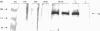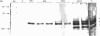Abstract
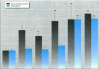
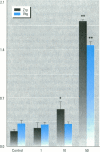
Environmental estrogens have recently caused great concern because of their ability to mimic natural hormones and influence vital endocrine functions in humans and wildlife. The induction of vitellogenin (Vtg) synthesis by environmental estrogens in viviparous vertebrates has been proposed as an effective and sensitive biomarker of estrogenicity. Immunochemical analysis of plasma from Atlantic salmon (Salmo salar) exposed to 4-nonylphenol (NP) or to effluent from oil refinery treatment plant (ORTP), shows that NP and ORTP effluent induces Vtg and zona radiata proteins (Zrp) in a dose-dependent manner. However, Zrp-beta cross-reactive proteins are more responsive than Zrp-alpha, Zrp-gamma, and Vtg. The sensitivity of Zrp induction points to the zona radiata proteins as alternate biomarkers of estrogenicity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold S. F., Klotz D. M., Collins B. M., Vonier P. M., Guillette L. J., Jr, McLachlan J. A. Synergistic activation of estrogen receptor with combinations of environmental chemicals. Science. 1996 Jun 7;272(5267):1489–1492. doi: 10.1126/science.272.5267.1489. [DOI] [PubMed] [Google Scholar]
- Bleil J. D., Wassarman P. M. Structure and function of the zona pellucida: identification and characterization of the proteins of the mouse oocyte's zona pellucida. Dev Biol. 1980 Apr;76(1):185–202. doi: 10.1016/0012-1606(80)90371-1. [DOI] [PubMed] [Google Scholar]
- Colborn T., vom Saal F. S., Soto A. M. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993 Oct;101(5):378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diano M., Le Bivic A., Hirn M. A method for the production of highly specific polyclonal antibodies. Anal Biochem. 1987 Oct;166(1):224–229. doi: 10.1016/0003-2697(87)90568-9. [DOI] [PubMed] [Google Scholar]
- Foster W. G. The reproductive toxicology of Great Lakes contaminants. Environ Health Perspect. 1995 Dec;103 (Suppl 9):63–69. doi: 10.1289/ehp.95103s963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillette L. J., Jr, Gross T. S., Gross D. A., Rooney A. A., Percival H. F. Gonadal steroidogenesis in vitro from juvenile alligators obtained from contaminated or control lakes. Environ Health Perspect. 1995 May;103 (Suppl 4):31–36. doi: 10.1289/ehp.95103s431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart N. H. Fertilization in teleost fishes: mechanisms of sperm-egg interactions. Int Rev Cytol. 1990;121:1–66. doi: 10.1016/s0074-7696(08)60658-0. [DOI] [PubMed] [Google Scholar]
- Hyllner S. J., Oppen-Berntsen D. O., Helvik J. V., Walther B. T., Haux C. Oestradiol-17 beta induces the major vitelline envelope proteins in both sexes in teleosts. J Endocrinol. 1991 Nov;131(2):229–236. doi: 10.1677/joe.0.1310229. [DOI] [PubMed] [Google Scholar]
- Hyllner S. J., Silversand C., Haux C. Formation of the vitelline envelope precedes the active uptake of vitellogenin during oocyte development in the rainbow trout, Oncorhynchus mykiss. Mol Reprod Dev. 1994 Oct;39(2):166–175. doi: 10.1002/mrd.1080390208. [DOI] [PubMed] [Google Scholar]
- Kavlock R. J., Daston G. P., DeRosa C., Fenner-Crisp P., Gray L. E., Kaattari S., Lucier G., Luster M., Mac M. J., Maczka C. Research needs for the risk assessment of health and environmental effects of endocrine disruptors: a report of the U.S. EPA-sponsored workshop. Environ Health Perspect. 1996 Aug;104 (Suppl 4):715–740. doi: 10.1289/ehp.96104s4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Norberg B., Haux C. Induction, isolation and a characterization of the lipid content of plasma vitellogenin from two Salmo species: rainbow trout (Salmo gairdneri) and sea trout (Salmo trutta). Comp Biochem Physiol B. 1985;81(4):869–876. doi: 10.1016/0305-0491(85)90081-1. [DOI] [PubMed] [Google Scholar]
- Oppen-Berntsen D. O., Gram-Jensen E., Walther B. T. Zona radiata proteins are synthesized by rainbow trout (Oncorhynchus mykiss) hepatocytes in response to oestradiol-17 beta. J Endocrinol. 1992 Nov;135(2):293–302. doi: 10.1677/joe.0.1350293. [DOI] [PubMed] [Google Scholar]
- Oppen-Berntsen D. O., Hyllner S. J., Haux C., Helvik J. V., Walther B. T. Eggshell zona radiata-proteins from cod (Gadus morhua): extra-ovarian origin and induction by estradiol-17 beta. Int J Dev Biol. 1992 Jun;36(2):247–254. [PubMed] [Google Scholar]
- Palmer B. D., Palmer S. K. Vitellogenin induction by xenobiotic estrogens in the red-eared turtle and African clawed frog. Environ Health Perspect. 1995 May;103 (Suppl 4):19–25. doi: 10.1289/ehp.95103s419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto A. M., Justicia H., Wray J. W., Sonnenschein C. p-Nonyl-phenol: an estrogenic xenobiotic released from "modified" polystyrene. Environ Health Perspect. 1991 May;92:167–173. doi: 10.1289/ehp.9192167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumpter J. P. Feminized responses in fish to environmental estrogens. Toxicol Lett. 1995 Dec;82-83:737–742. doi: 10.1016/0378-4274(95)03517-6. [DOI] [PubMed] [Google Scholar]
- Sumpter J. P., Jobling S. Vitellogenesis as a biomarker for estrogenic contamination of the aquatic environment. Environ Health Perspect. 1995 Oct;103 (Suppl 7):173–178. doi: 10.1289/ehp.95103s7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata J. R., Smith D. F. Vitellogenesis: a versatile model for hormonal regulation of gene expression. Recent Prog Horm Res. 1979;35:47–95. doi: 10.1016/b978-0-12-571135-7.50006-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassarman P. M. The biology and chemistry of fertilization. Science. 1987 Jan 30;235(4788):553–560. doi: 10.1126/science.3027891. [DOI] [PubMed] [Google Scholar]
- Wassarman P. M. Zona pellucida glycoproteins. Annu Rev Biochem. 1988;57:415–442. doi: 10.1146/annurev.bi.57.070188.002215. [DOI] [PubMed] [Google Scholar]
- White R., Jobling S., Hoare S. A., Sumpter J. P., Parker M. G. Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology. 1994 Jul;135(1):175–182. doi: 10.1210/endo.135.1.8013351. [DOI] [PubMed] [Google Scholar]
- Wiley H. S., Opresko L., Wallace R. A. New methods for the purification of vertebrate vitellogenin. Anal Biochem. 1979 Aug;97(1):145–152. doi: 10.1016/0003-2697(79)90338-5. [DOI] [PubMed] [Google Scholar]
- Yamagami K., Hamazaki T. S., Yasumasu S., Masuda K., Iuchi I. Molecular and cellular basis of formation, hardening, and breakdown of the egg envelope in fish. Int Rev Cytol. 1992;136:51–92. doi: 10.1016/s0074-7696(08)62050-1. [DOI] [PubMed] [Google Scholar]