Abstract
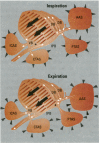
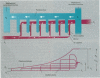
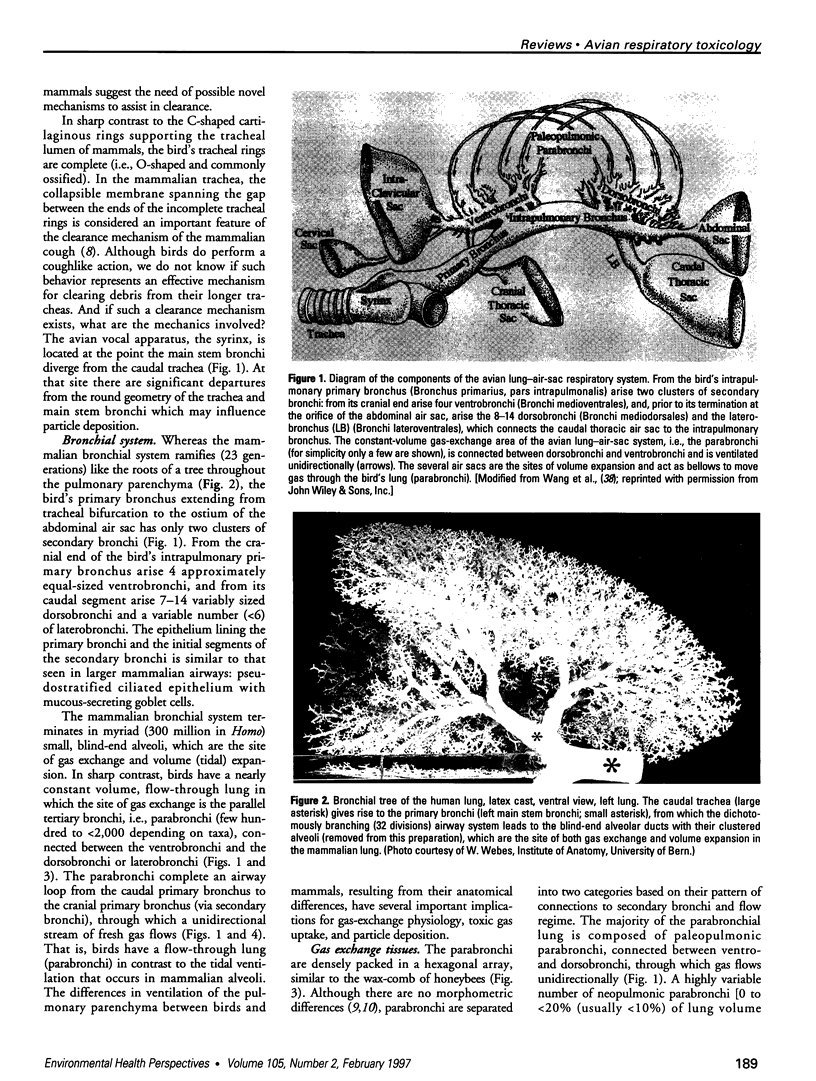
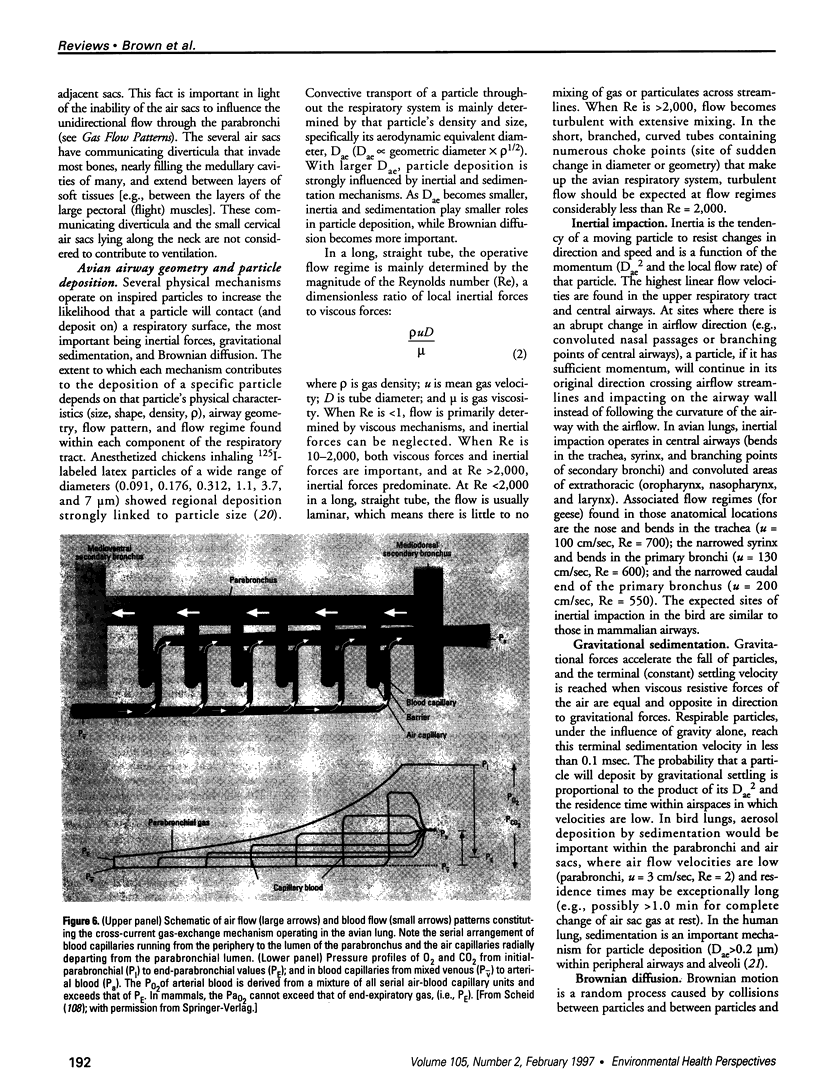
There are many distinct differences (morphologic, physiologic, and mechanical) between the bird's lung-air-sac respiratory system and the mammalian bronchoalveolar lung. In this paper, we review the physiology of the avian respiratory system with attention to those mechanisms that may lead to significantly different results, relative to those in mammals, following exposure to toxic gases and airborne particulates. We suggest that these differences can be productively exploited to further our understanding of the basic mechanisms of inhalant toxicology (gases and particulates). The large mass-specific gas uptake by the avian respiratory system, at rest and especially during exercise, could be exploited as a sensitive monitor of air quality. Birds have much to offer in our understanding of respiratory toxicology, but that expectation can only be realized by investigating, in a wide variety of avian taxa, the pathophysiologic interactions of a broad range of inhaled toxicants on the bird's unique respiratory system.
Full text
PDF


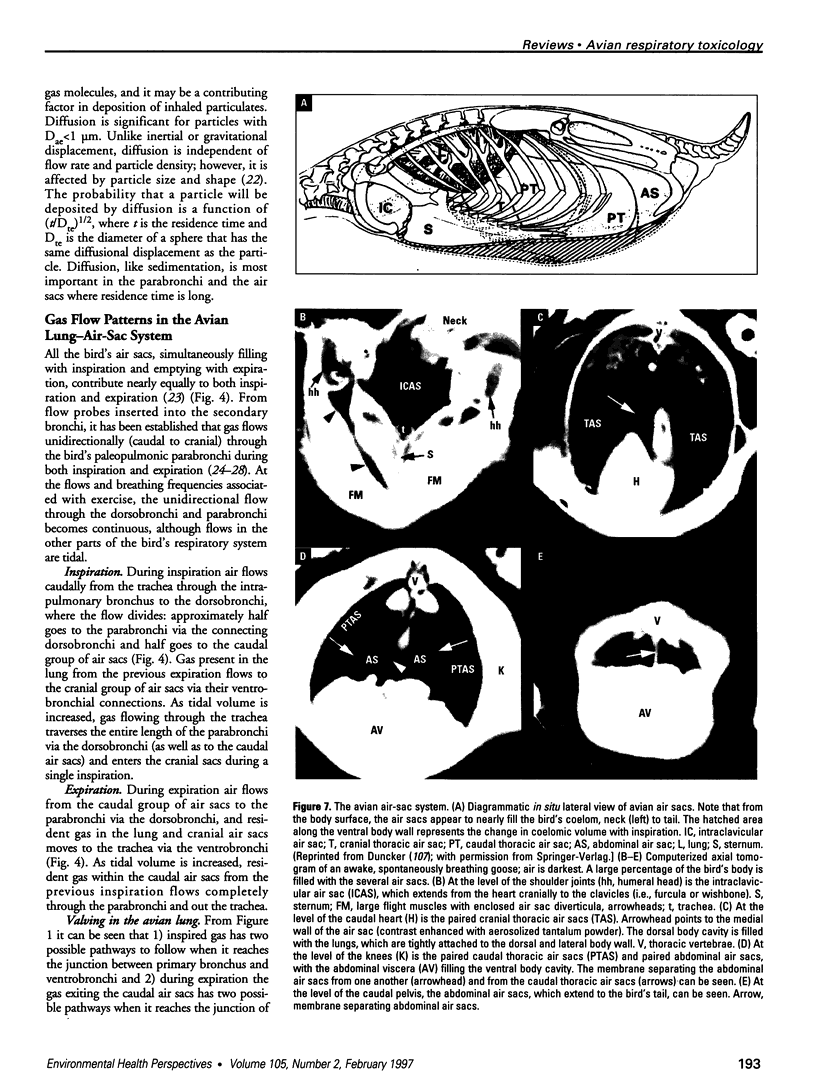







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. P., Beard C. W., Hanson R. P. Influence of poultry house dust, ammonia, and carbon dioxide on the resistance of chickens to Newcastle disease virus. Avian Dis. 1966 May;10(2):177–188. [PubMed] [Google Scholar]
- Anderson D. P., Wolfe R. R., Cherms F. L., Roper W. E. Influence of dust and ammonia on the development of air sac lesions in turkeys. Am J Vet Res. 1968 May;29(5):1049–1058. [PubMed] [Google Scholar]
- Aschoff J., Pohl H. Rhythmic variations in energy metabolism. Fed Proc. 1970 Jul-Aug;29(4):1541–1552. [PubMed] [Google Scholar]
- Banzett R. B., Butler J. P., Nations C. S., Barnas G. M., Lehr J. L., Jones J. H. Inspiratory aerodynamic valving in goose lungs depends on gas density and velocity. Respir Physiol. 1987 Dec;70(3):287–300. doi: 10.1016/0034-5687(87)90011-9. [DOI] [PubMed] [Google Scholar]
- Banzett R. B., Lehr J. L. Gas exchange during high-frequency ventilation of the chicken. J Appl Physiol Respir Environ Exerc Physiol. 1982 Dec;53(6):1418–1422. doi: 10.1152/jappl.1982.53.6.1418. [DOI] [PubMed] [Google Scholar]
- Barnas G. M., Gleeson M., Rautenberg W. Effect of bilateral vagotomy on arterial acid-base stability during panting in the pigeon. Respir Physiol. 1986 Dec;66(3):293–302. doi: 10.1016/0034-5687(86)90081-2. [DOI] [PubMed] [Google Scholar]
- Bartov I., Budowski P., Dror Y., Sandbank U., Bubis J. J. Effect of ozone exposure on growth, nutritional encephalopathy, and fatty acid composition of cerebellum and lungs in the young chick. Poult Sci. 1981 Mar;60(3):532–540. doi: 10.3382/ps.0600532. [DOI] [PubMed] [Google Scholar]
- Bland M. C., Nakaue H. S., Goeger M. P., Helfer D. H. Duration of exposure--histological effects on broiler lungs, performance, and house environment with Mt. St. Helens' volcanic ash dust. Poult Sci. 1985 Jan;64(1):51–58. doi: 10.3382/ps.0640051. [DOI] [PubMed] [Google Scholar]
- Bouverot P., Dejours P. Pathway of respired gas in the air sacs-lung apparatus of fowl and ducks. Respir Physiol. 1971 Dec;13(3):330–342. doi: 10.1016/0034-5687(71)90037-5. [DOI] [PubMed] [Google Scholar]
- Brambilla C., Abraham J., Brambilla E., Benirschke K., Bloor C. Comparative pathology of silicate pneumoconiosis. Am J Pathol. 1979 Jul;96(1):149–170. [PMC free article] [PubMed] [Google Scholar]
- Bretz W. L., Schmidt-Nielsen K. Bird respiration: flow patterns in the duck lung. J Exp Biol. 1971 Feb;54(1):103–118. doi: 10.1242/jeb.54.1.103. [DOI] [PubMed] [Google Scholar]
- Brown R, Kovacs C, Butler J, Wang N, Lehr J, Banzett R. The avian lung: is there an aerodynamic expiratory valve? J Exp Biol. 1995;198(Pt 11):2349–2357. doi: 10.1242/jeb.198.11.2349. [DOI] [PubMed] [Google Scholar]
- Buckpitt A. R., Boyd M. R. Metabolic activation of 4-ipomeanol by avian tissue microsomes. Toxicol Appl Pharmacol. 1982 Aug;65(1):53–62. doi: 10.1016/0041-008x(82)90361-1. [DOI] [PubMed] [Google Scholar]
- Buckpitt A. R., Statham C. N., Boyd M. R. In vivo studies on the target tissue metabolism, covalent binding, glutathione depletion, and toxicity of 4-ipomeanol in birds, species deficient in pulmonary enzymes for metabolic activation. Toxicol Appl Pharmacol. 1982 Aug;65(1):38–52. doi: 10.1016/0041-008x(82)90360-x. [DOI] [PubMed] [Google Scholar]
- Bunyan P. J., Stanley P. I. Toxic mechanisms in wildlife. Regul Toxicol Pharmacol. 1982 Jun;2(2):106–145. doi: 10.1016/0273-2300(82)90023-x. [DOI] [PubMed] [Google Scholar]
- Butler J. P., Banzett R. B., Fredberg J. J. Inspiratory valving in avian bronchi: aerodynamic considerations. Respir Physiol. 1988 May;72(2):241–255. doi: 10.1016/0034-5687(88)90010-2. [DOI] [PubMed] [Google Scholar]
- Callanan D., Dixon M., Widdicombe J. G., Wise J. C. Responses of geese to inhalation of irritant gases and injections of phenyl diguanide. Respir Physiol. 1974 Oct;22(1-2):157–166. doi: 10.1016/0034-5687(74)90054-1. [DOI] [PubMed] [Google Scholar]
- Conceiço M. A., Johnson H. E., Wathes C. M. Air hygiene in a pullet house: spatial homogeneity of aerial pollutants. Br Poult Sci. 1989 Dec;30(4):765–776. doi: 10.1080/00071668908417202. [DOI] [PubMed] [Google Scholar]
- Evans C. L. The toxicity of hydrogen sulphide and other sulphides. Q J Exp Physiol Cogn Med Sci. 1967 Jul;52(3):231–248. doi: 10.1113/expphysiol.1967.sp001909. [DOI] [PubMed] [Google Scholar]
- Fagerland J. A., Arp L. H. A morphologic study of bronchus-associated lymphoid tissue in turkeys. Am J Anat. 1990 Sep;189(1):24–34. doi: 10.1002/aja.1001890104. [DOI] [PubMed] [Google Scholar]
- Fedde M. R., Kuhlmann W. D. Cardiopulmonary responses to inhaled sulfur dioxide in the chicken. Poult Sci. 1979 Nov;58(6):1584–1591. doi: 10.3382/ps.0581584. [DOI] [PubMed] [Google Scholar]
- Ficken M. D., Edwards J. F., Lay J. C. Induction, collection, and partial characterization of induced respiratory macrophages of the turkey. Avian Dis. 1986 Oct-Dec;30(4):766–771. [PubMed] [Google Scholar]
- Hastings R. H., Powell F. L. Physiological dead space and effective parabronchial ventilation in ducks. J Appl Physiol (1985) 1986 Jan;60(1):85–91. doi: 10.1152/jappl.1986.60.1.85. [DOI] [PubMed] [Google Scholar]
- Hastings R. H., Powell F. L. Single breath CO2 measurements of deadspace in ducks. Respir Physiol. 1986 Feb;63(2):139–149. doi: 10.1016/0034-5687(86)90109-x. [DOI] [PubMed] [Google Scholar]
- Hayter R. B., Besch E. L. Airborne-particle deposition in the respiratory tract of chickens. Poult Sci. 1974 Jul;53(4):1507–1511. doi: 10.3382/ps.0531507. [DOI] [PubMed] [Google Scholar]
- Jaeger J. J., McGrath J. J. Effects of hypothermia on heart and respiratory responses of chicks to carbon monoxide. J Appl Physiol. 1973 May;34(5):564–567. doi: 10.1152/jappl.1973.34.5.564. [DOI] [PubMed] [Google Scholar]
- KLEINFELD M., GIEL C., ROSSO A. ACUTE HYDROGEN SULFIDE INTOXICATION; AN UNUSUAL SOURCE OF EXPOSURE. Ind Med Surg. 1964 Sep;33:656–660. [PubMed] [Google Scholar]
- Klentz R. D., Fedde M. R. Hydrogen sulfide: effects on avian respiratory control and intrapulmonary CO2 receptors. Respir Physiol. 1978 Mar;32(3):355–367. doi: 10.1016/0034-5687(78)90123-8. [DOI] [PubMed] [Google Scholar]
- Lasiewski R. C., Calder W. A., Jr A preliminary allometric analysis of respiratory variables in resting birds. Respir Physiol. 1971 Jan;11(2):152–166. doi: 10.1016/0034-5687(71)90020-x. [DOI] [PubMed] [Google Scholar]
- Leith D. E. Comparative mammalian respiratory mechanics. Physiologist. 1976 Nov;19(4):485–510. [PubMed] [Google Scholar]
- Madelin T. M., Wathes C. M. Air hygiene in a broiler house: comparison of deep litter with raised netting floors. Br Poult Sci. 1989 Mar;30(1):23–37. doi: 10.1080/00071668908417122. [DOI] [PubMed] [Google Scholar]
- McGrath J. J., Jaeger J. Effect of iodoacetate on the carbon monoxide tolerance of the chick. Respir Physiol. 1971 Apr;12(1):71–76. doi: 10.1016/0034-5687(71)90102-2. [DOI] [PubMed] [Google Scholar]
- Meinhart H., Fenske G. Untersuchungen über den Einfluss von Schwefeldioxid auf Leistung und Gesundheit von Broilern. Arch Exp Veterinarmed. 1977;31(4):591–602. [PubMed] [Google Scholar]
- Mensah G. A., Brain J. D. Deposition and clearance of inhaled aerosol in the respiratory tract of chickens. J Appl Physiol Respir Environ Exerc Physiol. 1982 Dec;53(6):1423–1428. doi: 10.1152/jappl.1982.53.6.1423. [DOI] [PubMed] [Google Scholar]
- O'donoghue J. G. Hydrogen Sulphide in Swine. Can J Comp Med Vet Sci. 1961 Sep;25(9):217–219. [PMC free article] [PubMed] [Google Scholar]
- Piiper J., Dejours P., Haab P., Rahn H. Concepts and basic quantities in gas exchange physiology. Respir Physiol. 1971 Dec;13(3):292–304. doi: 10.1016/0034-5687(71)90034-x. [DOI] [PubMed] [Google Scholar]
- Piiper J., Pfeifer K., Scheid P. Carbon monoxide diffusing capacity of the respiratory system in the domestic fowl. Respir Physiol. 1969 Apr;6(3):309–317. doi: 10.1016/0034-5687(69)90030-9. [DOI] [PubMed] [Google Scholar]
- Powell F. L., Mazzone R. W. Morphometrics of rapidly frozen goose lungs. Respir Physiol. 1983 Mar;51(3):319–332. doi: 10.1016/0034-5687(83)90026-9. [DOI] [PubMed] [Google Scholar]
- QUILLIGAN J. J., Jr, BOCHE R. D., FALK H. L., KOTIN P. The toxicity of ozone for young chicks. AMA Arch Ind Health. 1958 Jul;18(1):16–22. [PubMed] [Google Scholar]
- Qureshi M. A., Petitte J. N., Laster S. M., Dietert R. R. Avian macrophages: contribution to cellular microenvironment and changes in effector functions following activation. Poult Sci. 1993 Jul;72(7):1280–1284. doi: 10.3382/ps.0721280. [DOI] [PubMed] [Google Scholar]
- Scheid P. Mechanisms of gas exchange in bird lungs. Rev Physiol Biochem Pharmacol. 1979;86:137–186. doi: 10.1007/BFb0031533. [DOI] [PubMed] [Google Scholar]
- Scheid P., Piiper J. Cross-current gas exchange in avian lungs: effects of reversed parabronchial air flow in ducks. Respir Physiol. 1972 Dec;16(3):304–312. doi: 10.1016/0034-5687(72)90060-6. [DOI] [PubMed] [Google Scholar]
- Scheid P., Piiper J. Direct measurement of the aathway of respired gas in duck lungs. Respir Physiol. 1971 Mar;11(3):308–314. doi: 10.1016/0034-5687(71)90004-1. [DOI] [PubMed] [Google Scholar]
- Scheid P., Slama H., Willmer H. Volume and ventilation of air sacs in ducks studied by inert gas wash-out. Respir Physiol. 1974 Jul;21(1):19–36. doi: 10.1016/0034-5687(74)90004-8. [DOI] [PubMed] [Google Scholar]
- Smith A. J. Changes in the average weight and shell thickness of eggs produced by hens exposed to high environmental temperatures--a review. Trop Anim Health Prod. 1974 Nov;6(4):237–244. doi: 10.1007/BF02383283. [DOI] [PubMed] [Google Scholar]
- Smith B. L., Poole W. S., Martinovich D. Pneumoconiosis in the captive New Zealand kiwi. Vet Pathol. 1973;10(2):94–101. doi: 10.1177/030098587301000202. [DOI] [PubMed] [Google Scholar]
- Stahl W. R. Scaling of respiratory variables in mammals. J Appl Physiol. 1967 Mar;22(3):453–460. doi: 10.1152/jappl.1967.22.3.453. [DOI] [PubMed] [Google Scholar]
- Stearns R. C., Barnas G. M., Walski M., Brain J. D. Deposition and phagocytosis of inhaled particles in the gas exchange region of the duck, Anas platyrhynchos. Respir Physiol. 1987 Jan;67(1):23–36. doi: 10.1016/0034-5687(87)90004-1. [DOI] [PubMed] [Google Scholar]
- Stoltz J. H., Galey F., Johnson B. Sudden death in ten psittacine birds associated with the operation of a self-cleaning oven. Vet Hum Toxicol. 1992 Oct;34(5):420–421. [PubMed] [Google Scholar]
- Tschorn R. R., Fedde M. R. Cardiopulmonary responses to carbon monoxide breathing in the chicken. Respir Physiol. 1974 Jun;20(3):303–311. doi: 10.1016/0034-5687(74)90027-9. [DOI] [PubMed] [Google Scholar]
- Tucker V. A. Respiration during flight in birds. Respir Physiol. 1972 Mar;14(1):75–82. doi: 10.1016/0034-5687(72)90018-7. [DOI] [PubMed] [Google Scholar]
- Visser G. H., Zeinstra E. C., van Gasteren F., Beintema A. J. Gas conductance and metabolism of shorebird eggs: variation within and between species. Respir Physiol. 1995 Feb;99(2):273–281. doi: 10.1016/0034-5687(94)00097-j. [DOI] [PubMed] [Google Scholar]
- Wang N., Banzett R. B., Butler J. P., Fredberg J. J. Bird lung models show that convective inertia effects inspiratory aerodynamic valving. Respir Physiol. 1988 Jul;73(1):111–124. doi: 10.1016/0034-5687(88)90131-4. [DOI] [PubMed] [Google Scholar]
- Wang N., Banzett R. B., Nations C. S., Jenkins F. A., Jr An aerodynamic valve in the avian primary bronchus. J Exp Zool. 1992 Jul 1;262(4):441–445. doi: 10.1002/jez.1402620411. [DOI] [PubMed] [Google Scholar]
- Wangensteen O. D., Rahn H. Respiratory gas exchange by the avian embryo. Respir Physiol. 1970;11(1):31–45. doi: 10.1016/0034-5687(70)90100-3. [DOI] [PubMed] [Google Scholar]
- Wells R. E. Fatal toxicosis in pet birds caused by an overheated cooking pan lined with polytetrafluoroethylene. J Am Vet Med Assoc. 1983 Jun 1;182(11):1248–1250. [PubMed] [Google Scholar]
- Wells R. E., Slocombe R. F. Acute toxicosis of budgerigars (Melopsittacus undulatus) caused by pyrolysis products from heated polytetrafluoroethylene: microscopic study. Am J Vet Res. 1982 Jul;43(7):1243–1248. [PubMed] [Google Scholar]