Abstract
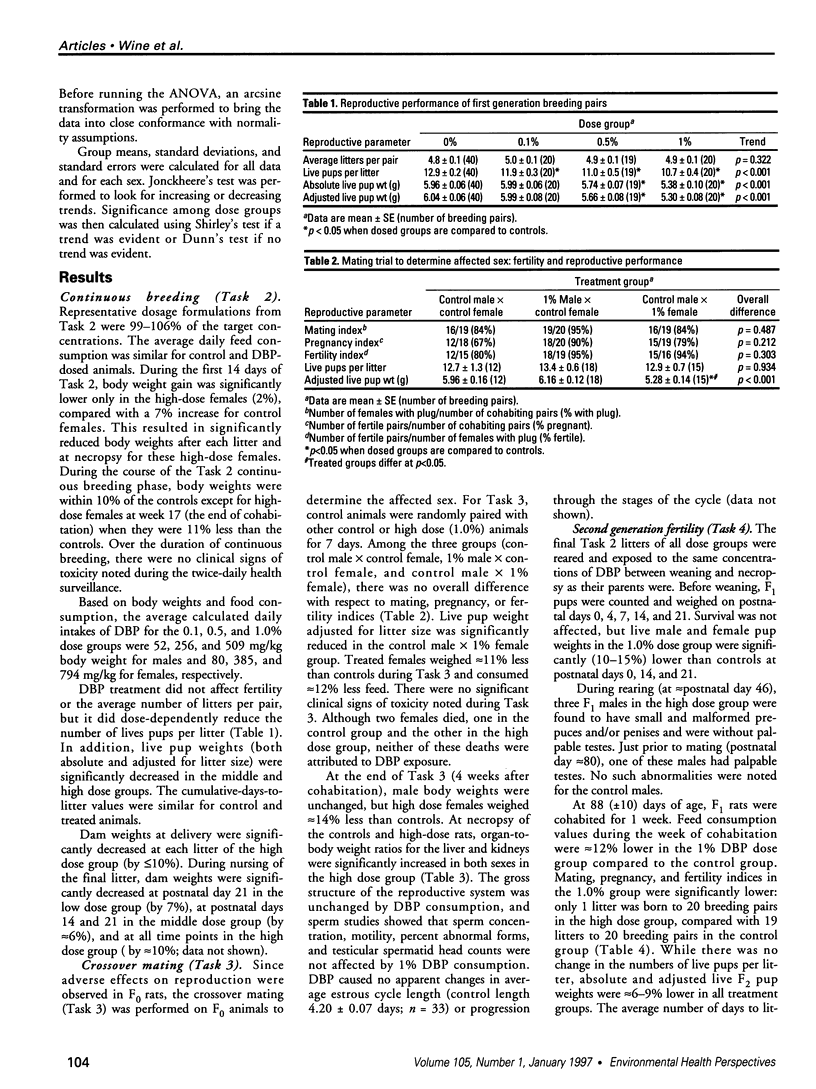
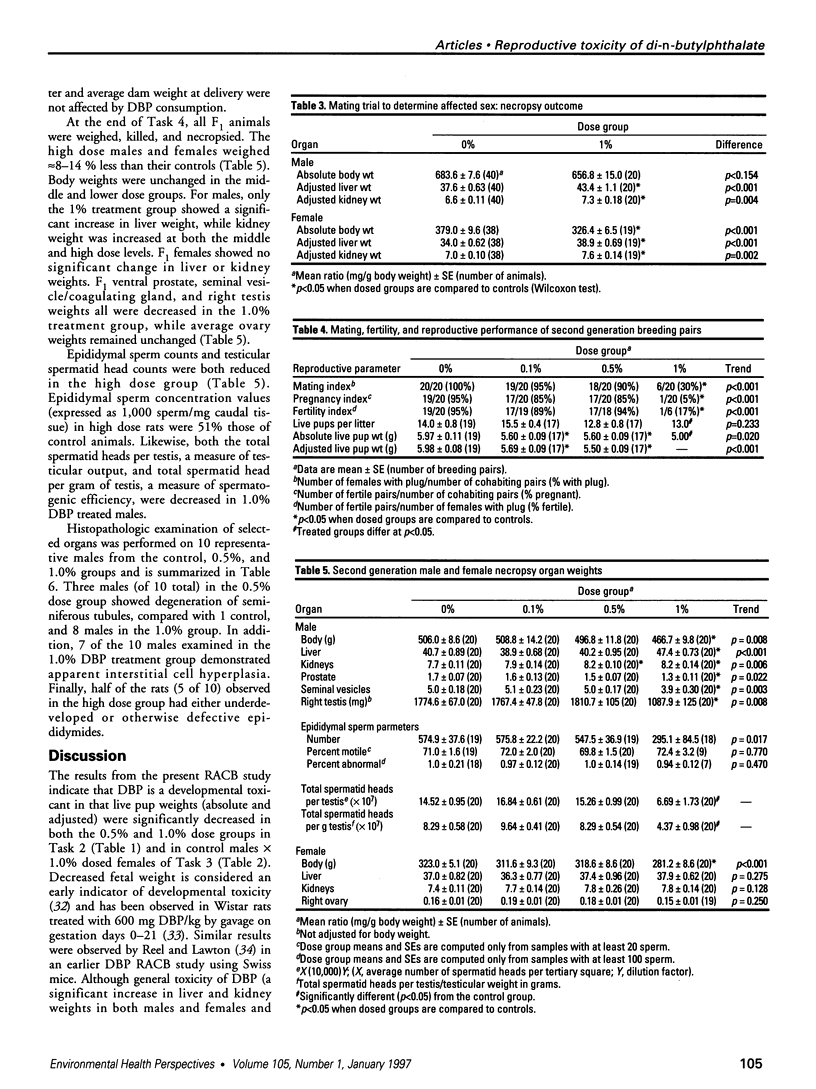
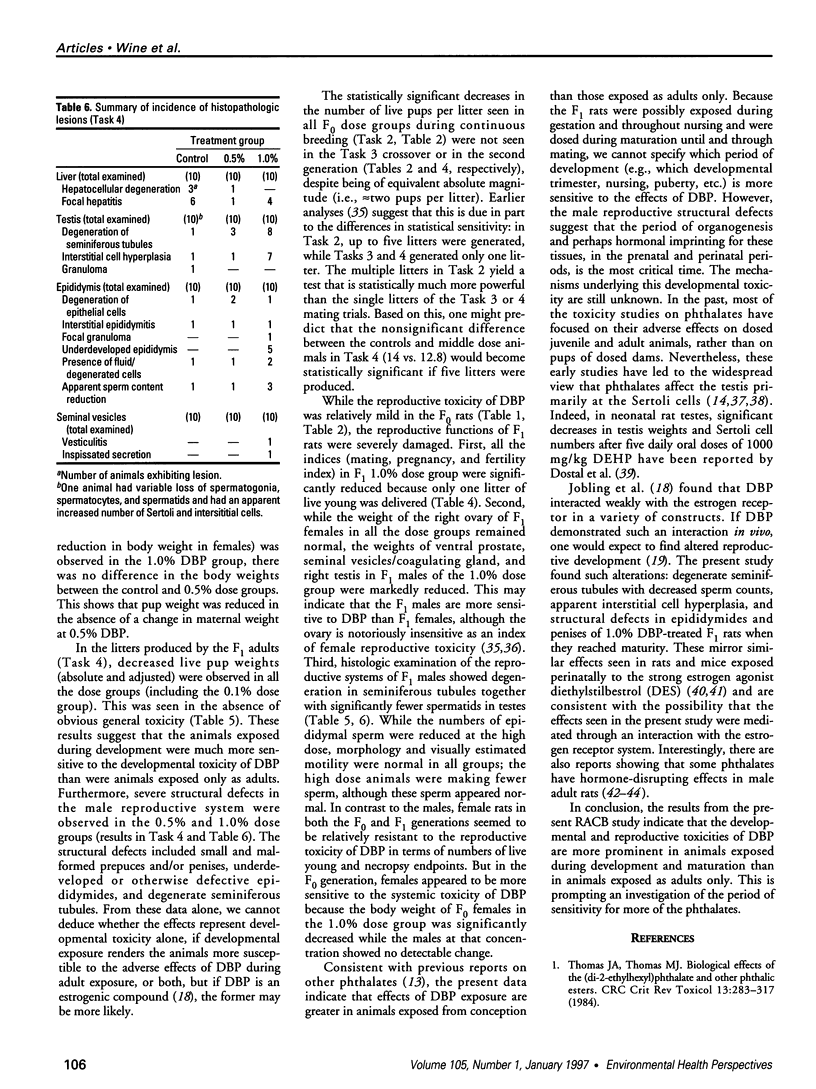
The phthalate ester di-n-butylphthalate (DBP) is used extensively in the manufacture of plastics; its reproductive toxicity was tested in rats by the National Toxicology Program's Reproductive Assessment by Continuous Breeding protocol. Levels of 0.1, 0.5, and 1.0% DBP in the diet were selected, and this dosing design yielded average daily DBP intakes of 52, 256, and 509 mg/kg for males and 80, 385, and 794 mg/kg for females, respectively. DBP consumption by F0 rats reduced the total number of live pups per litter in all treated groups by 8-17% and live pup weights in the 0.5% and 1.0% dose groups by < 13%. In tests to determine the affected sex, the number of offspring was unchanged, but the weights of pups from treated females were significantly decreased and offspring from treated males were unchanged. At necropsy, high-dose F0 females had a 14% reduction in body weight, and both sexes had approximately 10-15% increased kidney and liver to body weight ratios compared to controls. Sperm parameters and estrous cyclicity were not affected. In the F1 mating trial, indices of mating, pregnancy, and fertility in the 1.0% dose group were all sharply decreased (one live litter was delivered out of 20 cohabited pairs), concomitant with a 13% decrease in dam body weight. Live F2 pup weights were 6-8% lower in all dose groups. F1 necropsy results revealed that epididymal sperm counts and testicular spermatid head counts were significantly decreased in the 1.0% dose group. Histopathologic investigation showed that 8 of 10 F1 males consuming 1.0% DBP had degenerated seminiferous tubules and 5 of 10 had underdeveloped or otherwise defective epididymides. No ovarian or uterine lesions were observed. In conclusion, this study showed that DBP is a reproductive/developmental toxicant in Sprague-Dawley rats exposed both as adults and during development; it also indicates that the adverse reproductive/developmental effects of DBP on the second generation were greater than on the first generation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cater B. R., Cook M. W., Gangolli S. D., Grasso P. Studies on dibutyl phthalate-induced testicular atrophy in the rat: effect on zinc metabolism. Toxicol Appl Pharmacol. 1977 Sep;41(3):609–618. doi: 10.1016/s0041-008x(77)80014-8. [DOI] [PubMed] [Google Scholar]
- Creasy D. M., Beech L. M., Gray T. J., Butler W. H. The ultrastructural effects of di-n-pentyl phthalate on the testis of the mature rat. Exp Mol Pathol. 1987 Jun;46(3):357–371. doi: 10.1016/0014-4800(87)90056-6. [DOI] [PubMed] [Google Scholar]
- Dostal L. A., Chapin R. E., Stefanski S. A., Harris M. W., Schwetz B. A. Testicular toxicity and reduced Sertoli cell numbers in neonatal rats by di(2-ethylhexyl)phthalate and the recovery of fertility as adults. Toxicol Appl Pharmacol. 1988 Aug;95(1):104–121. doi: 10.1016/s0041-008x(88)80012-7. [DOI] [PubMed] [Google Scholar]
- Dostal L. A., Weaver R. P., Schwetz B. A. Transfer of di(2-ethylhexyl) phthalate through rat milk and effects on milk composition and the mammary gland. Toxicol Appl Pharmacol. 1987 Dec;91(3):315–325. doi: 10.1016/0041-008x(87)90054-8. [DOI] [PubMed] [Google Scholar]
- Ema M., Amano H., Ogawa Y. Characterization of the developmental toxicity of di-n-butyl phthalate in rats. Toxicology. 1994 Feb 7;86(3):163–174. doi: 10.1016/0300-483x(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Ema M., Kurosaka R., Amano H., Ogawa Y. Comparative developmental toxicity of n-butyl benzyl phthalate and di-n-butyl phthalate in rats. Arch Environ Contam Toxicol. 1995 Feb;28(2):223–228. doi: 10.1007/BF00217620. [DOI] [PubMed] [Google Scholar]
- Foster P. M., Thomas L. V., Cook M. W., Gangolli S. D. Study of the testicular effects and changes in zinc excretion produced by some n-alkyl phthalates in the rat. Toxicol Appl Pharmacol. 1980 Jul;54(3):392–398. doi: 10.1016/0041-008x(80)90165-9. [DOI] [PubMed] [Google Scholar]
- Foster P. M., Thomas L. V., Cook M. W., Walters D. G. Effect of DI-n-pentyl phthalate treatment on testicular steroidogenic enzymes and cytochrome P-450 in the rat. Toxicol Lett. 1983 Feb;15(2-3):265–271. doi: 10.1016/0378-4274(83)90226-6. [DOI] [PubMed] [Google Scholar]
- Fukuoka M., Kobayashi T., Hayakawa T. Mechanism of testicular atrophy induced by Di-n-butyl phthalate in rats. VI. A possible origin of testicular iron depletion. Biol Pharm Bull. 1994 Dec;17(12):1609–1612. doi: 10.1248/bpb.17.1609. [DOI] [PubMed] [Google Scholar]
- Fukuoka M., Kobayashi T., Zhou Y., Hayakawa T. Mechanism of testicular atrophy induced by di-n-butyl phthalate in rats. Part 4. Changes in the activity of succinate dehydrogenase and the levels of transferrin and ferritin in the Sertoli and germ cells. J Appl Toxicol. 1993 Jul-Aug;13(4):241–246. doi: 10.1002/jat.2550130406. [DOI] [PubMed] [Google Scholar]
- Fukuoka M., Tanimoto T., Zhou Y., Kawasaki N., Tanaka A., Ikemoto I., Machida T. Mechanism of testicular atrophy induced by di-n-butyl phthalate in rats. Part 1. J Appl Toxicol. 1989 Aug;9(4):277–283. doi: 10.1002/jat.2550090413. [DOI] [PubMed] [Google Scholar]
- Gray T. J., Gangolli S. D. Aspects of the testicular toxicity of phthalate esters. Environ Health Perspect. 1986 Mar;65:229–235. doi: 10.1289/ehp.8665229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati D. K., Hope E., Teague J., Chapin R. E. Reproductive toxicity assessment by continuous breeding in Sprague-Dawley rats: a comparison of two study designs. Fundam Appl Toxicol. 1991 Aug;17(2):270–279. doi: 10.1016/0272-0590(91)90218-s. [DOI] [PubMed] [Google Scholar]
- Hausen B. M., Milbrodt M., Koenig W. A. The allergens of nail polish. (I). Allergenic constituents of common nail polish and toluenesulfonamide-formaldehyde resin (TS-F-R). Contact Dermatitis. 1995 Sep;33(3):157–164. doi: 10.1111/j.1600-0536.1995.tb00537.x. [DOI] [PubMed] [Google Scholar]
- Jobling S., Reynolds T., White R., Parker M. G., Sumpter J. P. A variety of environmentally persistent chemicals, including some phthalate plasticizers, are weakly estrogenic. Environ Health Perspect. 1995 Jun;103(6):582–587. doi: 10.1289/ehp.95103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. B., Garside D. A., Liu R., Roberts J. C. The influence of phthalate esters on Leydig cell structure and function in vitro and in vivo. Exp Mol Pathol. 1993 Jun;58(3):179–193. doi: 10.1006/exmp.1993.1016. [DOI] [PubMed] [Google Scholar]
- Kluwe W. M. Overview of phthalate ester pharmacokinetics in mammalian species. Environ Health Perspect. 1982 Nov;45:3–9. doi: 10.1289/ehp.82453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lygre H., Solheim E., Gjerdet N. R. Leaching from denture base materials in vitro. Acta Odontol Scand. 1995 Apr;53(2):75–80. doi: 10.3109/00016359509005950. [DOI] [PubMed] [Google Scholar]
- Morrissey R. E., Lamb J. C., 4th, Morris R. W., Chapin R. E., Gulati D. K., Heindel J. J. Results and evaluations of 48 continuous breeding reproduction studies conducted in mice. Fundam Appl Toxicol. 1989 Nov;13(4):747–777. doi: 10.1016/0272-0590(89)90332-1. [DOI] [PubMed] [Google Scholar]
- Newbold R. R., Bullock B. C., McLachlan J. A. Testicular tumors in mice exposed in utero to diethylstilbestrol. J Urol. 1987 Dec;138(6):1446–1450. doi: 10.1016/s0022-5347(17)43672-x. [DOI] [PubMed] [Google Scholar]
- Nikonorow M., Mazur H., Piekacz H. Effect of orally administered plasticizers and polyvinyl chloride stabilizers in the rat. Toxicol Appl Pharmacol. 1973 Oct;26(2):253–259. doi: 10.1016/0041-008x(73)90259-7. [DOI] [PubMed] [Google Scholar]
- Oishi S., Hiraga K. Effect of phthalic acid esters on mouse testes. Toxicol Lett. 1980 May;5(6):413–416. doi: 10.1016/0378-4274(80)90024-7. [DOI] [PubMed] [Google Scholar]
- Rutledge L. C., Gupta R. K., Wirtz R. A., Buescher M. D. Evaluation of the laboratory mouse model for screening topical mosquito repellents. J Am Mosq Control Assoc. 1994 Dec;10(4):565–571. [PubMed] [Google Scholar]
- Schwetz B. A., Harris M. W. Developmental toxicology: status of the field and contribution of the National Toxicology Program. Environ Health Perspect. 1993 Apr;100:269–282. doi: 10.1289/ehp.93100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley E. A non-parametric equivalent of Williams' test for contrasting increasing dose levels of a treatment. Biometrics. 1977 Jun;33(2):386–389. [PubMed] [Google Scholar]
- Thomas J. A., Thomas M. J. Biological effects of di-(2-ethylhexyl) phthalate and other phthalic acid esters. Crit Rev Toxicol. 1984;13(4):283–317. doi: 10.3109/10408448409023761. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Wienckowski D. B., Gillies B. A., Thomas M. J., Youkilis E. J. Effects of phthalic acid esters (PAEs) on the neonate and aspects of teratogenic actions. Environ Health Perspect. 1986 Mar;65:243–248. doi: 10.1289/ehp.8665243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorherr H., Messer R. H., Vorherr U. F., Jordan S. W., Kornfeld M. Teratogenesis and carcinogenesis in rat offspring after transplacental and transmammary exposure to diethylstilbestrol. Biochem Pharmacol. 1979 Jun 15;28(12):1865–1877. doi: 10.1016/0006-2952(79)90638-5. [DOI] [PubMed] [Google Scholar]
- Williams D. A. A note on Shirley's nonparametric test for comparing several dose levels with a zero-dose control. Biometrics. 1986 Mar;42(1):183–186. [PubMed] [Google Scholar]
- Wu C. T., Pei X. T., Cao J. R., Xue H. H. A new pharmacological activity of dibutyl phthalate (DBP) on selective elimination of tumor cells from bone marrow. Leuk Res. 1993 Jul;17(7):557–560. doi: 10.1016/0145-2126(93)90084-x. [DOI] [PubMed] [Google Scholar]
- Wu C., Yang K., Pei X., Tang A., Wang F., Wang L. Bone marrow purging with dibutyl phthalate--experimental basis and a preliminary clinical application. Leuk Res. 1995 Aug;19(8):557–560. doi: 10.1016/0145-2126(95)00038-p. [DOI] [PubMed] [Google Scholar]


