Abstract
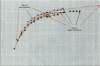
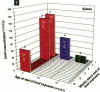
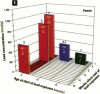
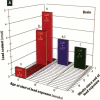
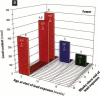
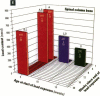
It has been hypothesized that the high rate of bone remodeling during childhood and the consequent high calcium and lead turnover result in a substantial reduction in bone lead stores so that much of the lead incorporated in bone during childhood does not persist into adulthood. We studied the effect of age at lead exposure on blood and organ concentrations of lead, calcium, and zinc 1-5 months after termination of lead ingestion. Blood and organ lead concentrations and contents 4 weeks after lead exposure ceased were significantly higher in the rats exposed beginning at 5 weeks of age than in those exposed beginning at 10 or 15 weeks old. Bone lead declined as the time since exposure increased. Despite this trend, the rats exposed when youngest had bone lead concentrations at 20 weeks after the termination of lead exposure that were higher than those of the other rats only 4 weeks after cessation of lead ingestion. Multiple regression analysis demonstrated that age at lead exposure remained a significant predictor of blood and organ lead concentrations and contents even after the inclusion of total lead consumed, body weight, and age at organ harvesting in the regression analysis. There were only small differences in organ calcium and zinc concentrations among treatment groups except for kidney calcium. The results do not support the hypothesis of rapid depletion of bone lead stores in young animals, but rather suggest that younger age at lead exposure is associated with greater lead retention and toxicity even in the absence of continued lead exposure.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bogden J. D., Gertner S. B., Christakos S., Kemp F. W., Yang Z., Katz S. R., Chu C. Dietary calcium modifies concentrations of lead and other metals and renal calbindin in rats. J Nutr. 1992 Jul;122(7):1351–1360. doi: 10.1093/jn/122.7.1351. [DOI] [PubMed] [Google Scholar]
- Bogden J. D., Gertner S. B., Kemp F. W., McLeod R., Bruening K. S., Chung H. R. Dietary lead and calcium: effects on blood pressure and renal neoplasia in Wistar rats. J Nutr. 1991 May;121(5):718–728. doi: 10.1093/jn/121.5.718. [DOI] [PubMed] [Google Scholar]
- Bogden J. D., Kemp F. W., Han S., Murphy M., Fraiman M., Czerniach D., Flynn C. J., Banua M. L., Scimone A., Castrovilly L. Dietary calcium and lead interact to modify maternal blood pressure, erythropoiesis, and fetal and neonatal growth in rats during pregnancy and lactation. J Nutr. 1995 Apr;125(4):990–1002. doi: 10.1093/jn/125.4.990. [DOI] [PubMed] [Google Scholar]
- Han S., Qiao X., Simpson S., Ameri P., Kemp F. W., Bogden J. D. Weight loss alters organ concentrations and contents of lead and some essential divalent metals in rats previously exposed to lead. J Nutr. 1996 Jan;126(1):317–323. doi: 10.1093/jn/126.1.317. [DOI] [PubMed] [Google Scholar]
- Hu H., Aro A., Payton M., Korrick S., Sparrow D., Weiss S. T., Rotnitzky A. The relationship of bone and blood lead to hypertension. The Normative Aging Study. JAMA. 1996 Apr 17;275(15):1171–1176. [PubMed] [Google Scholar]
- Hu H., Watanabe H., Payton M., Korrick S., Rotnitzky A. The relationship between bone lead and hemoglobin. JAMA. 1994 Nov 16;272(19):1512–1517. [PubMed] [Google Scholar]
- Kosnett M. J., Becker C. E., Osterloh J. D., Kelly T. J., Pasta D. J. Factors influencing bone lead concentration in a suburban community assessed by noninvasive K x-ray fluorescence. JAMA. 1994 Jan 19;271(3):197–203. [PubMed] [Google Scholar]
- Kostial K., Kello D., Jugo S., Rabar I., Maljković T. Influence of age on metal metabolism and toxicity. Environ Health Perspect. 1978 Aug;25:81–86. doi: 10.1289/ehp.782581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggett R. W. An age-specific kinetic model of lead metabolism in humans. Environ Health Perspect. 1993 Dec;101(7):598–616. doi: 10.1289/ehp.93101598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushak P., Davis J. M., Crocetti A. F., Grant L. D. Prenatal and postnatal effects of low-level lead exposure: integrated summary of a report to the U.S. Congress on childhood lead poisoning. Environ Res. 1989 Oct;50(1):11–36. doi: 10.1016/s0013-9351(89)80046-5. [DOI] [PubMed] [Google Scholar]
- Needleman H. L., Riess J. A., Tobin M. J., Biesecker G. E., Greenhouse J. B. Bone lead levels and delinquent behavior. JAMA. 1996 Feb 7;275(5):363–369. [PubMed] [Google Scholar]
- Nordberg G. F., Mahaffey K. R., Fowler B. A. Introduction and summary. International workshop on lead in bone: implications for dosimetry and toxicology. Environ Health Perspect. 1991 Feb;91:3–7. doi: 10.1289/ehp.91913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty E. J. Physiologically based models for bone-seeking elements. II. Kinetics of lead disposition in rats. Toxicol Appl Pharmacol. 1991 Nov;111(2):313–331. doi: 10.1016/0041-008x(91)90033-b. [DOI] [PubMed] [Google Scholar]
- O'Flaherty E. J. Physiologically based models for bone-seeking elements. V. Lead absorption and disposition in childhood. Toxicol Appl Pharmacol. 1995 Apr;131(2):297–308. doi: 10.1006/taap.1995.1072. [DOI] [PubMed] [Google Scholar]
- Pounds J. G. Effect of lead intoxication on calcium homeostasis and calcium-mediated cell function: a review. Neurotoxicology. 1984 Fall;5(3):295–331. [PubMed] [Google Scholar]
- Rader J. I., Celesk E. M., Peeler J. T., Mahaffey K. R. Retention of lead acetate in weanling and adult rats. Toxicol Appl Pharmacol. 1983 Jan;67(1):100–109. doi: 10.1016/0041-008x(83)90248-x. [DOI] [PubMed] [Google Scholar]
- Silbergeld E. K. Lead in bone: implications for toxicology during pregnancy and lactation. Environ Health Perspect. 1991 Feb;91:63–70. doi: 10.1289/ehp.919163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmers L. E., Jr, Aufderheide A. C., Wallgren J., Rapp G., Jr, Alich A. Lead in bone. IV. Distribution of lead in the human skeleton. Arch Environ Health. 1988 Nov-Dec;43(6):381–391. doi: 10.1080/00039896.1988.9935855. [DOI] [PubMed] [Google Scholar]