metabolites so as to develop a mechanistically based chemoprotective strategy that may be used in susceptible humans exposed to benzene. By doing biochemical risk analysis of bone marrow stromal cells from mice and rats and the human myeloid cell lines, HL-60 and ML-1; and by using buthionine sulfoximine and dicumarol we have observed that the susceptibility of these cell populations to hydroquinone (HQ) correlates with their concentration of glutathione (GSH) and activity of quinone reductase (QR). Accordingly, the induction of QR and GSH by 1,2-dithiole-3-thione (D3T) in these cell populations has resulted in a significant protection against the following hydroquinone-mediated toxicities: inhibition of cell proliferation and viability; reduced ability of stromal cells to support myelopoiesis; and altered differentiated of ML-1 cells to monocytes/macrophages. Preliminary in vivo experiments indicate that feeding mice D3T results in an induction of QR in the bone marrow compartment such that stromal cells are more resistant to hydroquinone-induced cytotoxicity in vitro. Overall, these studies suggest that in addition to hepatic cytochrome P4502E1, bone marrow QR and GSH are factors that could determine an individual's relative susceptibility to the toxic effects of benzene.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chuang L. F., Hinton D. E., Cheung A. T., Chuang R. Y. Induction of differentiation in human myeloblastic leukemia ML-1 cells by heptachlor, a chlorinated hydrocarbon insecticide. Toxicol Appl Pharmacol. 1991 Jun 1;109(1):98–107. doi: 10.1016/0041-008x(91)90194-j. [DOI] [PubMed] [Google Scholar]
- De Long M. J., Prochaska H. J., Talalay P. Induction of NAD(P)H:quinone reductase in murine hepatoma cells by phenolic antioxidants, azo dyes, and other chemoprotectors: a model system for the study of anticarcinogens. Proc Natl Acad Sci U S A. 1986 Feb;83(3):787–791. doi: 10.1073/pnas.83.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groopman J. D., DeMatos P., Egner P. A., Love-Hunt A., Kensler T. W. Molecular dosimetry of urinary aflatoxin-N7-guanine and serum aflatoxin-albumin adducts predicts chemoprotection by 1,2-dithiole-3-thione in rats. Carcinogenesis. 1992 Jan;13(1):101–106. doi: 10.1093/carcin/13.1.101. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P., Kim D. H., Iwasaki M. Role of human cytochrome P-450 IIE1 in the oxidation of many low molecular weight cancer suspects. Chem Res Toxicol. 1991 Mar-Apr;4(2):168–179. doi: 10.1021/tx00020a008. [DOI] [PubMed] [Google Scholar]
- Huggins C. B., Ueda N., Russo A. Azo dyes prevent hydrocarbon-induced leukemia in the rat. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4524–4527. doi: 10.1073/pnas.75.9.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Ohnishi S., Fujie K. Chromosome aberrations induced by aflatoxin B1 in rat bone marrow cells in vivo and their suppression by green tea. Mutat Res. 1989 Mar;222(3):253–261. doi: 10.1016/0165-1218(89)90141-9. [DOI] [PubMed] [Google Scholar]
- Johansson I., Ingelman-Sundberg M. Benzene metabolism by ethanol-, acetone-, and benzene-inducible cytochrome P-450 (IIE1) in rat and rabbit liver microsomes. Cancer Res. 1988 Oct 1;48(19):5387–5390. [PubMed] [Google Scholar]
- Jones T. D., Morris M. D., Young R. W., Kehlet R. A. A cell-kinetics model for radiation-induced myelopoiesis. Exp Hematol. 1993 Jun;21(6):816–822. [PubMed] [Google Scholar]
- Kensler T. W., Helzlsouer K. J. Oltipraz: clinical opportunities for cancer chemoprevention. p. J Cell Biochem Suppl. 1995;22:101–107. doi: 10.1002/jcb.240590813. [DOI] [PubMed] [Google Scholar]
- King A. G., Landreth K. S., Wierda D. Hydroquinone inhibits bone marrow pre-B cell maturation in vitro. Mol Pharmacol. 1987 Dec;32(6):807–812. [PubMed] [Google Scholar]
- Levay G., Pongracz K., Bodell W. J. Detection of DNA adducts in HL-60 cells treated with hydroquinone and p-benzoquinone by 32P-postlabeling. Carcinogenesis. 1991 Jul;12(7):1181–1186. doi: 10.1093/carcin/12.7.1181. [DOI] [PubMed] [Google Scholar]
- Li Y., Lafuente A., Trush M. A. Characterization of quinone reductase, glutathione and glutathione S-transferase in human myeloid cell lines: induction by 1,2-dithiole-3-thione and effects on hydroquinone-induced cytotoxicity. Life Sci. 1994;54(13):901–916. doi: 10.1016/0024-3205(94)00626-1. [DOI] [PubMed] [Google Scholar]
- Li Y., Trush M. A. Oxidation of hydroquinone by copper: chemical mechanism and biological effects. Arch Biochem Biophys. 1993 Jan;300(1):346–355. doi: 10.1006/abbi.1993.1047. [DOI] [PubMed] [Google Scholar]
- Longacre S. L., Kocsis J. J., Snyder R. Influence of strain differences in mice on the metabolism and toxicity of benzene. Toxicol Appl Pharmacol. 1981 Sep 30;60(3):398–409. doi: 10.1016/0041-008x(81)90324-0. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Jensen N. M., Levitt R. C., Felton J. S. Toxic chemical depression of the bone marrow and possible aplastic anemia explainable on a genetic basis. Clin Toxicol. 1980 Mar;16(1):99–122. doi: 10.3109/15563658008989927. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Levitt R. C., Jensen N. M., Lambert G. H., Felton J. S. Birth defects and aplastic anemia: differences in polycyclic hydrocarbon toxicity associated with the Ah locus. Arch Toxicol. 1977 Dec 30;39(1-2):109–132. doi: 10.1007/BF00343280. [DOI] [PubMed] [Google Scholar]
- Oliveira N. L., Kalf G. F. Induced differentiation of HL-60 promyelocytic leukemia cells to monocyte/macrophages is inhibited by hydroquinone, a hematotoxic metabolite of benzene. Blood. 1992 Feb 1;79(3):627–633. [PubMed] [Google Scholar]
- Paustenbach D. J., Bass R. D., Price P. Benzene toxicity and risk assessment, 1972-1992: implications for future regulation. Environ Health Perspect. 1993 Dec;101 (Suppl 6):177–200. doi: 10.1289/ehp.93101s6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter R., Böcker R., Beaune P. H., Iwasaki M., Guengerich F. P., Yang C. S. Hydroxylation of chlorzoxazone as a specific probe for human liver cytochrome P-450IIE1. Chem Res Toxicol. 1990 Nov-Dec;3(6):566–573. doi: 10.1021/tx00018a012. [DOI] [PubMed] [Google Scholar]
- Prochaska H. J., Talalay P. Regulatory mechanisms of monofunctional and bifunctional anticarcinogenic enzyme inducers in murine liver. Cancer Res. 1988 Sep 1;48(17):4776–4782. [PubMed] [Google Scholar]
- Qian G. S., Ross R. K., Yu M. C., Yuan J. M., Gao Y. T., Henderson B. E., Wogan G. N., Groopman J. D. A follow-up study of urinary markers of aflatoxin exposure and liver cancer risk in Shanghai, People's Republic of China. Cancer Epidemiol Biomarkers Prev. 1994 Jan-Feb;3(1):3–10. [PubMed] [Google Scholar]
- Rao K. P., Nandan B. D. Modification of benzo(a)pyrene induced chromosomal damage in mouse bone marrow by vitamin A. Bull Environ Contam Toxicol. 1990 Dec;45(6):829–832. doi: 10.1007/BF01701079. [DOI] [PubMed] [Google Scholar]
- Rembish S. J., Trush M. A. Further evidence that lucigenin-derived chemiluminescence monitors mitochondrial superoxide generation in rat alveolar macrophages. Free Radic Biol Med. 1994 Aug;17(2):117–126. doi: 10.1016/0891-5849(94)90109-0. [DOI] [PubMed] [Google Scholar]
- Renz J. F., Kalf G. F. Role for interleukin-1 (IL-1) in benzene-induced hematotoxicity: inhibition of conversion of pre-IL-1 alpha to mature cytokine in murine macrophages by hydroquinone and prevention of benzene-induced hematotoxicity in mice by IL-1 alpha. Blood. 1991 Aug 15;78(4):938–944. [PubMed] [Google Scholar]
- Sabourin P. J., Bechtold W. E., Birnbaum L. S., Lucier G., Henderson R. F. Differences in the metabolism and disposition of inhaled [3H]benzene by F344/N rats and B6C3F1 mice. Toxicol Appl Pharmacol. 1988 Jun 15;94(1):128–140. doi: 10.1016/0041-008x(88)90343-2. [DOI] [PubMed] [Google Scholar]
- Schlosser M. J., Shurina R. D., Kalf G. F. Metabolism of phenol and hydroquinone to reactive products by macrophage peroxidase or purified prostaglandin H synthase. Environ Health Perspect. 1989 Jul;82:229–237. doi: 10.1289/ehp.8982229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaton M. J., Schlosser P. M., Bond J. A., Medinsky M. A. Benzene metabolism by human liver microsomes in relation to cytochrome P450 2E1 activity. Carcinogenesis. 1994 Sep;15(9):1799–1806. doi: 10.1093/carcin/15.9.1799. [DOI] [PubMed] [Google Scholar]
- Seaton M. J., Schlosser P., Medinsky M. A. In vitro conjugation of benzene metabolites by human liver: potential influence of interindividual variability on benzene toxicity. Carcinogenesis. 1995 Jul;16(7):1519–1527. doi: 10.1093/carcin/16.7.1519. [DOI] [PubMed] [Google Scholar]
- Shertzer H. G., Vasiliou V., Liu R. M., Tabor M. W., Nebert D. W. Enzyme induction by L-buthionine (S,R)-sulfoximine in cultured mouse hepatoma cells. Chem Res Toxicol. 1995 Apr-May;8(3):431–436. doi: 10.1021/tx00045a015. [DOI] [PubMed] [Google Scholar]
- Thomas D. J., Reasor M. J., Wierda D. Macrophage regulation of myelopoiesis is altered by exposure to the benzene metabolite hydroquinone. Toxicol Appl Pharmacol. 1989 Mar 1;97(3):440–453. doi: 10.1016/0041-008x(89)90249-4. [DOI] [PubMed] [Google Scholar]
- Thomas D. J., Sadler A., Subrahmanyam V. V., Siegel D., Reasor M. J., Wierda D., Ross D. Bone marrow stromal cell bioactivation and detoxification of the benzene metabolite hydroquinone: comparison of macrophages and fibroblastoid cells. Mol Pharmacol. 1990 Feb;37(2):255–262. [PubMed] [Google Scholar]
- Twerdok L. E., Rembish S. J., Trush M. A. Induction of quinone reductase and glutathione in bone marrow cells by 1,2-dithiole-3-thione: effect on hydroquinone-induced cytotoxicity. Toxicol Appl Pharmacol. 1992 Feb;112(2):273–281. doi: 10.1016/0041-008x(92)90197-z. [DOI] [PubMed] [Google Scholar]
- Twerdok L. E., Rembish S. J., Trush M. A. Studies with 1,2-dithiole-3-thione as a chemoprotector of hydroquinone-induced toxicity to DBA/2-derived bone marrow stromal cells. Environ Health Perspect. 1993 Jun;101(2):172–177. doi: 10.1289/ehp.93101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twerdok L. E., Trush M. A. Differences in quinone reductase activity in primary bone marrow stromal cells derived from C57BL/6 and DBA/2 mice. Res Commun Chem Pathol Pharmacol. 1990 Mar;67(3):375–386. [PubMed] [Google Scholar]
- Yang C. S., Wang Z. Y. Tea and cancer. J Natl Cancer Inst. 1993 Jul 7;85(13):1038–1049. doi: 10.1093/jnci/85.13.1038. [DOI] [PubMed] [Google Scholar]
- Zhu H., Li Y., Trush M. A. Characterization of benzo[a]pyrene quinone-induced toxicity to primary cultured bone marrow stromal cells from DBA/2 mice: potential role of mitochondrial dysfunction. Toxicol Appl Pharmacol. 1995 Jan;130(1):108–120. doi: 10.1006/taap.1995.1015. [DOI] [PubMed] [Google Scholar]
- Zhu H., Li Y., Trush M. A. Differences in xenobiotic detoxifying activities between bone marrow stromal cells from mice and rats: implications for benzene-induced hematotoxicity. J Toxicol Environ Health. 1995 Oct;46(2):183–201. doi: 10.1080/15287399509532028. [DOI] [PubMed] [Google Scholar]


