Abstract
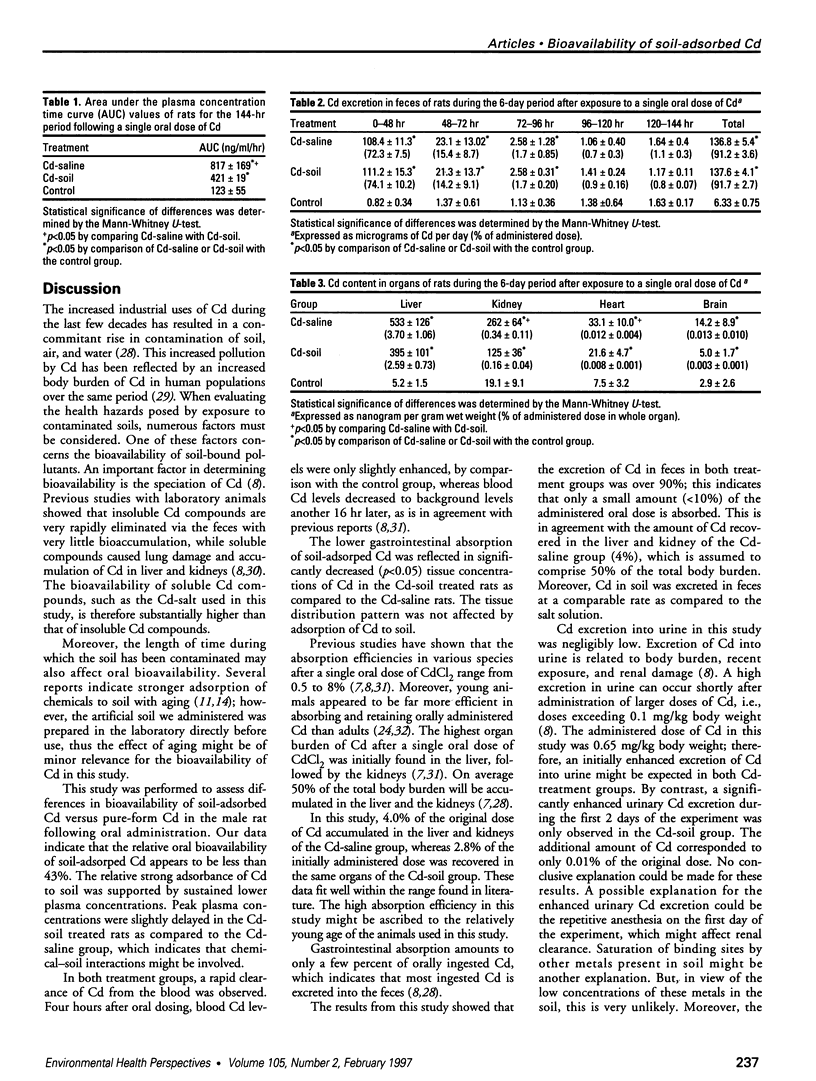
During the last few decades, the industrial production and use of Cd resulted in the release of significant quantities of Cd into the environment. Concern about health risks of human exposure to this toxic metal, which may be contained in soil and other environmental compartments, has increased significantly in recent years. Soil ingestion is a potentially important pathway of exposure to soil-absorbed environmental contaminants, especially for young children exhibiting hand-to-mouth behavior. Health risk assessments are usually based on unchanged bioavailability of soil-absorbed pollutants, e.g., heavy metals, neglecting interactions of metals with the soil matrix, which may lead to relatively lower bioavailability. This study was conducted to determine the bioavailability of Cd absorbed to soil in rats. Eight-week-old male Lewis rats were given either a soil polluted with CdCl2 (150 micrograms Cd/rat) dissolved in 5% gun acacia or an equal amount of Cd as CdCl2 dissolved in saline. Control rats were gavaged with isotonic saline. Cd concentrations in liver, kidney, brain, heart, and blood, as well as Cd content of urine and feces were analyzed using graphite furnace atomic absorption spectrometry. Tissue Cd concentrations in soil-treated animals were significantly lower than the tissue concentrations in the Cd-saline group; in the liver and kidneys of the Cd-saline and Cd-soil groups, 4 and 2.7% respectively, of the original doses were recovered. Relative bioavailability, calculated on the basis of blood Cd levels for the Cd-soil group as compared to the Cd-saline group, appeared to be 43%. No differences in the excretion pattern of Cd into feces were observed between the Cd-saline and Cd-soil groups. After 6 days, over 91% of the original dose was recovered in the feces of both Cd-treated groups. Cd excretion via urine was very low, but in the Cd-soil group a significant increase in urinary Cd was observed as compared to the control group. However, the amount of Cd excreted into urine of the Cd-soil group during the experimental period corresponded to only 0.01% of the original dose. In the Cd-saline group, no additional Cd was excreted into urine as compared to the control group. These results indicate that the soil matrix significantly reduced the absorption of Cd in the gastrointestinal tract. Consequently, exposure assessment models, assuming an unaffected bioavailability of soil-absorbed Cd, overestimate the internal dose and thereby overestimate health risks associated with direct ingestion of soil particles.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhattacharyya M. H., Whelton B. D., Peterson D. P. Gastrointestinal absorption of cadmium in mice during gestation and lactation. I. Short-term exposure studies. Toxicol Appl Pharmacol. 1981 Dec;61(3):335–342. doi: 10.1016/0041-008x(81)90354-9. [DOI] [PubMed] [Google Scholar]
- Cherian M. G., Goyer R. A., Valberg L. S. Gastrointestinal absorption and organ distribution of oral cadmium chloride and cadmium-metallothionein in mice. J Toxicol Environ Health. 1978 Sep-Nov;4(5-6):861–868. doi: 10.1080/15287397809529707. [DOI] [PubMed] [Google Scholar]
- Coogan T. P., Bare R. M., Waalkes M. P. Cadmium-induced DNA strand damage in cultured liver cells: reduction in cadmium genotoxicity following zinc pretreatment. Toxicol Appl Pharmacol. 1992 Apr;113(2):227–233. doi: 10.1016/0041-008x(92)90118-c. [DOI] [PubMed] [Google Scholar]
- Davis S., Waller P., Buschbom R., Ballou J., White P. Quantitative estimates of soil ingestion in normal children between the ages of 2 and 7 years: population-based estimates using aluminum, silicon, and titanium as soil tracer elements. Arch Environ Health. 1990 Mar-Apr;45(2):112–122. doi: 10.1080/00039896.1990.9935935. [DOI] [PubMed] [Google Scholar]
- Engström B., Nordberg G. F. Dose dependence of gastrointestinal absorption and biological half-time of cadmium in mice. Toxicology. 1979 Aug;13(3):215–222. doi: 10.1016/s0300-483x(79)80025-6. [DOI] [PubMed] [Google Scholar]
- Engström B., Nordberg G. F. Factors influencing absorption and retention of oral 109Cd in mice: age, pretreatment and subsequent treatment with non-radioactive cadmium. Acta Pharmacol Toxicol (Copenh) 1979 Oct;45(4):315–324. doi: 10.1111/j.1600-0773.1979.tb02399.x. [DOI] [PubMed] [Google Scholar]
- Freeman G. B., Johnson J. D., Killinger J. M., Liao S. C., Feder P. I., Davis A. O., Ruby M. V., Chaney R. L., Lovre S. C., Bergstrom P. D. Relative bioavailability of lead from mining waste soil in rats. Fundam Appl Toxicol. 1992 Oct;19(3):388–398. doi: 10.1016/0272-0590(92)90178-k. [DOI] [PubMed] [Google Scholar]
- Groen K., Vaessen H. A., Kliest J. J., de Boer J. L., van Ooik T., Timmerman A., Vlug R. F. Bioavailability of inorganic arsenic from bog ore-containing soil in the dog. Environ Health Perspect. 1994 Feb;102(2):182–184. doi: 10.1289/ehp.94102182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadry A. M., Skowronski G. A., Turkall R. M., Abdel-Rahman M. S. Comparison between oral and dermal bioavailability of soil-adsorbed phenanthrene in female rats. Toxicol Lett. 1995 Jul;78(2):153–163. doi: 10.1016/0378-4274(95)03250-o. [DOI] [PubMed] [Google Scholar]
- Kadry A. M., Turkall R. M., Skowronski G. A., Abdel-Rahman M. S. Soil adsorption alters kinetics and bioavailability of trichloroethylene in orally exposed female rats. Toxicol Lett. 1991 Nov;58(3):337–346. doi: 10.1016/0378-4274(91)90046-9. [DOI] [PubMed] [Google Scholar]
- Kimbrough R. D., Falk H., Stehr P., Fries G. Health implications of 2,3,7,8-tetrachlorodibenzodioxin (TCDD) contamination of residential soil. J Toxicol Environ Health. 1984;14(1):47–93. doi: 10.1080/15287398409530562. [DOI] [PubMed] [Google Scholar]
- Klimisch H. J. Lung deposition, lung clearance and renal accumulation of inhaled cadmium chloride and cadmium sulphide in rats. Toxicology. 1993 Nov 12;84(1-3):103–124. doi: 10.1016/0300-483x(93)90111-5. [DOI] [PubMed] [Google Scholar]
- LaGoy P. K. Estimated soil ingestion rates for use in risk assessment. Risk Anal. 1987 Sep;7(3):355–359. doi: 10.1111/j.1539-6924.1987.tb00471.x. [DOI] [PubMed] [Google Scholar]
- Lamphere D. N., Dorn C. R., Reddy C. S., Meyer A. W. Reduced cadmium body burden in cadmium-exposed calves fed supplemental zinc. Environ Res. 1984 Feb;33(1):119–129. doi: 10.1016/0013-9351(84)90013-6. [DOI] [PubMed] [Google Scholar]
- Neathery M. W., Miller W. J. Metabolism and toxicity of cadmium, mercury, and lead in animals: a review. J Dairy Sci. 1975 Dec;58(12):1767–1781. doi: 10.3168/jds.S0022-0302(75)84785-0. [DOI] [PubMed] [Google Scholar]
- Sheehan P. J., Meyer D. M., Sauer M. M., Paustenbach D. J. Assessment of the human health risks posed by exposure to chromium-contaminated soils. J Toxicol Environ Health. 1991 Feb;32(2):161–201. doi: 10.1080/15287399109531476. [DOI] [PubMed] [Google Scholar]
- Shore R. F., Douben P. E. The ecotoxicological significance of cadmium intake and residues in terrestrial small mammals. Ecotoxicol Environ Saf. 1994 Oct;29(1):101–112. doi: 10.1016/0147-6513(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Stanek E. J., 3rd, Calabrese E. J. Daily estimates of soil ingestion in children. Environ Health Perspect. 1995 Mar;103(3):276–285. doi: 10.1289/ehp.95103276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkall R. M., Skowronski G. A., Abdel-Rahman M. S. Differences in kinetics of pure and soil-adsorbed toluene in orally exposed male rats. Arch Environ Contam Toxicol. 1991 Feb;20(2):155–160. doi: 10.1007/BF01055899. [DOI] [PubMed] [Google Scholar]
- Turkall R. M., Skowronski G., Gerges S., Von Hagen S., Abdel-Rahman M. S. Soil adsorption alters kinetics and bioavailability of benzene in orally exposed male rats. Arch Environ Contam Toxicol. 1988 Mar;17(2):159–164. doi: 10.1007/BF01056020. [DOI] [PubMed] [Google Scholar]
- Waalkes M. P., Coogan T. P., Barter R. A. Toxicological principles of metal carcinogenesis with special emphasis on cadmium. Crit Rev Toxicol. 1992;22(3-4):175–201. doi: 10.3109/10408449209145323. [DOI] [PubMed] [Google Scholar]
- Waalkes M. P., Rehm S., Riggs C. W., Bare R. M., Devor D. E., Poirier L. A., Wenk M. L., Henneman J. R., Balaschak M. S. Cadmium carcinogenesis in male Wistar [Crl:(WI)BR] rats: dose-response analysis of tumor induction in the prostate and testes and at the injection site. Cancer Res. 1988 Aug 15;48(16):4656–4663. [PubMed] [Google Scholar]
- van Gestel C. A., van Dis W. A., van Breemen E. M., Sparenburg P. M. Development of a standardized reproduction toxicity test with the earthworm species Eisenia fetida andrei using copper, pentachlorophenol and 2,4-dichloroaniline. Ecotoxicol Environ Saf. 1989 Dec;18(3):305–312. doi: 10.1016/0147-6513(89)90024-9. [DOI] [PubMed] [Google Scholar]